Translate this page into:
Dehydrated human amnion/chorion membrane treatment of venous leg ulcers
2 Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy
3 Department of Human Pathology in Adulthood and Childhood “G. Barresi”, University Hospital of Messina, Messina, Italy
Correspondence Address:
Fausto Fama
Residential Complex MITO – Ginestre F/2, 98151 Messina
Italy
| How to cite this article: Mazzei S, Sindoni A, Fama F, Buizon NJ, Shafei MA. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers. Indian J Dermatol Venereol Leprol 2020;86:212-214 |
Sir,
Venous leg ulcers are associated with important morbidities and worsen the quality of life of the affected individuals, as their healing is often a long and painful process. Their prevalence is expected to increase due to the rise in aging population and increase in risk factors such as obesity and congestive heart failure. The aim of therapy is ulcer healing, improvement of venous hypertension/insufficiency and prevention of recurrences. Therapeutic approaches include graduated compression, limb elevation, wound debridement and topical wound dressing. In patients affected by venous leg ulcers, human amnion/chorion tissue products have been evaluated.[1],[2],[3] Dehydrated human amnion/chorion membrane allograft is made up of multiple layers of epithelial cells and avascular connective tissue matrix. Tissues require stringent donor screening process and laboratory testing to avoid transmission of infectious diseases. All tissues are obtained after full informed consent of the donors (mothers of newborns). After cleaning tissues in buffered solutions, membrane grafts can be prepared as fresh or preserved allografts. While using the former method, immediate transplantation is necessary; whereas, when using preserved allografts, cryopreservation or dehydration techniques are used to increase storage time, as in our case. The allograft is minimally manipulated, dehydrated and processed using the PURION® Process, a unique approach which makes it safe and easy to use. Dehydrated human amnion/chorion membrane allografts have been found to contain angiogenic cytokines and growth factors, such as the epidermal growth factor, basic fibroblast growth factor, heparin-binding epidermal growth factor, platelet-derived growth factor BB and vascular endothelial growth factor.[3]
The purpose of our report is to show the safety and efficacy of dehydrated human amnion/chorion membrane in a 38-year-old woman, diagnosed with systemic lupus erythematosus (treated with systemic glucocorticoid therapy) with non-healing ulcers on the left leg. The patient was referred to the hyperbaric oxygen therapy and wound care department of Al Zahra Hospital, Dubai. Her past history included a road traffic accident with major trauma of the left leg in 2002, treated by surgery and autologous skin graft in another country. In January 2018, she underwent vascular surgery for venous insufficiency (Doppler-ultrasound before surgery showed common femoral vein/great-saphenous vein valve insufficiency with reflux). However, the same open wounds persisted and the patient underwent hyperbaric therapy and compression bandaging. Clinical examination when she presented to us, in October 2018, revealed three ulcers, one of 3 cm and the remaining two of 1 cm in maximum diameter [Figure - 1]a. X-ray of the left lower leg ruled out bone abnormalities; culture swab showed growth of Staphylococcus aureus, requiring antibiotic therapy. Biopsy from the largest lesion ruled out vasculitic changes associated with lupus which could have complicated the ulceration. Blood tests revealed high levels of C-reactive protein. The patient underwent surgical debridement until the layer of muscular fascia, followed by application of saline-moistened dehydrated human amnion/chorion membrane allograft (size 4 cm × 4 cm). This was positioned on the wounds and secured with adhesive strips, as a one-time procedure [Figure - 1]b, followed by a moist dressing as supportive medical therapy. Three weeks after surgery, healing was visible. The patient underwent follow-up for skin debridement and bandage changes; after 2 months, wounds had completely healed [Figure - 1]c; at 6-month follow-up, no sign of recurrence was found.
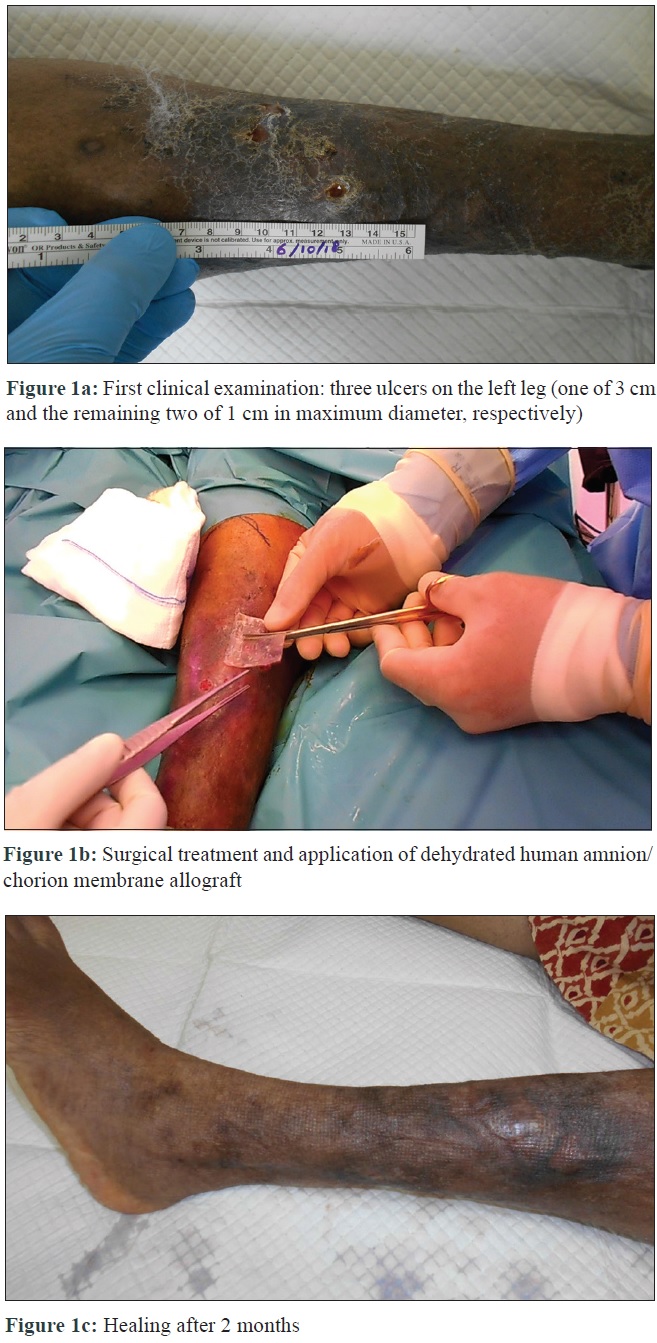 |
| Figure 1: |
Slow healing and recurrence of venous leg ulcers cause disability and require continuous care, causing psychosocial and economic burdens. A correlation between intermediate wound reduction (40% of wound area reduction after 4 weeks of treatment) and complete healing at 24 weeks, in patients affected by venous leg ulcers was demonstrated by Serena et al.[4] Recently, Caporusso et al. have reported reduced average healing time for venous leg ulcers treated with dehydrated human amnion/chorion membrane.[1] A recent literature review also demonstrated that patients treated with amniotic membrane products show better wound healing than patients treated with standard approaches, for burns, diabetic foot ulcers, fistulas, ocular defects and venous leg ulcers.[5]
The unique features in our case are: the clinical history of venous leg ulcers co-existing with systemic lupus erythematosus which could slow down the healing process; the allograft being able to reactivate the healing process; elastic tissue formation in the wound bed; healing accelerated with complete re-epithelialisation being achieved in a few weeks, unlike previous treatments; reduction of health care-related costs.
In conclusion, as venous leg ulcers often have a long healing time, identification of patients at risk of non-responsiveness to standard treatment approaches allows for therapeutical choices of more advanced wound care products, which can improve these patients' quality of life and reduce their suffering. Evaluating new treatments based on dehydrated human amnion/chorion membrane could be a successful option to achieve the goal of a wound healing in a shorter time than standard approaches, as in our case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Caporusso J, Abdo R, Karr J, Smith M, Anaim A. Clinical experience using a dehydrated amnion/chorion membrane construct for the management of wounds. Wounds 2019;31:S19-27.
[Google Scholar]
|
| 2. |
Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT; EpiFix VLU Study Group. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen 2014;22:688-93.
[Google Scholar]
|
| 3. |
Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353-62.
[Google Scholar]
|
| 4. |
Serena TE, Yaakov R, DiMarco D, Le L, Taffe E, Donaldson M, et al. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: Correlation between 4-week and 24-week outcomes. J Wound Care 2015;24:530-4.
[Google Scholar]
|
| 5. |
Kogan S, Sood A, Granick MS. Amniotic membrane adjuncts and clinical applications in wound healing: A review of the literature. Wounds 2018;30:168-73.
[Google Scholar]
|
Fulltext Views
3,995
PDF downloads
2,490





