Translate this page into:
A case of febrile ulceronecrotic Mucha-Habermann disease with comorbidities
Correspondence Address:
Mukta Shriram Tulpule
26, Sahawas Society, Karvenagar, Pune - 411 052, Maharashtra
India
| How to cite this article: Bhide DS, Tulpule MS, Pethe SV. A case of febrile ulceronecrotic Mucha-Habermann disease with comorbidities. Indian J Dermatol Venereol Leprol 2019;85:660-663 |
Abstract
Febrile ulceronecrotic Mucha-Habermann disease is a very rare and severe variant of pityriasis lichenoides et varioliformis acuta. Adult cases are difficult to diagnose as in the early course they can mimic erythema multiforme or lymphomatoid papulosis. We report a case of a 38-year-old woman who presented with 90% body surface area involvement, fever, diarrhea, malaise and associated comorbidities. She was treated with systemic steroids and methotrexate but suffered a fatal outcome. So far, a total of 65 cases are reported in the literature.
Introduction
We report a case of a 38-year-old woman with febrile ulceronecrotic Mucha-Habermann disease with multiple comorbid conditions. Many challenges are faced by the dermatologists due to rarity of condition, difficulty in diagnosis, unavailability of specific diagnostic tests, lack of treatment guidelines and the fulminant nature of the disease. Associated comorbidities can make it even more challenging to manage such cases. According to our search of literature, this is the second case report of febrile ulceronecrotic Mucha-Habermann disease from India.[1]
Case Report
A 38-year-old woman presented to our hospital with a painful rash that started on her abdomen and was spreading rapidly to her extremities, trunk, and face over a period of 3 weeks. She was treated with oral prednisone 40 mg once daily, oral acyclovir and injection ceftriaxone for 7 days at another institute. Previous biopsy report showed necrotic keratinocytes, lymphohistiocytic infiltrate and edema in papillary dermis.
However, there was no improvement and the painful rash worsened. Hence, for further management she was referred to our institute 3 weeks after the onset of the disease with complaints of fever, irritability, progressive itchy and painful skin lesions and loose motion.
She gave a history of abdominal tuberculosis and rheumatic heart disease. She had undergone a hemicolectomy due to intestinal obstruction. She had also suffered an episode of urticaria due to diclofenac sodium and sulfa drugs. On examination, she was febrile (100°F) and had tachycardia (>120 beats/min). There was pedal edema, pallor and a single inguinal lymph node.
Systemic examination was within normal limits.
On cutaneous examination, erythematous plaques and papules with scaling and hemorrhagic crusts were present in a generalized distribution involving scalp, flexures, palms and soles with sparing of mucosae. There was marked skin tenderness. She also had superficial necrotic ulcers predominantly in pressure areas, antecubital and popliteal fossae.
A differential diagnosis of erythema multiforme major, Stevens–Johnson syndrome (SJS), disseminated herpes zoster, lymphomatoid papulosis and pityriasis lichenoides et varioliformis acuta were considered.
On admission, her haemoglobin was 7.5 gm/dl, total white cell count was 6300/cu mm. Liver enzymes were deranged. Serology for human immunodeficiency virus antibodies I and II, Hepatitis B, Hepatitis C, VDRL were negative. Toxoplasma and Epstein–Barr IgM as well as Weil–Felix for Rickettsia were negative. Skin swab showed no growth of organisms, blood culture and stool culture were negative. Serum procalcitonin was 0.12 ng/ml, c-reactive protein 22.7 mg/dl and anti-streptolysin O titer was 200 IU/ml, rheumatoid factor was negative. ANA, ANA blot studies; c-ANCA and p-ANCA were negative. Chest X-ray was clear, electrocardiogram was normal, two-dimensional echo revealed a low ejection fraction (<50%). Ultrasonogram of abdomen showed mild hepatosplenomegaly.
New lesions kept occurring in crops, prominently over the lower extremities. They started as purpuric macules evolving to form coalescing ulcers with black crusts and necrotic center. Some lesions evolved into hemorrhagic bullae [Figure - 1]. The ulcers healed with hypopigmented, atrophic or varioliform scars [Figure - 2]. Over the period of next 3 weeks, the lesions on lower extremity worsened. There was desquamation from the scaly plaques. The ulcers became deep and foul smelling due to secondary infection. There was black eschar-like formation on a few lesions [Figure - 3].
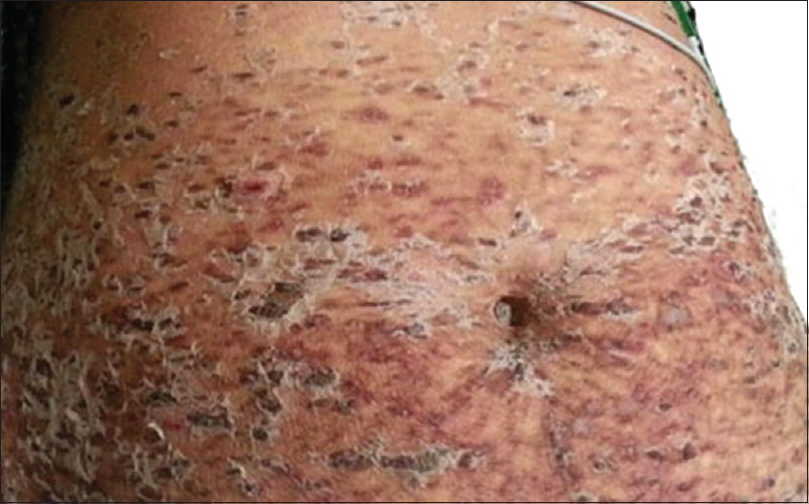 |
| Figure 1: On admission, scaly crusted papules coalescing to form plaques |
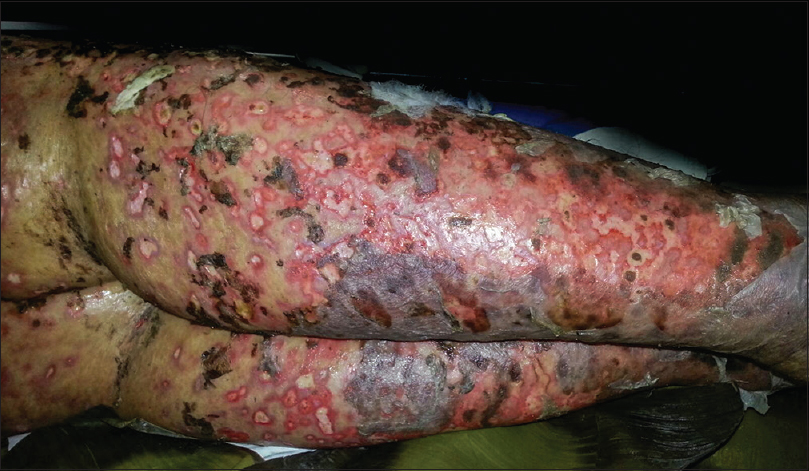 |
| Figure 2: Healing with atrophic, varioliform scarring |
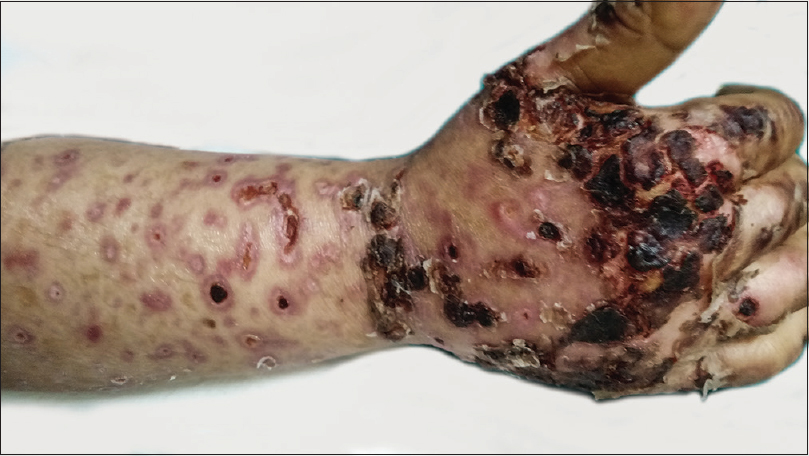 |
| Figure 3: Evolution to black crusts and eschar formation |
Skin biopsy done twice in our institute showed parakeratosis, necrotic keratinocytes, basal cell vacuolation, pigment incontinence and lymphocytic vaculitis [Figure - 4] and [Figure - 5].
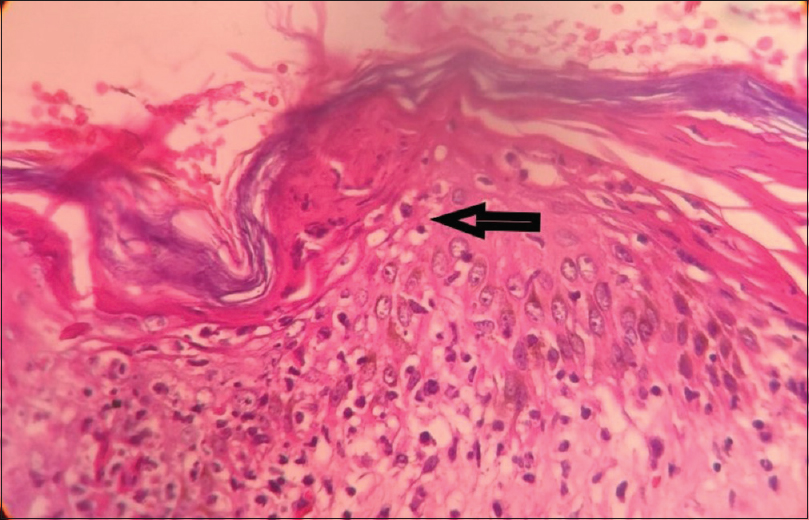 |
| Figure 4: Arrow showing necrotic keratinocytes, basal layer vacuolation, pigment incontinence (H and E, ×100) |
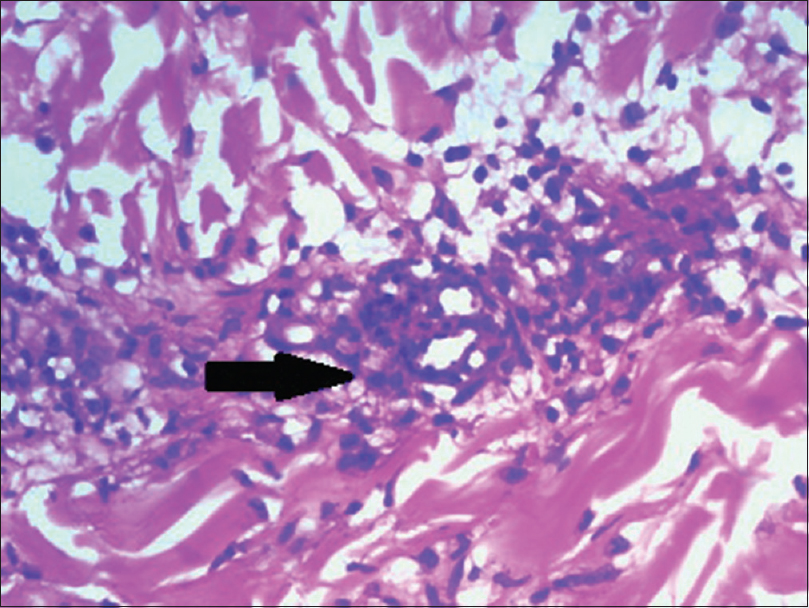 |
| Figure 5: Arrow showing lymphocytic vasculitis (H and E, ×400) |
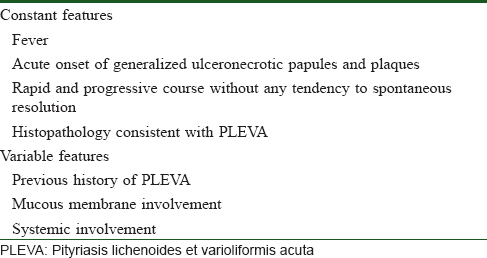
According to the diagnostic criteria for febrile ulceronecrotic Mucha-Habermann disease proposed by Nofal et al. [Table - 1], our case fulfilled all four major criteria and one variable feature of systemic involvement.[2]
The patient was managed in the intensive care unit with strict barrier nursing with simultaneous management by physician, intensivist and counselling by psychiatrist. She was started on oral prednisolone 1 mg/kg (60 mg/day) for 15 days that was tapered slowly by 5 mg every 3 days. The Weinstein regimen for administering methotrexate was used as patient's white blood cell and platelet counts were fluctuating and she had a history of drug reactions. Intravenous antibiotics like meropenem, teicoplanin, tigecycline and colistin were started, and modified according to the culture and sensitivity reports done once a week which revealed growth of Escherichia coli and Pseudomonas aeruginosa. Lesions dried up following the dose, and showed temporary improvement. High-grade fever episodes continued despite antibiotics. Steroid pulse was not given, fearing reactivation of tuberculosis and current state of septicemia.
Despite all efforts to save the patient, her condition deteriorated due to uncontrolled gram-negative septicemia and she passed away at the end of 7 weeks after admission.
The challenges faced during treatment were:
- Patient's history of abdominal tuberculosis and rheumatic heart disease with low ejection fraction and uncontrolled skin sepsis limited the use of biologicals and pulse steroids.
- Lack of standard guidelines for the diagnosis and treatment of this condition when she presented to our institute.
- Ongoing inflammation leading to the formation of ulcerative lesions despite two immunosuppressive agents.
- Inability to establish the etiology with available diagnostic resources.
- Immunohistochemistry studies to establish T-cell clonality were not performed.
We report a case of febrile ulceronecrotic Mucha-Habermann disease with fever, gastrointestinal involvement and histopathological features of lymphocytic vasculitis, managed with high dose prednisolone and weekly methotrexate, who suffered a fatal outcome.
Discussion
The term pityriasis lichenoides et varioliformis acuta was described by Mucha in 1916 and Habermann in 1925, as an acute eruption of ulceronecrotic papules and plaques.[3] Later in 1966, Degos et al., described a rare and severe variant of the term with a fulminant course and termed it febrile ulcernecrotic Mucha-Habermann disease.[3]
Of the 65 cases reported so far in literature as of 2016, 34 cases including ours are adult cases.[2] Childhood cases have better prognosis and only 1 fatality has been reported so far. The reason for better outcome in children is not established.
An analysis of total 34 adult cases reveals:
- ale:Female ratio = 2.4:1
- A total 9 (26.4%) cases reported fatal outcome, four were males (average age = 66.7 years) and five were females (average age = 50 years).
- Most common cause for fatality was sepsis in 5 (62.5%) cases, followed by pneumonia and disseminated intravascular coagulation.
- Mucosal involvement was seen in 9 (26.4%) patients.
- Antibiotics with systemic steroids were the most common treatment modalities.
- Methotrexate showed good success rate.
The etiopathogenesis of pityriasis lichenoides et varioliformis acuta and febrile ulceronecrotic Mucha-Habermann disease is uncertain. Various theories like hypersensitivity to Epstein–Barr virus, herpes simplex virus, human immunodeficiency virus, measles virus, parvovirus B19 and cytomegalovirus have been proposed.[4],[5] In a few cases, secondary infection by Staphylococcus aureus and Pseudomonas aeruginosa was also reported.[6]
Miyamoto et al. proposed the theory of T-cell clonality and that febrile ulceronecrotic Mucha-Habermann disease is an aggressive lymphoproliferative disorder based on three fatal case reports.[7],[8]
López-Estebaranz et al., however, proposed that it is an inflammatory process rather than lymphoproliferative process based on T-cell receptor gene analysis.[9]
High tumor necrosis factor alpha levels have also been noted by some authors.[10] Hence, anti-tumor necrosis factor alpha drugs may be used for treatment in the future. The lesions present in a generalized distribution with flexural accentuation, papules that evolve into ulceronecrotic papules and plaques and healing with varioliform atrophic scarring. Involvement of mucosa may be present. Black-shellac like crust and eschar formation may be present. Recurrences have been reported in literature, though are less severe than the primary episode.
Histopathology of febrile ulceronecrotic Mucha-Habermann disease is similar to that of pityriasis lichenoides et varioliformis acuta with parakeratosis, spongiosis, hydropic degeneration of basal cell layer, exocytosis of red blood cells, dyskeratosis and perivasuclar lymphocytic infiltrate. Epidermal necrosis and lymphocytic vasculitis may also be seen. Immunohistochemistry is nonspecific.
Febrile ulceronecrotic Mucha-Habermann disease poses a diagnostic challenge. It can be confused wtih SJS if there is mucosal involvement along with extensive keratinocyte necrosis on histology.[11]
Various treatment modalities have been tried including systemic corticosteroids, acyclovir, methotrexate, antibiotics, phototherapy, dapsone, cyclosporine and intravenous immunoglobulins.
Maintaining adequate hydration, pain management and dressing and debridement of wounds is necessary. General management with paraffin gauze dressing or banana leaf dressing prevents sticking.
Optimum treatment guidelines that can be used for all patients have not yet been stated, and treatment is individualized.
Conclusion
Febrile ulceronecrotic Mucha-Habermann disease remains an enigma due to unestablished etiology, lack of data and few reported cases, lack of guidelines for management and possibility of fatal outcome. A high index of suspicion must be kept for cases of pityriasis lichenoides et varioliformis acuta presenting with fever.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initial will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Virdi SK, Kanwar AJ, Saikia UN. Pityriasis lichenoides with ulceronecrosis and hyperthermia: A rare variant of pityriasis lichenoides et varioliformis acuta. Indian J Dermatol Venereol Leprol 2010;76:172-5.
[Google Scholar]
|
| 2. |
Nofal A, Assaf M, Alakad R, Amer H, Nofal E, Yosef A, et al. Febrile ulceronecrotic Mucha-Habermann disease: Proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol 2016;55:729-38.
[Google Scholar]
|
| 3. |
Degos R, Duperrat B, Daniel F. [The ulceronecrotic parapsoriasis with hyperthermia.] Ann Dermatol Venereol 1966;93:481-96.
[Google Scholar]
|
| 4. |
Tsai KS, Hsieh HJ, Chow KC, Lin TY, Chiang SF, Huang HH, et al. Detection of cytomegalovirus infection in a patient with febrile ulceronecrotic Mucha-Habermann's disease. Int J Dermatol 2001;40:694-8.
[Google Scholar]
|
| 5. |
Nanda A, Alshalfan F, Al-Otaibi M, Al-Sabah H, Rajy JM. Febrile ulceronecrotic Mucha-Habermann disease (pityriasis lichenoides et varioliformis acuta fulminans) associated with parvovirus infection. Am J Dermatopathol 2013;35:503-6.
[Google Scholar]
|
| 6. |
De Cuyper C, Hindryckx P, Deroo N. Febrile ulceronecrotic pityriasis lichenoides et varioliformis acuta. Dermatology 1994;189 Suppl 2:50-3.
[Google Scholar]
|
| 7. |
Miyamoto T, Takayama N, Kitada S, Hagari Y, Mihara M. Febrile ulceronecrotic Mucha-Habermann disease: A case report and a review of the literature. J Clin Pathol 2003;56:795-7.
[Google Scholar]
|
| 8. |
Cozzio A, Hafner J, Kempf W, Häffner A, Palmedo G, Michaelis S, et al. Febrile ulceronecrotic Mucha-Habermann disease with clonality: A cutaneous T-cell lymphoma entity? J Am Acad Dermatol 2004;51:1014-7.
[Google Scholar]
|
| 9. |
López-Estebaranz JL, Vanaclocha F, Gil R, García B, Iglesias L. Febrile ulceronecrotic Mucha-Habermann's disease. J Am Acad Dermatol 1993;29:903-6.
[Google Scholar]
|
| 10. |
Kim HS, Yu DS, Kim JW. A case of febrile ulceronecrotic Mucha-Habermann's disease successfully treated with oral cyclosporin. J Eur Acad Dermatol Venereol 2007;21:272-3.
[Google Scholar]
|
| 11. |
Kaufman WS, McNamara EK, Curtis AR, Kosari P, Jorizzo JL, Krowchuk DP, et al. Febrile ulceronecrotic Mucha-Habermann disease (pityriasis lichenoides et varioliformis acuta fulminans) presenting as Stevens-Johnson syndrome. Pediatr Dermatol 2012;29:135-40.
[Google Scholar]
|
Fulltext Views
4,839
PDF downloads
2,515





