Translate this page into:
Efficacy and safety of intralesional triple combination versus intralesional triamcinolone acetonide for the treatment of keloids: A randomised controlled trial
Corresponding author: Dr. Jude Ernest Dileep, Department of Dermatology, Venereology and Leprosy, Aarupadai Veedu Medical College and Hospital, Kirumampakkam, Puducherry, India. judegenext@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Menon TSK, Dileep JE, Kuruvila S, Kaliyaperumal D, Sadasivam IP, Dharanisankar S, et al. Efficacy and safety of intralesional triple combination versus intralesional triamcinolone acetonide for the treatment of keloids: A randomised controlled trial. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1263_2024
Abstract
Background
Treatment of keloids presents a significant therapeutic challenge due to their tendency to recur and their impact on a patient’s quality of life. This randomised controlled trial aimed to compare the effectiveness of intralesional triple combination regimen versus intralesional triamcinolone acetonide monotherapy in treating keloids.
Aims
To compare the efficacy and safety of intralesional triple combination versus intralesional triamcinolone acetonide monotherapy in treating keloids at any site.
Methods
This study was conducted in the outpatient department of Dermatology, Venereology and Leprosy in a tertiary care hospital at Puducherry. Seventy two patients aged ≥18 years with a clinical diagnosis of keloids of any duration, involving any site and without any prior treatment were included in the study. Patients were randomised into two groups: Group A received intralesional triple combination (triamcinolone acetonide, 5-fluorouracil and hyaluronidase), while Group B received intralesional triamcinolone acetonide monotherapy. Treatments were administered every three weeks for four sessions or till complete flattening, whichever was earlier. The Vancouver Scar Scale was used for assessment at baseline and every three weeks for four sessions, and monthly for three months post treatment.
Results
Both groups showed significant improvement in the Vancouver Scar Scale scores at each follow-up compared to baseline. The mean (percentage) improvement in the Vancouver Scar Scale score in Group A was 0.58 ± 0.5 (7.08%) at three weeks, which progressively increased to 4.47 ± 1.29 (54.55%) at the final follow-up. In Group B, the improvement was lesser, with 0.08 ± 0.28 (0.95%) at three weeks, increasing to 3.08 ± 0.81 (36.65%) at the final follow-up. This improvement was significantly more in Group A at all time points compared to Group B (p < 0.05). Post-procedure pain, which lasted for a few hours, was noted in three and two patients in Groups A and B, respectively (p = 0.642). None of the patients had a recurrence of keloids during the study.
Limitations
Limitations of this study include small sample size, single centre design, short follow-up period, lack of blinding and patient-reported outcome measures, which may impact the generalisability of the findings.
Conclusion
Intralesional triple combination is more effective than triamcinolone acetonide monotherapy in treating keloids, offering significantly superior improvements in the Vancouver Scar Scale scoring.
Keywords
Keloid
triamcinolone acetonide
5-fluorouracil
hyaluronidase
Introduction
Present therapeutic options for the treatment of keloids consist of topical and intralesional treatments, surgical procedures, cryotherapy, radiation therapy and laser therapy.1–3 It has been documented that the addition of 0.6 ml of 5-fluorouracil 50 mg/mL to 0.4 mL of triamcinolone acetonide 40 mg/mL prevents the production of type I collagen by fibroblast and results in a rapid response in terms of scar flattening and reduction of adverse effects.4 Further addition of 1500 IU hyaluronidase in conjunction with triamcinolone acetonide and 5-fluorouracil was observed to dissolve the excessive quantity of collagen that had been irregularly deposited into the dermis to form a keloid.5
The triple combination used here consists of three medications with different mechanisms of action, acting synergistically in treating keloids. These include triamcinolone acetonide, a corticosteroid with anti-inflammatory action; 5-fluorouracil, an anti-metabolite which interferes with fibroblast proliferation; and hyaluronidase, an enzyme which dissolves hyaluronic acid fibrous bands.6,7 Currently, there is insufficient evidence comparing the efficacy and side-effects of intralesional triple combination to triamcinolone acetonide monotherapy. The triple combination is a relatively new modality that could exhibit promising and long-lasting effects in the treatment of keloids.8
The primary objective of this study was to compare the efficacy of intralesional triple combination versus triamcinolone acetonide monotherapy in treating keloids using the Vancouver Scar Scale and the secondary objective was to compare their side effects.
Methods
This was a hospital-based randomised controlled trial conducted in the Department of Dermatology, Venereology and Leprosy in a tertiary care hospital at Puducherry, India, between November 2022 and April 2024. The study was approved by the Institutional Human Ethics Committee (AV/IHEC/2022/072) and registered with the Clinical Trials Registry – India (CTRI No: CTRI/061930). Keloid was diagnosed clinically and differentiated from hypertrophic scar by its extension beyond the wound margins, presence of pain and itching and lack of spontaneous regression. All patients aged ≥18 years of both genders with a clinical diagnosis of keloid of any duration, site and without a prior treatment history were included in the study after obtaining written informed consent. Exclusion criteria included pregnancy, lactation, heart disease, liver and kidney disease, immunocompromised status and patients unable to commit to the follow-up schedule of the study [Figure 1].

- Consort flow chart.
The sample size of 72 (36 in each group) was calculated using the statistical formula for the comparison of two independent means with an expected mean difference in the Vancouver Scar Scale score of 3 and a standard deviation of 4, based on a similar study by Goyal et al.,8 with the level of significance and power as 5% and 80%, respectively. A non-probability convenience sampling technique was used to recruit the study participants. Simple randomisation was done using computer-generated random numbers to allocate the participants to Group A – intralesional triple combination and Group B – intralesional triamcinolone acetonide monotherapy. Allocation concealment was done using sealed envelopes. [Figure 1].
Patients allocated to Group A received intralesional triple combination. This was prepared by adding 0.4 mL of triamcinolone acetonide (40 mg/mL) to 0.6 mL of 5-fluorouracil (250 mg/5mL) and further incorporating this mixture into a vial of vacuum-dried tablet of hyaluronidase (1500 IU). Those in Group B received intralesional triamcinolone acetonide (40 mg/mL) alone. For those who had multiple keloids in Group A, the largest keloid was given intralesional triple combination while the rest were treated with intralesional triamcinolone acetonide monotherapy, which is the standard of care. The procedure was performed four times, once every three weeks (zero, three, six and nine weeks) or till complete flattening of the keloid, whichever was earlier, and patients were followed up monthly for three months (24 weeks from baseline). All patients were followed up until 24 weeks, even if the scar flattening was achieved earlier. Scar evaluation at each stage was done by the Vancouver Scar Scale by a single observer. Vascularity was rated based on visual inspection and the refill rate after blanching. Pigmentation was assessed after blanching and comparing the scar colour with the surrounding skin. Pliability was subjectively assessed by palpation and the height was accurately measured with callipers.
The data was analysed using the Statistical Package for the Social Sciences version 21. Categorical variables were summarised as frequency with percentage and continuous variables were summarised as mean (± standard deviation). Chi-square test was used to compare the association between the categorical variables. The normality of the data was checked using the Shapiro-Wilk test. Mann Whitney U test was performed to compare the Vancouver Scar Scale between the groups and for the association of other continuous variables. Paired t-test was used to compare the scale during the follow-up period. A p-value of <0.05 was considered statistically significant.
Results
The mean age was 29.83 ± 13.69 years in Group A and 28.31 ± 14.07 years in Group B. The mean length, breadth and height were 3.26 ± 3.36, 1.34 ± 1.22 and 0.27 ±0.11 centimeters, respectively in Group A and 3.69 ± 3.98, 1.40 ± 1.40 and 0.29 ± 0.21 centimeters, respectively in Group B. The mean duration of keloids was 5.78 ± 4.01 years in Group A and 5.11 ± 4.37 years in Group B. The demographic characteristics of the study population, including age, gender, number, site and size of keloids, were comparable between both groups, indicating a well-matched cohort [Table 1].
| Parameters | Group A | Group B | p-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age (years) | < 20 | 13 | 36.1 | 18 | 50 | 0.520 |
| 21–40 | 13 | 36.1 | 8 | 22.2 | ||
| 41–60 | 8 | 22.2 | 7 | 19.4 | ||
| 61 | 2 | 5.6 | 3 | 8.3 | ||
| Gender | Male | 17 | 47.2 | 19 | 53.8 | 0.637 |
| Female | 19 | 53.8 | 17 | 47.2 | ||
| Number | Single | 32 | 88.9 | 31 | 86.1 | 0.722 |
| Multiple | 4 | 11.1 | 5 | 13.9 | ||
| Site | Abdomen | 2 | 5.6 | 2 | 5.6 | 0.812 |
| Arm | 3 | 8.3 | 5 | 13.9 | ||
| Breast | 2 | 5.6 | 0 | 0 | ||
| Chest | 15 | 41.7 | 12 | 33.3 | ||
| Ear lobe | 1 | 2.8 | 3 | 8.3 | ||
| Elbow | 1 | 2.8 | 3 | 8.3 | ||
| Face | 3 | 8.3 | 2 | 5.6 | ||
| Forearm | 1 | 2.8 | 2 | 5.6 | ||
| Neck | 1 | 2.8 | 2 | 5.6 | ||
| Shoulder | 4 | 11.1 | 3 | 8.3 | ||
| Wrist | 3 | 8.3 | 2 | 5.6 | ||
| Size (centimetres) | ≤5 | 27 | 75 | 29 | 80.6 | 0.571 |
| >5 | 9 | 25 | 7 | 19.4 | ||
| Duration (years) | <1 | 0 | 0 | 2 | 5.6 | 0.014 |
| 1–5 | 6 | 16.7 | 6 | 16.7 | ||
| 6–10 | 30 | 83.3 | 21 | 58.3 | ||
| >10 | 0 | 0 | 7 | 19.4 | ||
Both groups demonstrated an improvement in the Vancouver Scar Scale score compared to the baseline. Group A demonstrated a mean (percentage) improvement in the Vancouver Scar Scale score of 0.58 ± 0.5 (7.08%) at three weeks, 1.39 ± 0.49 (16.83%) at six weeks and 2.08 ± 0.99 (25.40%) at nine weeks. By the first follow-up, mean (percentage) improvement reached 2.58 ± 1.23 (31.48%), increasing to 3.75 ± 1.20 (45.78%) and 4.47 ± 1.29 (54.55%) at the second and third follow-up, respectively. This reduction was statistically significant at all visits (three weeks, six weeks and nine weeks and the first, second and third follow-ups), with p values <0.001 from three weeks [Table 2].
| Vancouver scar scale | Group A | Group B | p-value of difference in improvement from baseline between Groups A and B | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Improvement from baseline Mean ± SD (%) | p-value of improvement from baseline | Mean ± SD | Improvement from baseline Mean ± SD (%) | p-value of improvement from baseline | ||
| Zero week (baseline) | 8.19 ± 1.14 | 8.39 ± 0.96 | |||||
| Three weeks | 7.61 ± 1.55 | 0.58 ± 0.5 (07.08) | < 0.001* | 8.31 ± 1.04 | 0.08 ± 0.28 (0.95) | 0.083 | 0.002* |
| Six weeks | 6.81 ± 0.82 | 1.39 ± 0.49 (16.83) | < 0.001* | 7.58 ± 1.05 | 0.81 ± 0.62 (9.66) | < 0.001* | 0.002* |
| Nine weeks | 6.11 ± 0.32 | 2.08 ± 0.99 (25.40) | < 0.001* | 7.14 ± 1.02 | 1.25 ± 0.25 (14.89) | < 0.001* | 0.000* |
| First follow-up post treatment (4 weeks) | 5.61 ± 0.49 | 2.58 ± 1.23 (31.48) | < 0.001* | 6.58 ± 1.02 | 1.81 ± 0.67 (21.56) | < 0.001* | 0.000* |
| Second follow-up post treatment (8 weeks) | 4.44 ± 0.56 | 3.75 ± 1.20 (45.78) | < 0.001* | 5.94 ± 1.09 | 2.44 ± 0.81 (29.21) | < 0.001* | 0.000* |
| Third follow-up post treatment (12 weeks) | 3.72 ± 0.57 | 4.47 ± 1.29 (54.55) | < 0.001* | 5.31 ± 1.04 | 3.08 ± 0.81 (36.65) | < 0.001* | 0.000* |
SD: Standard Deviation, *: Significant p-values for this study, Group A: Intralesional triple combination, Group B: Intralesional triamcinolone acetonide monotherapy
In Group B, the improvement was lesser, with 0.08 ± 0.28 (0.95%) at three weeks, 0.81 ± 0.62 (9.66%) at six weeks and 1.25 ± 0.25 (14.89%) at nine weeks. This further increased to 1.81 ± 0.67 (21.56%) at the first follow-up, rising to 2.44 ± 0.81 (29.21%) and 3.08 ± 0.81 (36.65%) at the second and third follow-ups, respectively. This improvement was statistically significant from six weeks onwards (p < 0.001) [Table 2].
The improvement of the mean Vancouver Scar Scale score was significantly higher in Group A compared to Group B at all the time points (three weeks, six weeks and nine weeks and the first, second and third follow-ups) (p < 0.05) [Figure 2 and Table 2].
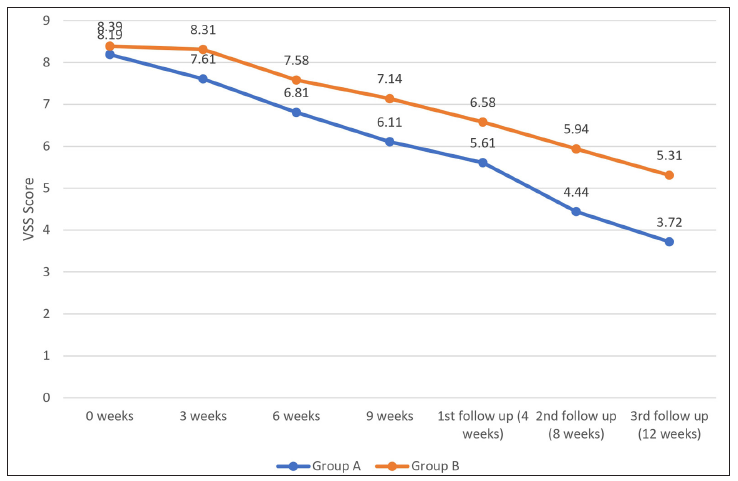
- Graph demonstrating the Vancouver Scar Scale score distribution among groups A & B.
Both the groups experienced a reduction in all four aspects of the Vancouver Scar Scale, that is, vascularity, pigmentation, pliability and height scores, compared to baseline. Among the four components of the Vancouver Scar Scale, a significantly higher improvement in pliability was noted in Group A compared to Group B from three weeks (p < 0.05). Significantly higher improvements in vascularity, pigmentation and height were noted in Group A during the second and third follow-ups. Three patients in Group A achieved complete flattening and none of the patients in both Group A and Group B showed any recurrence. [Table 3].
| Duration | Vascularity | Pigmentation | Pliability | Height | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | p-value | Group A | Group B | p-value | Group A | Group B | p-value | Group A | Group B | p-value | |
| Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | |||||
| 0 weeks | 2.08 (± 0.77) | 1.97 (±0.81) | 0.601 | 1.42 (±0.69) | 1.44 (± 0.69) | 0.99 | 2.61 (±1.15) | 3.06 (±1.17) | 0.117 | 2.08 (± 0.81) | 1.92 (± 0.77) | 0.312 |
| Three weeks | 1.83 (± 0.81) | 1.97 (±0.81) | 0.399 | 1.31 (±0.67) | 1.44 (± 0.69) | 0.387 | 2.42 (±1.16) | 2.97 (±2.25) | 0.053* | 2.06 (±0.79) | 1.92 (± 0.77) | 0.385 |
| Six weeks | 1.69 (± 0.67) | 1.78 (±0.76) | 0.576 | 1.28 (±0.66) | 1.36 (± 0.59) | 0.632 | 1.97 (± 0.77) | 2.61 (±1.08) | 0.006* | 1.86 (±0.72) | 1.83 (± 0.81) | 0.730 |
| Nine weeks | 1.53 (± 0.56) | 1.67 (±0.68) | 0.318 | 1.08 (±0.65) | 1.36 (± 0.59) | 0.068 | 1.78 (±0.68) | 2.36 (±0.96) | 0.004* | 1.72 (±0.74) | 1.75 (± 0.73) | 0.971 |
| First follow-up post treatment (4 weeks) | 1.42 (± 0.55) | 1.56 (±0.65) | 0.287 | 1.06 (±0.63) | 1.28 (±0.61) | 0.134 | 1.56 (±0.56) | 2.11 (±0.82) | 0.001* | 1.58 (±0.73) | 1.64 (± 0.64) | 0.700 |
| Second follow-up post treatment (8 weeks) | 1.14 (± 0.35) | 1.42 (±0 65) | 0.029* | 0.72 (±0.57) | 1.17 (± 0.65) | 0.004* | 1.31 (± 0.47) | 1.83 (±0.70) | 0.000* | 1.28 (±0.61) | 1.53 (± 0.61) | 0.094* |
| Third follow-up post treatment (12 weeks) | 1.03 (± 0.17) | 1.31 (±0.58) | 0.006* | 0.53 (±0.56) | 1.03 (±0.61) | 0.001* | 1.08 (± 0.44) | 1.56 (±0.69) | 0.001* | 1.08 (±0.50) | 1.42 (± 0.55) | 0.013* |
Although all patients experienced pain during the procedure, only three patients in Group A and two patients in Group B had pain which lasted for a few hours. This was not significantly different between the groups (p = 0.642).
In this 24 weeks study, there were no reports of any atrophy, hypopigmentation, ulceration or infection for both groups. None of the patients came back with recurrence in this 24 weeks study. Three patients in Group A and none of the patients in Group B achieved complete flattening during the third review.
Discussion
This study compared intralesional triple combination versus triamcinolone acetonide monotherapy in terms of efficacy and safety for treating keloids.
The assessment of keloid characteristics, including vascularity, pigmentation, pliability, height and overall scar severity using the Vancouver Scar Scale, provided comprehensive insights into the efficacy of the treatment modalities. Both intralesional triple combination (Group A) and triamcinolone acetonide monotherapy (Group B) showed improvement in these parameters over the course of the study, indicating the effectiveness of the treatment interventions. However, notable differences were observed between the two groups in vascularity, pigmentation, pliability and height severity scores, as evidenced by the significant differences in mean scores at various time points [Table 3, and Figures 3–6].
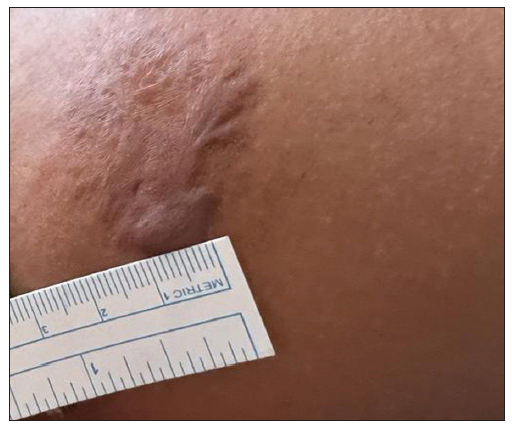
- Photograph at the baseline of a patient with four years history of keloid over the right upper arm before treatment with triple combination. (VSS Score = 10)
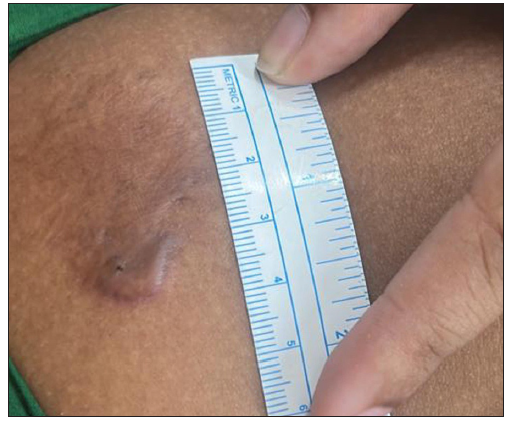
- Photograph at the end of 24 weeks of a patient with four years history of keloid over the right upper arm treated with triple combination. (VSS Score = 5)
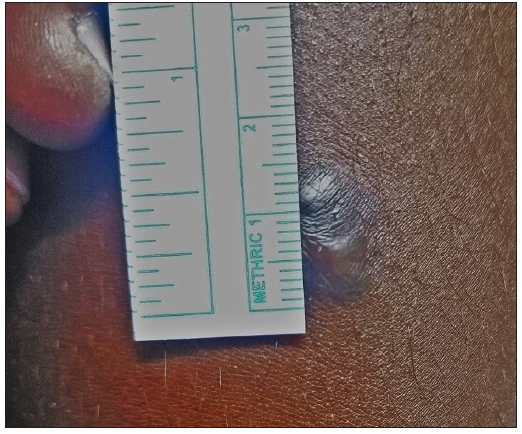
- Photograph at the baseline of a patient with seven years history of keloid over the left arm before treatment with triamcinolone acetonide. (VSS Score = 10)
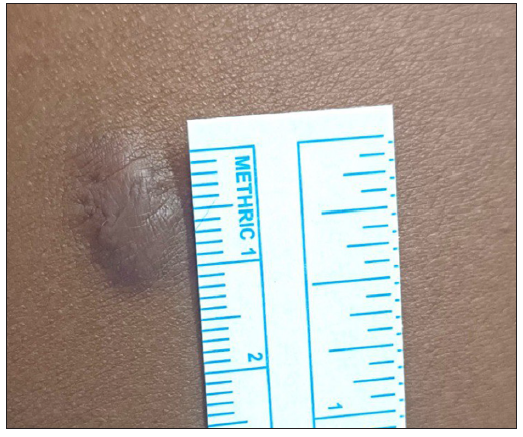
- Photograph at the end of 24 weeks of a patient with seven years history of keloid over the left arm treated with triamcinolone acetonide. (VSS Score = 7)
Intralesional triple combination (Group A) exhibited a greater reduction in the Vancouver Scar Scale scores compared to triamcinolone acetonide (Group B) [Table 2]. These findings suggest that intralesional triple combination may offer superior efficacy in keloid treatment, leading to more favourable outcomes in terms of scar appearance and texture.
The progressive improvement observed in Group A’s vascularity, pigmentation, pliability, and height severity scores over time underscores the sustained efficacy of intralesional triple combination. The steady improvement suggests ongoing remodelling and maturation of the scar tissue, resulting in better overall scar outcomes [Figures 3 and 4]. Treatment with intralesional triamcinolone acetonide monotherapy resulted in slower and less pronounced improvement in vascularity, pigmentation, pliability, and height severity scores [Figures 5 and 6].
Previous clinical trials have evaluated different intralesional injectable medications, alone or in combinations. These include triamcinolone acetonide, botulinum toxin type A, 5-fluorouracil, pentoxifylline, cryotherapy, radiofrequency, verapamil hydrochloride, and hyaluronidase.
Srivastava et al.9 compared intralesional therapy with a combination of triamcinolone acetonide and 5-fluorouracil and monotherapy with either drug in 60 patients. They administered intralesional injections every three weeks till 24 weeks (eight sessions), and the final follow-up was done at 30 weeks and they observed that combination therapy yielded faster improvements in keloid height, vascularity, and pliability compared to monotherapy with either agent. Khalid et al.6 evaluated the efficacy of 5-fluorouracil with triamcinolone acetonide (45 mg + 5 mg, respectively) versus triamcinolone acetonide monotherapy among 108 patients in both groups, with eight injections at a weekly interval and patient evaluation at baseline, fourth week and eighth week during treatment and four weeks after the end of treatment and found that the combination of 5-fluorouracil and triamcinolone acetonide resulted in greater reductions in scar height and recurrence rates compared to triamcinolone acetonide alone. Mohamed et al.7 investigated a combination therapy involving triamcinolone acetonide (0.3 mL), 5-fluorouracil (2.7 mL of 50 mg/mL), and hyaluronidase (500 IU) among 30 patients for a period of 12 weeks with eight weekly injections and evaluation at four, eight and 12 weeks and found significant improvements in scar-related parameters which included pain, itching, stiffness, colour and thickness of the scar as compared to triamcinolone acetonide alone. Goyal et al.8 were the first to evaluate the triple combination used in our study, comprising triamcinolone acetonide (0.4 mL of 40 mg/mL), 5-fluorouracil (0.6 mL of 50 mg/mL), and hyaluronidase (1500 IU), among 20 patients with four monthly sessions and monthly follow-up for one year and demonstrated its tolerability and potential efficacy in treating keloids. Despite some injection-related discomfort, the triple combination was well tolerated by patients, with no serious adverse events. The results observed in this study were similar to the group A arm of our study. The anti-inflammatory action of triamcinolone acetonide, the anti-metabolite action of 5-fluorouracil through inhibition of fibroblast proliferation, and the tissue dissolving property of hyaluronidase contribute to the synergistic action of this combination in the treatment of keloids.6–9
Although all patients experienced minimal pain during the procedure, only three patients in Group A and two patients in Group B had pain which lasted for a few hours. This was not significantly different between the groups (p = 0.642). None of the patients had recurrence during the study period. This suggests that both intralesional triple combination and triamcinolone acetonide monotherapy are well tolerated and effective. Overall, the findings of this study support the use of intralesional triple combination as a potentially superior treatment modality for keloids compared to triamcinolone acetonide monotherapy [Table 2]. The significant improvements observed in vascularity, pigmentation, pliability, height, and scar severity scores in Group A highlight the promising efficacy of this treatment approach in promoting scar resolution and optimising cosmetic outcomes [Table 3]. Further research, including larger randomised controlled trials with longer follow-up periods, is warranted to confirm these findings and establish the optimal keloid treatment protocol.
Limitations
Limitations of this study include, single centre design, short follow-up period (three months), lack of blinding and patient-reported outcome measures, which might impact the generalisability of the findings. Larger multicentre studies with longer follow-up periods, including patient-centred outcomes such as quality of life, satisfaction, and cosmetic appearance, are recommended to assess the overall impact of treatment on patients’ lives.
Conclusion
This study compared the efficacy and safety of intralesional triple combination versus triamcinolone acetonide monotherapy for keloid treatment, and both treatment modalities proved effective. However, the intralesional triple combination showed favourable advantages, including a faster and more pronounced response than intralesional triamcinolone acetonide monotherapy. None of the patients in either group had any recurrence during the follow-up period. These findings suggest that the intralesional triple combination could be a valuable alternative or adjunctive therapy for keloid management, offering potentially better patient outcomes. Furthermore, studies with longer follow-up periods are to be carried out since keloids are known for recurrence over time.
Ethical approval
The research/study was approved by the Institutional Review Board at (Aarupadai Veedu Medical College) AVMC Institutional Human Ethics Committee, number IHEC/072, dated 18/08/2022 and CTRI No: CTRI/061930.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Pruritus, pain, and small nerve fiber function in keloids: A controlled study. J Am Acad Dermatol. 2004;51:1002-6.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433-8.
- [CrossRef] [PubMed] [Google Scholar]
- DLQI scores in patients with keloids and hypertrophic scars: A prospective case control study. J Dtsch Dermatol Ges. 2009;7:688-92.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative efficacy and safety of common therapies in keloids and hypertrophic scars: A systematic review and meta-analysis. Aesthetic Plast Surg. 2020;44:207-18.
- [CrossRef] [PubMed] [Google Scholar]
- Keloids: A review of etiology, prevention, and treatment. J Clin Aesthet Dermatol. 2020;13:33-43.
- [PubMed] [PubMed Central] [Google Scholar]
- Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: Randomised control trial. Burns. 2019;45:69-75.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the efficacy and safety of intralesional triamcinolone injection with the combination of triamcinolone, 5 fluorouracil, and hyaluronidase enzyme in the treatment of keloids and hypertrophic scars. Egyptian J Surgery. 2023;42:1007-16.
- [Google Scholar]
- A novel triple medicine combination injection for the resolution of keloids and hypertrophic scars. J Clin Aesthet Dermatol. 2014;7:31-4.
- [PubMed] [PubMed Central] [Google Scholar]
- Comparison of intralesional triamcinolone acetonide, 5-fluorouracil, and their combination in treatment of keloids. World J Plast Surg. 2018;7:212-9.
- [PubMed] [PubMed Central] [Google Scholar]






