Translate this page into:
Paraffin-embedded micrographic surgery for the treatment of dermatofibrosarcoma protuberans: Analysis of 33 patients
2 Department of Pathology, Hospital Universitario de Guadalajara, Guadalajara, Spain
Correspondence Address:
Adriana Martín-Fuentes
Department of Dermatology, Hospital Universitario de Guadalajara, C/Donantes de Sangre, s/n, 19002 Guadalajara
Spain
| How to cite this article: Martín-Fuentes A, De Eusebio-Murillo E, Herreros CS, Ballano-Ruiz A, Blázquez EJ, Cuevas-Santos J. Paraffin-embedded micrographic surgery for the treatment of dermatofibrosarcoma protuberans: Analysis of 33 patients. Indian J Dermatol Venereol Leprol 2018;84:298-303 |
Abstract
Background: Dermatofibrosarcoma protuberans is a rare malignant tumor with a high rate of recurrence after surgery. Moh's micrographic surgery allows examination of all surgical margins to ensure complete removal.
Objective: To evaluate the use of Moh's micrographic surgery using paraffin-embedded sections for the treatment of dermatofibrosarcoma protuberans.
Methods: We performed a retrospective analysis of 33 patients with dermatofibrosarcoma protuberans treated in our department with paraffin-embedded micrographic surgery between January 2002 and June 2015.
Our cases included patients with primary untreated disease and also those with persistent disease previously treated surgically elsewhere, with histologically positive margins.
Results: Tumors were most commonly located on the trunk. After the first stage of micrographic surgery, including an initial lateral margin, 20 (60.6%) tumors were completely excised, 11 (33.3%) tumors required two stages and one tumor each (3.0%) required 4 and 6 stages respectively. Patients were monitored for recurrence for a mean duration of 6.5 years. There was no recurrence in any of our 33 patients.
Conclusions: Our results indicate that Moh's micrographic surgery with paraffin-embedded sections may be the method of choice to treat dermatofibrosarcoma protuberans with a low recurrence rate, while preserving surrounding normal healthy tissue.
Introduction
Dermatofibrosarcoma protuberans is a rare, low-grade soft tissue tumor which is locally aggressive, but has a low risk of metastasis. It accounts for <0.1% of all malignancies and approximately 1% of all soft tissue sarcomas.[1] This tumor is usually diagnosed in young to middle-aged adults, although some cases have been reported in children.[2]
Clinically, it presents as an asymptomatic skin-colored plaque or tumor and diagnosis is frequently delayed because of its indolent nature. As the tumor grows, lateral and deep infiltration begins and protuberant nodules develop on the surface [Figure - 1].[3]
 |
| Figure 1: Skin-colored plaque with nodules on the surface |
The most common anatomic location is the trunk, followed by the extremities and the head and neck.[4]
Histological features include a dense collection of spindle cells arranged in a storiform pattern in the dermis infiltrating the subcutaneous fat, and with tentacle-like projections into fascia or muscle.[4],[5] Immunohistochemical markers are used to improve diagnostic accuracy. CD34 antigen is selectively expressed in dermatofibrosarcoma protuberans [Figure - 2]. CD34 can help to differentiate this tumor from dermatofibroma which is CD34 negative and positive for factor XIIIa. In addition, CD68 selectively stains for malignant fibrous histiocytoma and atypical fibroxanthoma, but not for dermatofibrosarcoma protuberans.[6],[7]
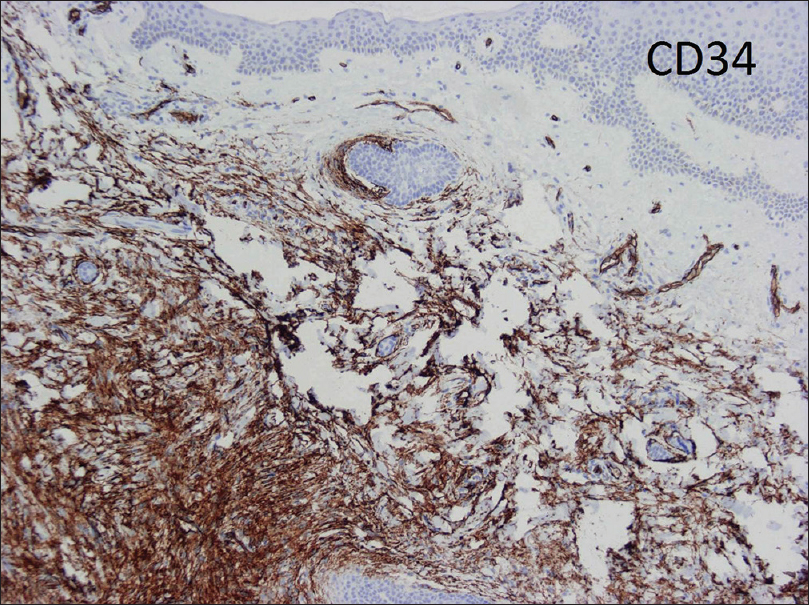 |
| Figure 2: Immunochemical stain: CD34 antibody expressed in dermatofibrosarcoma protuberans |
The aim of treatment should be complete removal of the tumor due to its high rate of recurrence. Even with extensive resections including lateral margins of up to 3 cm, recurrence rates of up to 15% are reported.[8] Moh's micrographic surgery is followed by a complete histological evaluation of the tumor's surgical margins.[9],[10] Two techniques of histological margin examination have been described: Moh's micrographic surgery and peripheral margin examination as described by Breuninger et al.[11] Traditional Moh's micrographic surgery [12] is performed on frozen sections; modified Moh's micrographic surgery uses paraffin-embedded sections. There are no randomized studies comparing wide local excision with Moh's micrographic surgery but evidence available from retrospective studies has shown higher recurrence rates for wide local excision (7.3%) than for Moh's micrographic surgery (1%).[3]
We present our experience in treating 33 patients with dermatofibrosarcoma protuberans using paraffin-embedded micrographic surgery.
Methods
A retrospective review was performed on patients with dermatofibrosarcoma protuberans treated with paraffin-embedded micrographic surgery in our department between January 2002 and June 2015.
In all cases, histological diagnosis from a tumor biopsy preceded the micrographic excision.
Data compiled from each case included patient age, sex, anatomic site distribution, number of stages of Moh's micrographic surgery necessary to achieve negative margins, the incidence of recurrence and the duration of follow-up.
During the first stage, the tumor or residual scar with a margin of 10-20mm (10mm for head and neck and 20mm for trunk and extremities) is removed [Figure - 3]. The defined margin of tissue and underlying fat was removed and mapped for precise anatomical orientation [Figure - 4].
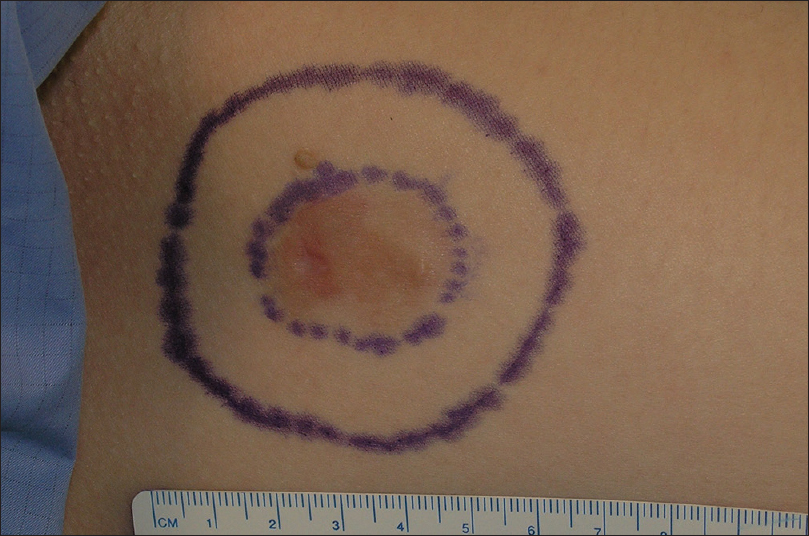 |
| Figure 3: Delimitation of the visible tumor and the 2 cm lateral margin for the first stage of Moh's micrographic surgery |
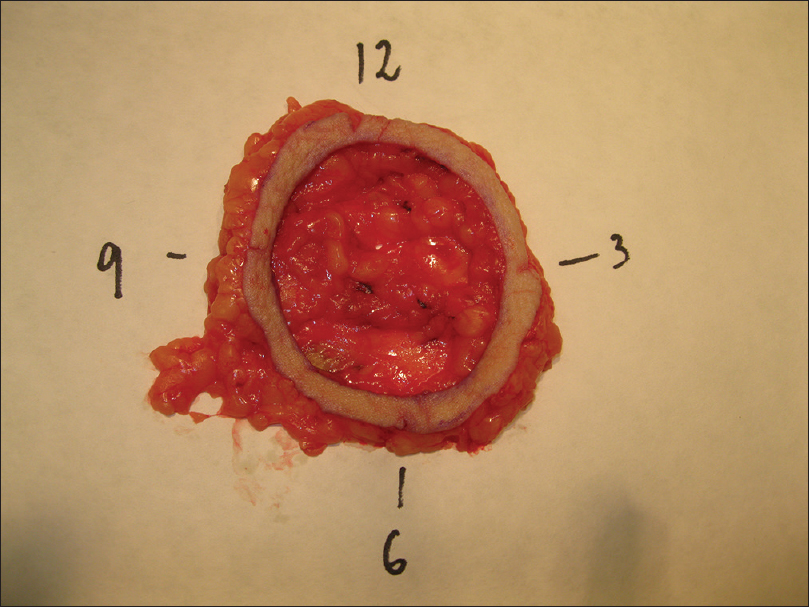 |
| Figure 4: Pathological method: marked tissue for a correct orientation |
The pathologist marked the tissue for formalin fixation during the first 24 hours [Figure - 5]. Thereafter, tissue was divided into multiple specimens that were color coded for anatomical orientation and placed in cassettes for paraffin-embedded sections [Figure - 6]. The tissue was sectioned horizontally by a pathology technician and stained with hematoxylin and eosin [Figure - 7]. Tissue margins were evaluated by both surgeon and pathologist, within 2 or 3 days.
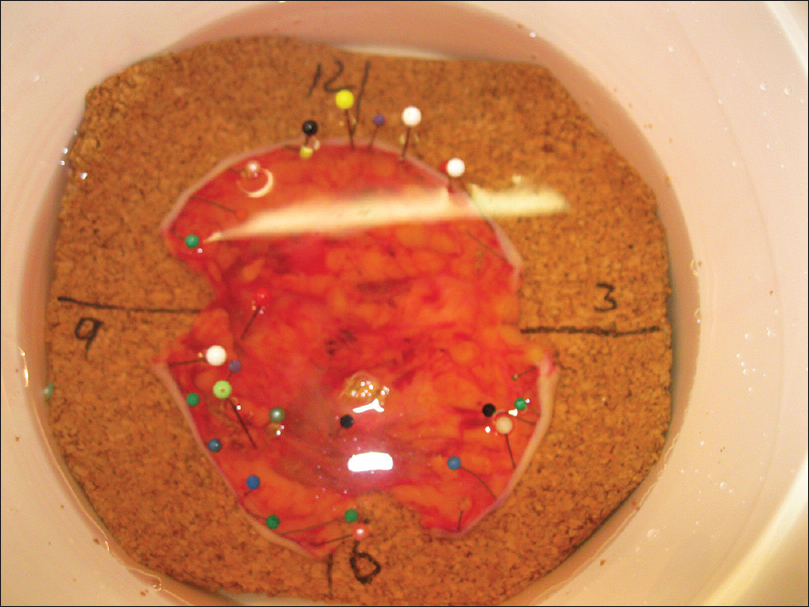 |
| Figure 5: Marked tissue for formalin fixation during 24 h |
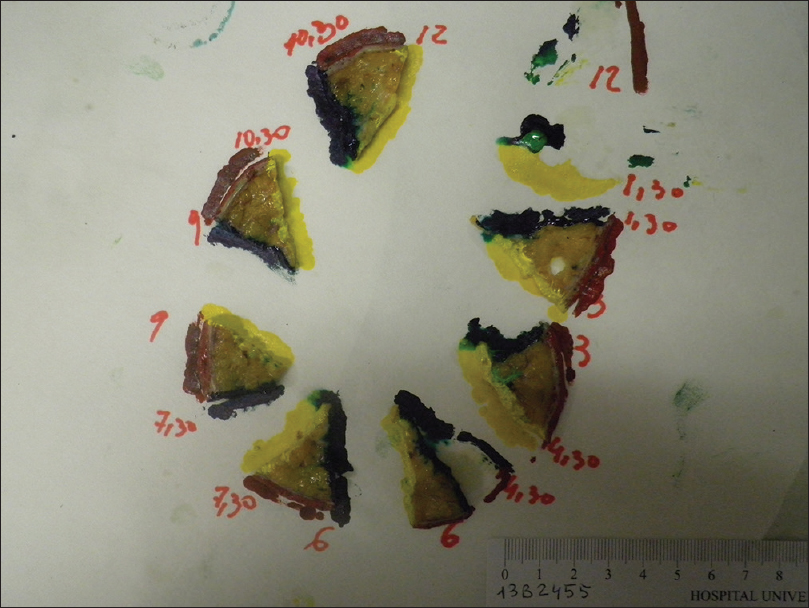 |
| Figure 6: Multiple tissue sections color coded for anatomical orientation |
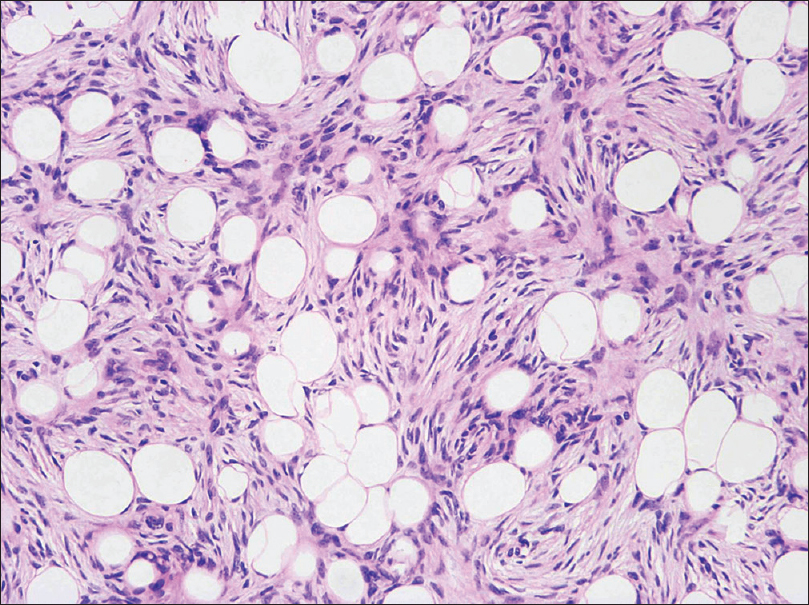 |
| Figure 7: High-power Moh's section: spindle cells in a storiform pattern invading subcutaneous tissue (H and E, ×400) |
The process of excision, mapping and microscopic examination was repeated until no tumor was found microscopically. Immunostaining with CD34 antibody was only employed in persistent cases to discriminate between scar tissue and residual tumor or in hypocellular tumors.
We placed a purse-string suture and temporarily covered the skin defects with synthetic wound dressing until complete excision was proven [Figure - 8].
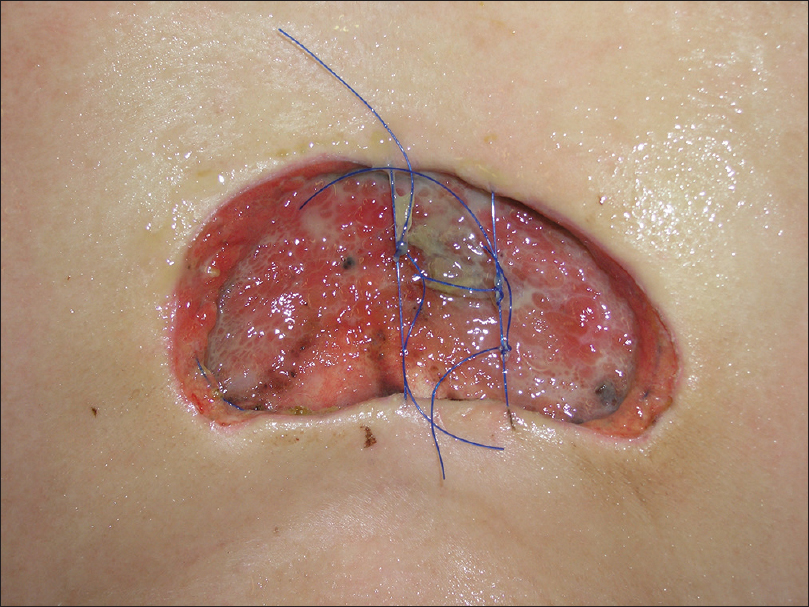 |
| Figure 8: Purse-string suture placed until the final outcome of Moh's micrographic surgery |
Results
Between 2002 and 2015, we treated 33 patients with histologically confirmed dermatofibrosarcoma protuberans using paraffin-embedded micrographic surgery [Table - 1]. Twenty three (69.6%) were women and 11 (33.4%) were men. The mean patient age at surgery was 41 years (ranging from 20 to 71 years). Nineteen (57.5%) patients presented with tumors on the trunk, 7 (21.2%) on the upper limbs, 4 (12.2%) on the head and neck and 3 (9.1%) on the lower limbs. Twenty seven had primary tumors and 6 were referred for Moh's micrographic surgery after previous incomplete excisions.
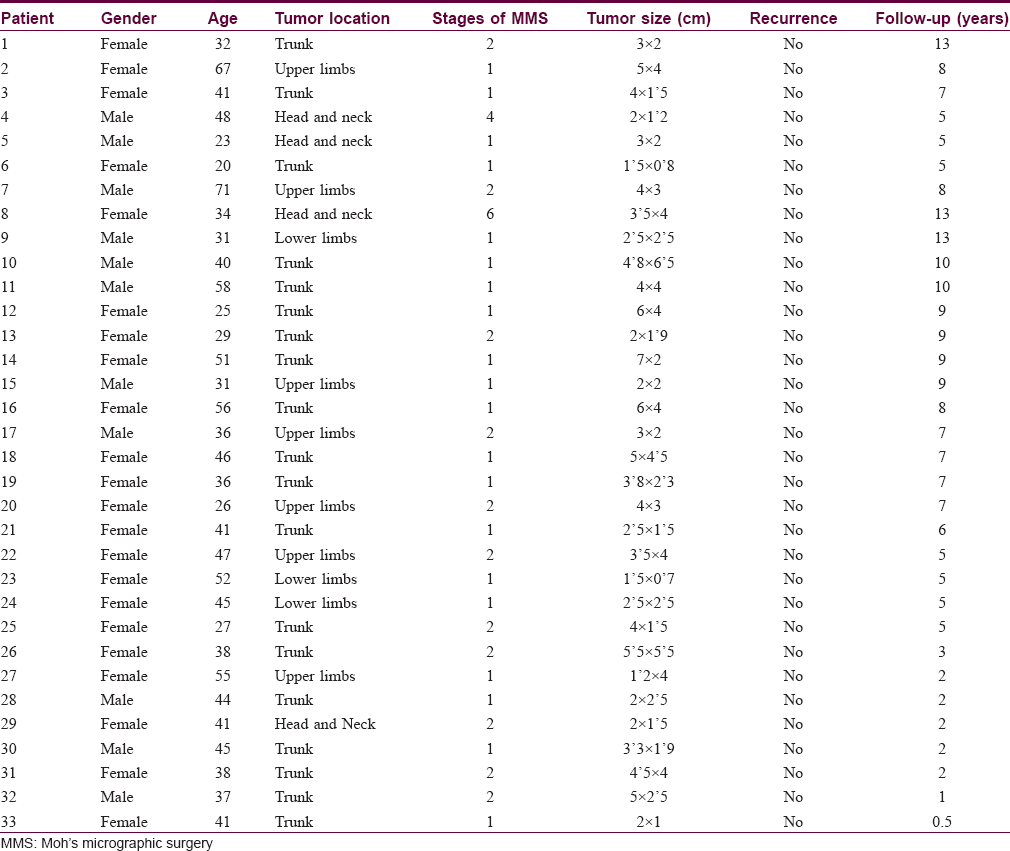
There were no cases of myxoid or fibrosarcomatous variants in our patient group.
All patients had either primary disease, defined as previously untreated lesions or persistent disease, which included patients who had undergone prior surgery with histologically positive margins. In our series, there were no cases of recurrent disease.
After the first stage of micrographic surgery, including an initial lateral margin, 20 (60.6%) tumors were completely excised, 11 (33.3%) tumors required two stages, and one tumor each (3.0%) four and six stages respectively. We used immunochemical staining with CD34 antibody in four patients.
We undertook defect reconstructions with direct closures, local random flaps and full-thickness skin grafts [Figure - 9], [Figure - 10], [Figure - 11].
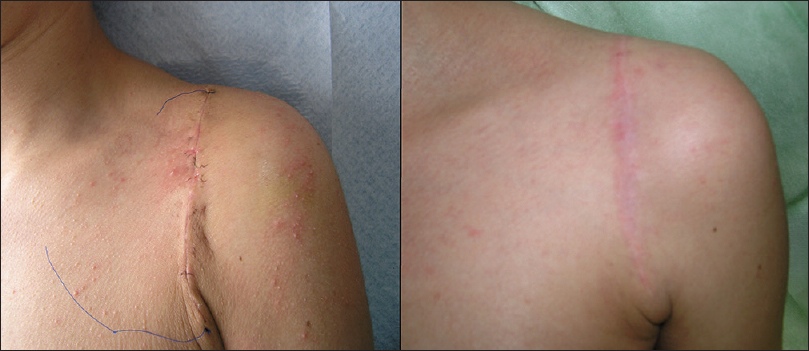 |
| Figure 9: Surgical defect reconstruction with direct closure |
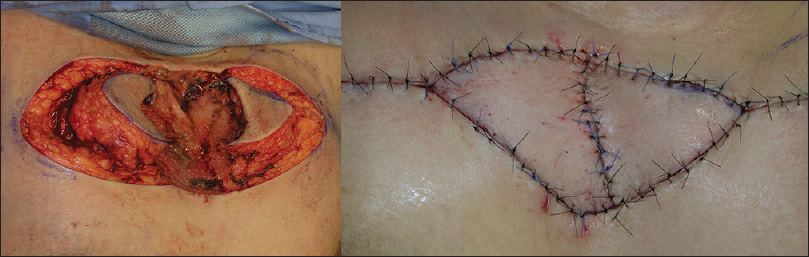 |
| Figure 10: Surgical defect reconstruction with local random flap |
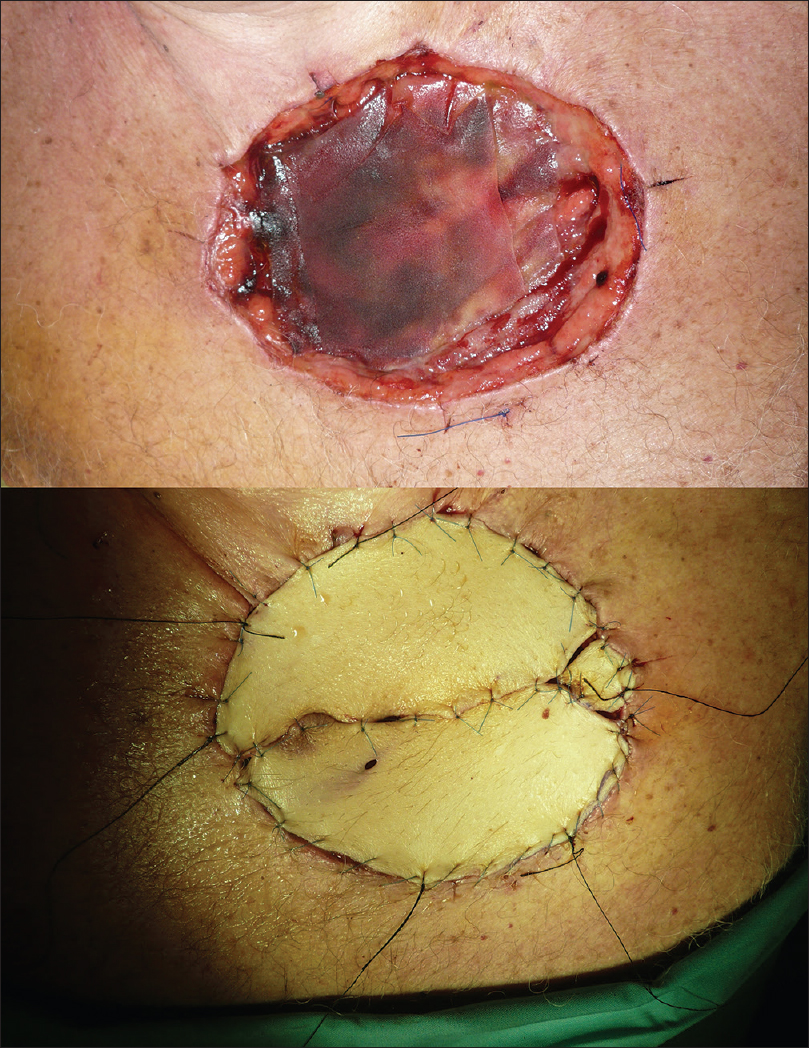 |
| Figure 11: Surgical defect reconstruction with full-thickness skin graft |
Patients were prophylactically treated with antibiotics according to our hospital protocol. None presented with surgical wound infection.
Patients were monitored for recurrence every 3 months during the first year following the procedure and every 6 months thereafter. Clinical evidence of local recurrence has not been noted in any of these patients so far, after a mean post-procedure interval of 78 months (range, 5 to 156 months).
Discussion
Dermatofibrosarcoma protuberans is a rare dermal tumor with a high risk of local recurrence. Its aggressiveness depends on four risk factors: Involvement of the head, depth of tumor invasion, degree of atypical, myxoid or sarcomatous histologic differentiation and recurrence after previous treatment.[13]
Surgical excision is the main treatment modality; however, the recurrence rates for this tumor after local excision range from 10% to 60%.[3] In the past few years, wide local excision has been widely used and has been the treatment of choice with an overall recurrence rate of 7.3% (range 0–46%). The surgical margins included in wide local excision range from <2 to 5 cm or greater in some studies and hence the popularity of the procedure is marred by the increasing loss of healthy surrounding tissue.[3],[14]
The goal of Moh's micrographic surgery is to histologically inspect 100% of the margins of removed tissue. Positive margins result in the removal of a deeper layer until all margins are free of malignancy. Several retrospective reviews have shown that the recurrence rate of dermatofibrosarcoma protuberans treated with Moh's micrographic surgery is approximately 1%.[3],[15],[16]
Traditional Moh's micrographic surgery uses frozen sections. We perform paraffin-embedded micrographic surgery because our patient group includes persistent tumors that have been previously treated at other institutions, with histologically positive margins. Advantages of the paraffin-embedded method are the high quality of the resulting slides, accurate discrimination of fine tumor strands from normal skin and the possibility of performing immunohistochemistry for CD34 in uncertain cases.
Using frozen sections, it may be difficult to distinguish between residual tumor and scar with reactive fibroblast proliferation, especially in the setting of a recurrent tumor.
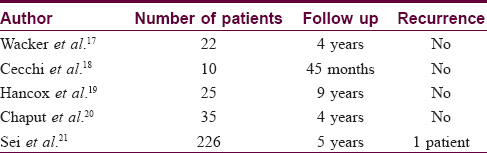
Our data strongly support the utility of Moh's micrographic surgery with paraffin-embedded sections for treating dermatofibrosarcoma protuberans. There have been no recurrences in any of our 33 patients treated with paraffin-embedded micrographic surgery. Our results are in keeping with other case series of patients treated with paraffin-embedded micrographic surgery. Our results are in keeping with other case series of patients treated with paraffin-embedded micrographic surgery [Table - 2].[17],[18],[19],[20],[21]
Tan et al. performed a review of the literature and found that five out of six cases of recurrent dermatofibrosarcoma protuberans following micrographic surgery, occurred after Moh's micrographic surgery using frozen sections.[14]
Loghdey et al. describe 76 patients treated with frozen-section Moh's micrographic surgery with one recurrence (rate 1.5%).[22]
Some institutions try to resolve this problem by taking an extra layer after completion of traditional frozen Moh's micrographic surgery and sending it for permanent paraffin sectioning for additional screening with CD34 immunostaining.[13],[23]
The main limitation of using paraffin-embedded sections is time, increasing inconvenience for patients who do not live locally and who require admission. Another potential disadvantage is the discomfort from open wounds between stages and the increased risk of infection with delayed closure.
Since 70% of dermatofibrosarcoma protuberans may extend microscopically at least 1 cm from the edge of the tumor, we recommend an initial lateral margin of at least 1 cm.[8] In our study, 3 of the 4 patients treated with a 1 cm initial margin required more than one stage and all of them had lateral and deep involvement. On the other hand, 10 of the 29 patients with an initial margin of 2 cm needed more than one stage but only 2 of them had lateral involvement and both were persistent tumors. In a recent study, Serra-Guillén et al. calculated that the minimum margin needed to achieve complete clearance in 74 cases of primary dermatofibrosarcoma protuberans was 1.34 cm.[24] According to the latest publications and the results of our study, we now perform initial excisions with a 1 cm margin for primary and persistent tumors of the head and neck, a1.5 cm margin for the rest of the body and a 2 cm margin only in cases of persistent tumors.
The majority of local recurrences of this tumor occur within the first 3 years with about half presenting within 1 year of surgery.[8] Thirty two of our 33 patients have been followed up for at least 1 year and 26 of them for more than 3 years, all of whom remain tumor free at the time of publication. All of our patients continue to be monitored every 6 months for recurrences because after Moh's micrographic surgery, late local recurrences (after more than 10 years) may occur.[13] Accordingly, we believe that patients with dermatofibrosarcoma protuberans should be followed up for life.
Non-surgical treatment modalities include radiotherapy which has been used alone or as an adjunct to surgery to decrease local recurrence rates.[25]
Ninety percent of all dermatofibrosarcoma protuberans contain the translocation t (17;22) which results in fusion of the collagen 1A1 gene from chromosome 17 with the platelet-derived growth factor B gene on chromosome 22. Imatinib mesylate is a tyrosine kinase inhibitor that is approved for use in dermatofibrosarcoma protuberans refractory to surgery and for metastatic disease.[3],[26]
Conclusions
We have presented 33 patients with dermatofibrosarcoma protuberans treated with paraffin-embedded micrographic surgery, in which there has been no case of recurrence after up to 13 years of review. Paraffin-embedded micrographic surgery appears to be an effective treatment for this condition with a low risk of local recurrence, while preserving surrounding normal healthy tissue. Our experience, in conjunction with other studies, suggests that paraffin-embedded micrographic surgery should be the method of choice when treating dermatofibrosarcoma protuberans, especially for tumors on the head and neck region or other areas where tissue preservation is important.
We prefer paraffin-embedded micrographic surgery over traditional frozen Moh's micrographic surgery because it allows immunohistochemistry in doubtful cases and because we have not found any recurrences till date in our case series.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol 2007;56:968-73.
[Google Scholar]
|
| 2. |
Farma JM, Ammori JB, Zager JS, Marzban SS, Bui MM, Bichakjian CK, et al. Dermatofibrosarcoma protuberans: How wide should we resect?Ann Surg Oncol 2010;17:2112-8.
[Google Scholar]
|
| 3. |
Bogucki B, Neuhaus I, Hurst EA. Dermatofibrosarcoma protuberans: A review of the literature. Dermatol Surg 2012;38:537-51.
[Google Scholar]
|
| 4. |
Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. A study of 115 cases. Cancer 1962;15:717-25.
[Google Scholar]
|
| 5. |
Llombart B, Sanmartín O, López-Guerrero JA, Monteagudo C, Serra C, Requena C, et al. Dermatofibrosarcoma protuberans: Clinical, pathological, and genetic (COL1A1-PDGFB) study with therapeutic implications. Histopathology 2009;54:860-72.
[Google Scholar]
|
| 6. |
Johnson-Jahangir H, Ratner D. Advances in management of dermatofibrosarcoma protuberans. Dermatol Clin 2011;29:191-200.
[Google Scholar]
|
| 7. |
Mentzel T. Sarcomas of the skin in the elderly. Clin Dermatol 2011;29:80-90.
[Google Scholar]
|
| 8. |
Ratner D, Thomas CO, Johnson TM, Sondak VK, Hamilton TA, Nelson BR, et al. Moh's micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol 1997;37:600-13.
[Google Scholar]
|
| 9. |
Paradisi A, Abeni D, Rusciani A, Cigna E, Wolter M, Scuderi N, et al. Dermatofibrosarcoma protuberans: Wide local excision vs. Moh's micrographic surgery. Cancer Treat Rev 2008;34:728-36.
[Google Scholar]
|
| 10. |
Meguerditchian AN, Wang J, Lema B, Kraybill WG, Zeitouni NC, Kane JM 3. Wide excision or Moh's micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol 2010;33:300-3.
[Google Scholar]
|
| 11. |
Breuninger H, Thaller A, Schippert W. Subclinical spread of dermatofibrosarcoma protuberans and resulting treatment modalities. Hautarzt 1994;45:541-5.
[Google Scholar]
|
| 12. |
Moh's FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978. p. 1-29.
[Google Scholar]
|
| 13. |
Snow SN, Gordon EM, Larson PO, Bagheri MM, Bentz ML, Sable DB. Dermatofibrosarcoma protuberans: A report on 29 patients treated by Moh's micrographic surgery with long-term follow-up and review of the literature. Cancer 2004;101:28-38.
[Google Scholar]
|
| 14. |
Tan WP, Barlow RJ, Robson A, Kurwa HA, McKenna J, Mallipeddi R. Dermatofibrosarcoma protuberans: 35 patients treated with Moh's micrographic surgery using paraffin sections. Br J Dermatol 2011;164:363-6.
[Google Scholar]
|
| 15. |
Nelson RA, Arlette JP. Moh's micrographic surgery and dermatofibrosarcoma protuberans: A multidisciplinary approach in 44 patients. Ann Plast Surg 2008;60:667-72.
[Google Scholar]
|
| 16. |
Roh MR, Bae B, Chung KY. Moh's' micrographic surgery for dermatofibrosarcoma protuberans. Clin Exp Dermatol 2010;35:849-52.
[Google Scholar]
|
| 17. |
Wacker J, Khan-Durani B, Hartschuh W. Modified Moh's micrographic surgery in the therapy of dermatofibrosarcoma protuberans: Analysis of 22 patients. Ann Surg Oncol 2004;11:438-44.
[Google Scholar]
|
| 18. |
Cecchi R, Rapicano V. Micrographic surgery (Tübingen technique) for the treatment of dermatofibrosarcoma protuberans: A single-centre experience. Eur J Dermatol 2007;17:543-4.
[Google Scholar]
|
| 19. |
Hancox JG, Kelley B, Greenway HT Jr. Treatment of dermatofibroma sarcoma protuberans using modified Moh's micrographic surgery: No recurrences and smaller defects. Dermatol Surg 2008;34:780-4.
[Google Scholar]
|
| 20. |
Chaput B, Filleron T, Le Guellec S, Meresse T, Courtade-Saïdi M, Grolleau JL, et al. Dermatofibrosarcoma protuberans: Margins reduction using slow-Moh's micrographic surgery. Experience with 35 patients. Ann Chir Plast Esthet 2014;59:219-25.
[Google Scholar]
|
| 21. |
Sei JF, Chaussade V, Tchakerian A, Serra M, Zimmermann U, Clerici T, et al. Treatment of dermatofibrosarcoma protuberans (DFSP) with fixed Moh's micrographic surgery (f-MMS). J Clin Oncol 2015;33:(suppl):abstr e20066. Available from: http://meetinglibrary.asco.org/content/145704-156. [Last accessed on 2016 Aug 19].
[Google Scholar]
|
| 22. |
Loghdey MS, Varma S, Rajpara SM, Al-Rawi H, Perks G, Perkins W. Moh's micrographic surgery for dermatofibrosarcoma protuberans (DFSP): A single-centre series of 76 patients treated by frozen-section Moh's micrographic surgery with a review of the literature. J Plast Reconstr Aesthet Surg 2014;67:1315-21.
[Google Scholar]
|
| 23. |
Nouri K, Lodha R, Jimenez G, Robins P. Moh's micrographic surgery for dermatofibrosarcoma protuberans: University of Miami and NYU experience. Dermatol Surg 2002;28:1060-4
[Google Scholar]
|
| 24. |
Serra-Guillén C, Llombart B, Nagore E, Guillén C, Requena C, Traves V, et al. Moh's micrographic surgery in dermatofibrosarcoma protuberans allows tumour clearance with smaller margins and greater preservation of healthy tissue compared with conventional surgery: A study of 74 primary cases. Br J Dermatol 2015;172:1303-7.
[Google Scholar]
|
| 25. |
Suit H, Spiro I, Mankin HJ, Efird J, Rosenberg AE. Radiation in management of patients with dermatofibrosarcoma protuberans. J Clin Oncol 1996;14:2365-9.
[Google Scholar]
|
| 26. |
Han A, Chen EH, Niedt G, Sherman W, Ratner D. Neoadjuvant imatinib therapy for dermatofibrosarcoma protuberans. Arch Dermatol 2009;145:792-6.
[Google Scholar]
|
Fulltext Views
3,929
PDF downloads
4,430





