Translate this page into:
Superficial thrombophlebitis during secukinumab treatment in a patient with psoriasis
2 Department of Radiology, Akdeniz University School of Medicine, Antalya, Turkey
3 Department of Pathology, Akdeniz University School of Medicine, Antalya, Turkey
Correspondence Address:
Erkan Alpsoy
Department of Dermatology and Venerology, Akdeniz University School of Medicine, Antalya 07059
Turkey
| How to cite this article: Mammadli K, Ceken K, Unal B, Karakas AA, Yilmaz E, Alpsoy E. Superficial thrombophlebitis during secukinumab treatment in a patient with psoriasis. Indian J Dermatol Venereol Leprol 2020;86:699-701 |
Sir,
Secukinumab, the fully humanized monoclonal antibody selectively neutralizing interleukin-17A, is used to treat moderate to severe psoriasis patients who do not respond to conventional systemic therapies and/or those who can not use conventional systemic therapies due to their side effects. Previous studies have demonstrated that secukinumab is an effective and safe therapeutic option in psoriasis. Nasopharyngitis, headache and upper respiratory tract infection are the most common side effects. Other infections, including mucocutaneous candidiasis, herpes labialis, tinea pedis, conjunctivitis and otitis media, are also frequently seen. Rhinorrhea, diarrhea, anaphylactic reaction, urticaria, inflammatory bowel disease and neutropenia are rare side effects.[1] Here, we present an extremely rare case of superficial thrombophlebitis developing under secukinumab treatment.
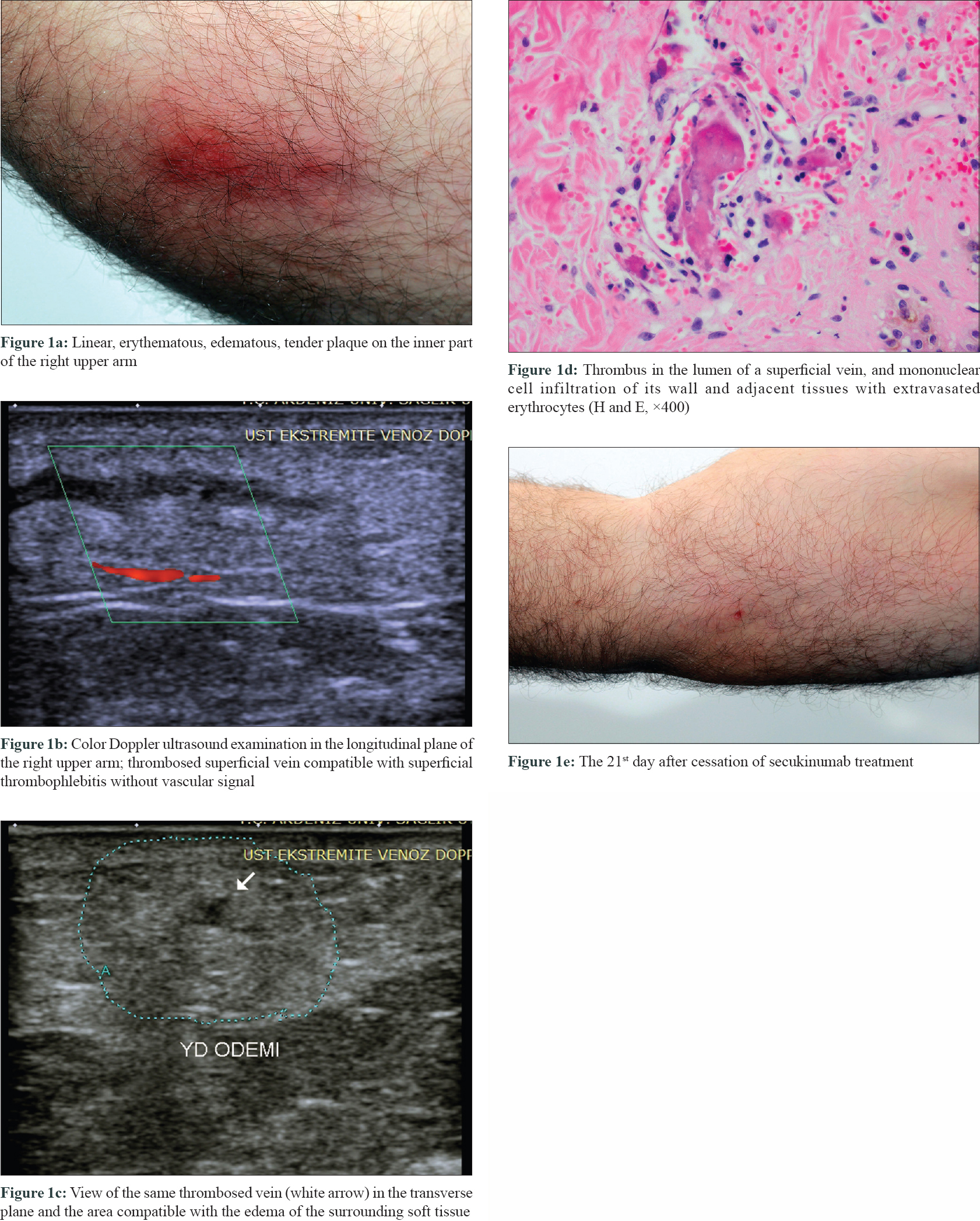
A 48-year-old male patient with plaque psoriasis was being treated in our clinic since October 2014. There was no known additional disease affecting the patient other than psoriatic arthritis. Due to the lack of response or recurrences to conventional and some biological treatments (adalimumab, etanercept and infliximab), secukinumab treatment was initiated. After the second dose of induction therapy with standard psoriasis treatment dosage (300 mg), the patient presented to our clinic with redness and swelling in the inner part of the right upper arm. Dermatologic examination revealed a linear, erythematous, edematous, tender plaque [Figure - 1]a at the site. Doppler ultrasonographic examination was consistent with the clinical diagnosis of superficial thrombophlebitis [Figure - 1]b and [Figure - 1]c. The diagnosis was confirmed by histopathological examination which revealed thrombus in the lumen of a superficial vein, and mononuclear cell infiltration of its wall and adjacent tissues with extravasated erythrocytes [Figure - 1]d. The patient had no past history of thrombophlebitis in his medical history. He did not define any physical triggers prior to the thrombophlebitis attack. The patient was retired (not working actively). Detailed investigations for the conditions that may cause a tendency to thrombosis (protein C/S, antithrombin III, factor V Leiden, prothrombin values, etc.) were within normal limits. After stopping secukinumab treatment, superficial thrombophlebitis completely regressed on the 21st day of follow-up [Figure - 1]e. Ustekinumab treatment was started 1 month later due to the exacerbation of psoriasis. The psoriatic lesions resolved within 1 month, and superficial thrombophlebitis did not recur at the 6-month follow-up with ustekinumab treatment.
 |
Psoriasis is a chronic, immune-mediated inflammatory disease affecting 2% of the world's population. Several comorbidities may accompany psoriasis, and it has a profound impact on the quality of life of the patient.[2] While the complex immune pathogenesis is still not fully defined, the available evidence supports the critical role of Th 17 pathway. The interleukin-17 cytokine. Interleukin-17A is the most potent member of the interleukin-17 family and plays an active role in many cells including keratinocytes, endothelial cells, chondrocytes, fibroblasts and monocytes.[1]
Interleukin-17A-mediated thrombosis is believed to occur in two steps: platelet aggregation and coagulation activation in endothelial cells. Interleukin-17A increases adenosine diphosphate-induced platelet activation and Von Willebrand factor production, which is a marker of endothelial dysfunction and mediates platelet aggregation from endothelial cells. Interleukin-17A alone or together with tumor necrosis factor-alpha activates tissue factor and coagulation, reducing CD39 and thrombomodulin, thus preventing anticoagulation. Therefore, the tendency to coagulation and thrombosis increases. Besides, interleukin-17A induces endothelial damage through increased apoptosis, reducing the mitochondrial transmembrane potential.[3] In controlled trials, increased interleukin-17A levels were reported at the beginning of treatment in patients receiving secukinumab therapy. The highest median concentration is 2 weeks after the first dose, followed by slowly declining concentrations during a 6-month period.[4] In our case, there was a significant correlation between secukinumab therapy and superficial thrombophlebitis which occurred after the second dose of induction therapy. Briefly, the chronological relationship of superficial thrombophlebitis with secukinumab treatment, the spontaneous regression of superficial thrombophlebitis after discontinuation of secukinumab, the absence of any laboratory markers causing to a predisposition to thrombosis and the absence of similar complaints at 6 months of follow-up after treatment with ustekinumab in our case confirmed the relationship of thrombophlebitis with secukinumab treatment. Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism, have been reported to be increased among psoriasis patients.[5] In our patient, besides typical clinical features, radiological and histopathological findings were consistent with superficial thrombophlebitis. Moreover, thrombotic markers were negative.
In conclusion, the appearance of both superficial thrombophlebitis and secukinumab treatment in the presented case may be a coincidental association. On the other hand, superficial thrombophlebitis may also be a rare complication of secukinumab treatment. Increased interleukin-17A levels at the beginning of secukinumab treatment might be associated with platelet aggregation and coagulation activation in endothelial cells leading to superficial thrombophlebitis. Besides the individual characteristics of the patient, the suppression of the interleukin-17 pathway, an essential element of the immune system, may have led to the predominance of other inflammatory pathways such as interleukin-1 and tumor necrosis factor-α, leading to endothelial cell damage and development of superficial thrombophlebitis. Although chronologically incompatible with our case, these proinflammatory cytokines which are responsible for leukocyte adhesion, procoagulant activity and platelet-activating factor activation, might cause a tendency to coagulation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal the identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Frieder J, Kivelevitch D, Menter A. Secukinumab: A review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis 2018;9:5-21.
[Google Scholar]
|
| 2. |
Alpsoy E, Polat M, Fettahlioglu-Karaman B, Karadag AS, Kartal-Durmazlar P, YalCın B, et al. Internalized stigma in psoriasis: A multicenter study. J Dermatol 2016;44:885-91.
[Google Scholar]
|
| 3. |
Robert M, Miossec P. Effects of ınterleukin 17 on the cardiovascular system. Autoimmun Rev 2017;16:984-91.
[Google Scholar]
|
| 4. |
US Food and Drug Administration. Center for Drug Evaluation and Research. Application number125504Orig1s000: Pharmacology Review (s). From FDAwebsite. Available: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125504Orig1s000ClinPharmR.pdf. Submission Date: 10/24/2013 Accesible from 23 Jan 2015.
[Google Scholar]
|
| 5. |
Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: A systematic review and meta-analysis. QJM 2014;107:793-7.
[Google Scholar]
|
Fulltext Views
4,808
PDF downloads
2,004





