Translate this page into:
Rational use of laboratory tests in dermatology
Correspondence Address:
Saumya Panda
Department of Dermatology, KPC Medical College and Hospital, 1F, Raja Subodh Chandra Mullick Road, Jadavpur, Kolkata - 700 032, West Bengal
India
| How to cite this article: Panda S. Rational use of laboratory tests in dermatology. Indian J Dermatol Venereol Leprol 2018;84:377-383 |
Background
Laboratory and in vivo test results greatly influence diagnosis and treatment. Therefore, it is essential to select appropriate tests in the diagnostic process and keep in mind that each test can produce false positive and false negative results. The selection of an optimal test requires a thorough understanding of the concepts delineating the efficacy of a diagnostic test such as sensitivity, specificity, positive and negative predictive value and likelihood ratios for a positive and negative test.[1]
While accurate interpretation of laboratory tests often depends on the use of statistical concepts we learned during medical training, many of us find it difficult, for different reasons, to incorporate these principles into a busy practice. We need to be aware of the fact that without correct interpretation laboratory results are hardly meaningful. Laboratory mistakes are not yet defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that happen every year.[2]
Overuse of laboratory tests is problematic. Because “normal range” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside the range.[3] It is important to order only the tests we really need as extra testing automatically means more false positive results. The risk of false positive results increases up to 40% when a patient is exposed to ten unnecessary laboratory investigations.[4]
Unfortunately, such misuse of laboratory tests is widespread. In a systematic review of 44 eligible published studies measuring inappropriate laboratory utilization in the light of methodological criteria, there was widespread prevalence but large variations in the estimates of inappropriate laboratory use (4.5%–95%).[5] Though data obtained from this review is two decades old, more recent studies have hardly shown any improvement in this regard. In a study carried out in the intensive care unit of a general hospital in Brazil, 41% (n = 719) of the 1,750 lab tests ordered were found to be unnecessary.[6] In a South African study, in 59.1% of cases, the test results were incorporated into the physicians' management plan for the patient, that is, in 41.9% of cases the results had no bearing on further management of the patients.[7] Effective use of lab tests is really the key as there is overwhelming evidence that such use significantly improves diagnosis and treatment at every setting and every level of medical care.[8]
With the rapidly increasing volume of medical research being conducted and clinical laboratory tests being developed, physicians are challenged increasingly on how best to integrate clinical and laboratory evidence in making decisions about the day-to-day care of their patients.[9] No sure method exists for eliminating biases in medical decision making, but there is some evidence that the adoption of an evidence-based medicine approach or the incorporation of formal decision analytic tools can improve the quality of physicians' reasoning.[10]
In one of the rare studies conducted among patients in dermatology outpatient clinics who had been advised laboratory tests, the cognitive or psychological effect of the examinations were tested according to the hypothesis that performing laboratory tests increases the patients' fear of morbidity and mortality, and therefore, has a positive effect on the patients' attitude toward the doctors' recommendations and willingness to accept them. Patients who had undergone laboratory tests one week before the survey had a tendency to show even lower positive attitude toward the doctor's recommendations and less intention to follow the recommendations. In contrast to the hypothesis that also happens to be the conventional wisdom, performing laboratory tests does not subliminally increase patients' fears or anxieties about their disease or their compliance with doctors' recommendations.[11]
This editorial will seek to refresh the relevant statistical concepts. We shall also discuss certain tests commonly employed by dermatologists in day-to-day practice and exemplify how the test results are reliable only if comorbidities, pre- and post-test probabilities and clinical context are carefully considered. Thus, we hope a rational understanding of the laboratory tests that we employ may develop.
Key Concepts
What are the rational indications of performing a test? In a nutshell, these are:
- Diagnosis of disease
-
- Occult disease
- Early diagnosis
- Differential diagnosis
- Prognosis of disease
- Staging of disease
- Estimating activity of disease
- Detecting recurrence
- Monitoring effect of therapy
- Prediction of response to therapy
- Prediction of drug toxicity
- Monitoring for adverse drug reactions
- Genetic counselling
- Medicolegal problems (e.g., paternity).
Thus, laboratory or in-vivo tests are an integral part of our diagnostic techniques. However, it is not mandatory to order tests for every patient, irrespective of the clinical situation.
If we review strategies of clinical diagnosis, we find that a diagnosis may be made by specific clinical features alone (viz., erythema multiforme), lab tests alone (diabetes mellitus), clinical features aided by histopathology (e.g., vasculitis, panniculitis and sundry other inflammatory and neoplastic dermatoses), clinical features aided by lab test (e.g., connective tissue diseases), clinical criteria alone (atopic dermatitis according to UK Working Party's diagnostic criteria) and by means of clinical and lab criteria (atopic dermatitis according to Hanifin and Rajka's criteria).
In an ideal world, an ideal laboratory test should have the following characteristics:
- Positive in every patient with the disease
- Negative in every patient without the disease
- Positive from an early or very early stage
- Vary synchronously with corresponding disease activity.[12]
As we live in a world far from ideal, we need to ask some questions whenever we order a lab test. These are:
- What is the reason for the test?
- What are the consequences of not ordering the test?
- How is the test result interpreted:
- In patients with the disease, what proportion tests positive?
- In patients without the disease, what proportion tests negative?
- If a patient tests positive, what is the probability of having the disease?
- If a patients tests negative, what is the probability of not having the disease?
- On the basis of our interpretation, is the test adequate for differentiating people who are diseased from the nondiseased?
- Whether the test result will change the diagnosis or prognosis or therapy?
- Will the result provide a better understanding of the disease process that is going on in the patient?
- How will the test results affect the patient's life?[13]
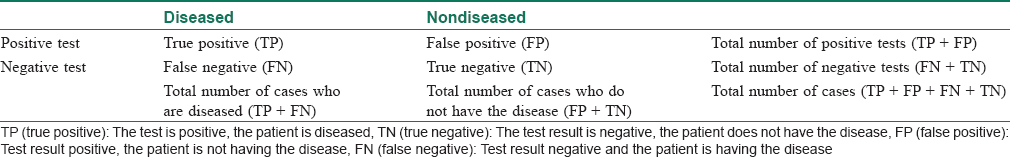
In good clinical practice, it is necessary to realize that every test can be a source of false positive and false negative results. This is expressed as a classic 2 × 2 table and is interpreted as such [Table - 1]. The risk of such errors is usually described using indicators of sensitivity and specificity. [Table - 2] provides the definitions of terms useful in determining the effectiveness of laboratory tests using those indicators.

Rational uses of Diagnostic Tests: A Few Examples
Here, we shall discuss a few examples of the rational use of commonly used in-vitro tests. In all these cases, the test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered.
How to interpret abnormal liver function tests for patients on methotrexate therapy
Abnormal liver function tests should never be presumed to be caused by methotrexate. The available evidence indicates that methotrexate-related liver adverse events are rarely serious, particularly in the short term, whereas many other causes of abnormal liver function tests may be serious. An evaluation for other potential causes should follow identical pathways and similar rigor to that applied to a patient who is not taking methotrexate.[14]
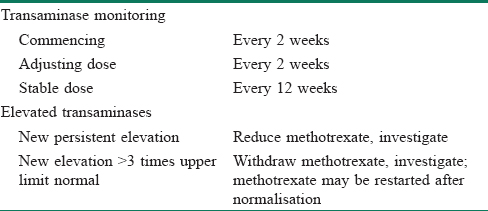
If after exhaustive investigation no cause other than methotrexate is identifiable, the treatment approach recommended in guidelines depends on the degree of transaminase elevation. The baseline transaminase levels prior to methotrexate institution are also important; a previously elevated transaminase level that has not changed following institution of methotrexate is unlikely to need further intervention. The threshold for immediately interrupting methotrexate use differs by the respective guideline; however, levels greater than three times the upper limit of normal are often used.[15] Persistent lower degree elevations may also require intervention particularly if the trend is for a progressive increase in the transaminases.[16] A proposed approach to suspected methotrexate hepatotoxicity is outlined in [Table - 3].[14]
Take home message
Methotrexate use is associated with an increased risk of elevated transaminase levels; however, the risk of an increased risk of serious liver adverse events with modern methotrexate monitoring protocols appears to be extremely low at present. Large increases are rare, should be taken seriously and the medication stopped.
How to interpret antinuclear antibody tests
Antinuclear antibodies are autoantibodies that react with antigens in the nucleoplasm. They probably occur in the circulation of all human beings. Tests for antinuclear antibodies are only considered positive if they occur in concentration significantly above the normal serum level. Pathogenetic role is attributed to only a few antibodies. Most are thought to be caused by the disease (epiphenomena) rather than the cause of the disease (etiopathogenetic in origin).[17] Antinuclear antibodies are markers for various connective tissue diseases, which have considerable overlap among each other. Very few antinuclear antibodies are specific for a single clinical entity. All connective tissue disorders are diagnosed by a set of clinical features, aided by lab tests such as antinuclear antibodies. If the clinical criteria for the diagnosis of these disorders are not fulfilled (low pre-test probability), these tests are not useful and should not be carried out.
Fluorescent antinuclear antibodies test is considered the gold standard for screening suspected cases of systemic lupus erythematosus. A positive fluorescent antinuclear antibodies test is arbitrarily defined as the level of antinuclear antibodies that exceeds the level seen in 95% of normal healthy individuals. The sensitivity of positive antinuclear antibodies test in systemic lupus erythematosus is 99%. Thus, it is a very good screening test for systemic lupus erythematosus. However, 1% of active systemic lupus erythematosus patients will show false negative result. So will the patients with isolated anti-Ro antibodies and patients with end-stage renal disease on dialysis. At the same time, many patients without any connective tissue diseases may show false positive antinuclear antibodies, e.g. those with viral infections such as HIV and hepatitis C, other autoimmune disorders such as autoimmune thyroiditis and primary biliary cholangitis (previously known as primary biliary cirrhosis), and malignancies such as lymphoma, etc.[12]
At an endpoint titre of 1:40, 32% of normal individuals are positive. At titres of 1:80, 1:160 and 1:320, the percentages of normal individuals showing positivity drop down to 13%, 5% and 3%, respectively.[18] To calculate the positive predictive value of a test, we need to know the disease prevalence in the population. In other words, in a population, the prevalence of the disease is the pretest probability (probability of a person having a disease before the test result in known). For example, we know from the point prevalence data of a population survey carried out in North India that the prevalence of systemic lupus erythematosus in India is comparatively low (3.2 per 100,000 population).[19] Taking the background positivity rate of antinuclear antibodies at 1:160 as 5% and the prevalence in India roughly as 3 per 100,000, if 100,000 randomly selected Indians are tested with ANA, three persons will be true positive and 5000 will have false positive results at a titre of 1:160. Thus, the positive predictive value, Total positive/Total positive + false positive, will be 3/5003 or. 06%. This goes on to demonstrate that, when pretest probability (in this case, prevalence of the disease in a population) is low, a positive result is more likely to be false positive. Thus, one needs to take a conservative approach when ordering antinuclear antibodies to screen for systemic lupus erythematosus in India.
However, irrespective of the population and prevalence rates, antinuclear antibodies testing is only warranted when pretest probability is high. In a retrospective study, more than 90% of patients who were referred to a tertiary rheumatology clinic for a positive antinuclear antibodies test result had no evidence for an antinuclear antibody-associated rheumatic disease. The poor predictive value of a positive antinuclear antibody in this cohort was largely attributable to unnecessary testing in patients with low pretest probabilities for antinuclear antibody-associated rheumatic disease.[20]
Take home message
Testing for antinuclear antibodies should only be done when there is strong clinical suspicion. For any specific connective tissue disorder, screening has to be primarily done with sensitive tests and, if positive, more specific tests are to be added to the diagnostic work-up. When pretest probability is low, positive tests are likely to be false positive. Antinuclear antibodies may be present normally. Negative antinuclear antibodies may help exclude systemic lupus erythematosus. Antinuclear antibody titres do not predict disease activity. An exception is the anti-dsDNA test in systemic lupus erythematosus. Fluorescent antinuclear antibody testing should usually be ordered only once. Positive antinuclear antibody tests do not need to be repeated. Negative tests need to be repeated only if either there is a strong suspicion of an evolving connective tissue disease or a change in the patient's clinical findings suggesting that the diagnosis should be revised.[21] The sundry autoimmunity test panels, offered by various laboratories, should be shunned as their use in the absence of clinical suspicion may cause confusion and unnecessary expenditure.[12]
Thyroid function testing: When should one test?
Thyroid-stimulating hormone is the first-line test when investigating presumed hyper- or hypothyroidism.[22] However, thyroid-stimulating hormone levels exhibit diurnal variation and are affected by other medications, including steroids, opiates and some antihistamines, among others, as well as comorbidities. Chronic and acute conditions unrelated to thyroid disease can cause transient changes in thyroid-stimulating hormone concentrations and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins. Thus, thyroid-stimulating hormone should be ordered only when clinical suspicion of a thyroid problem exists.[23] The United States Preventive Services Task Force recommends against routine thyroid-stimulating hormone screening for asymptomatic adults.[24]
For patients found to have abnormal thyroid-stimulating hormone levels, free T4 is the next test to order.[25] A free T4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.[23]
Triiodothyronine measures can be useful in diagnosing Graves' disease, in which triiodothyronine toxicosis may be the initial symptom or an indication of a relapse. Because triiodothyronine is often a peripheral product, nonthyroid illnesses and medications can cause abnormal results as an artifact.[23]
Other thyroid-specific labs include thyroid antibodies such as antithyroid peroxidase, antithyroglobulin and thyroid-stimulating hormone receptor, both blocking and stimulating. Retesting thyroid-stimulating hormone to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.[26]
Take home message
Thyroid studies can be very difficult to interpret. Thyroid-stimulating hormone should be the first test ordered.[27] However, if thyroid-stimulating hormone values do not match the clinical picture, free T4, antithyroid peroxidase antibody and other thyroid tests that are less affected by external factors can be useful.
Hemoglobin A1c and anemia
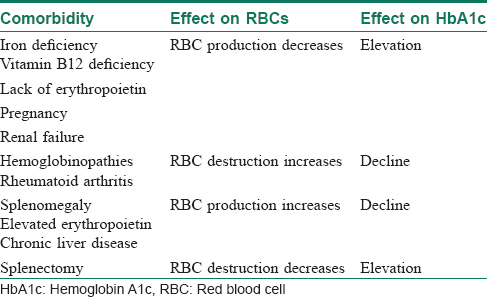
The two primary variables influencing glycosylated hemoglobin (hemoglobin A1c) are the average glucose level and the average lifespan of red blood cells.[23] Normally, there is a direct correlation between average serum glucose and hemoglobin A1c.[28] In patients with anemia, however, this relationship does not hold good, and may be affected by erythropoiesis and red blood cell destruction.[29] In iron-deficiency anemia, hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated hemoglobin A1c.[30] In at least one study, haemoglobin A1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with iron supplementation.[31]
Increased destruction as well as increased production of red blood cells lower their lifespan and in turn decreases hemoglobin A1c levels [Table - 4].[23] This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels, thereby making hemoglobin A1c testing unreliable.
Take home message
In a country where diseases such as iron deficiency anemia and thalassemia are endemic in large parts, it is crucial to order for testing hemoglobin levels whenever ordering for hemoglobin A1c.
Serum ferritin: A marker of iron deficiency and an acute phase reactant
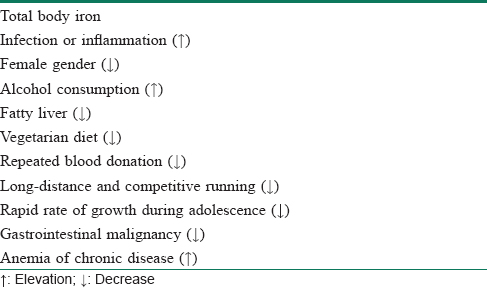
Serum ferritin is the preferred serologic marker of iron stores.[32] As iron deficiency develops, low serum ferritin appears long before anemia. There are two other conditions besides iron deficiency that may be associated with low serum ferritin – hypothyroidism and scurvy. In both these situations, by the time serum ferritin falls below normal, the patient has anemia and other clinical features suggesting the diagnosis. A ferritin level less than 15 ng/mL is, thus, virtually diagnostic of iron deficiency anemia with a specificity of 99%.[33] However, a cut-off of 41 ng/mL yields sensitivity and specificity of 98% each.[34] Dermatologists, in India as well as elsewhere, frequently employ this test whenever a chronic diffuse telogen hair loss is suspected. This is particularly relevant in the Indian context because of the widespread prevalence of iron deficiency anemia that is one of the foremost reasons behind this kind of hair loss.[35] However, because low serum ferritin is a marker of subclinical anemia, a question arises regarding how to interpret low ferritin and normal hemoglobin levels. If a patient with low serum ferritin and normal hemoglobin has a low mean corpuscular volume, then (s)he has iron deficiency; if his/her mean corpuscular volume is normal, (s)he is iron-depleted. The most serious cause of the latter is gastrointestinal malignancy. Much common, and more banal, causes of iron deficiency are vegetarian diet, repeated blood donation, long-distance and competitive running and rapid rate of growth during adolescence. Interestingly, serum ferritin may be elevated in anemia of chronic disease.[36]
Unfortunately, serum ferritin is also an acute phase reactant being elevated in chronic inflammation [Table - 5].[32] It has been suggested that a ferritin of less than 40 can diagnose iron deficiency anemia in patients without inflammation and less than 70 was indicative of deficiency for those with an inflammatory condition.[33]
Because of such a multitude of influencing factors behind serum ferritin, the relationship of hair loss with iron deficiency (low serum ferritin) without anemia or only mild anemia is complex and controversial.[37] While low serum ferritin and low hemoglobin is a straightforward scenario, for which iron supplementation is recommended, a ferritin level of more than 70 ng/mL rules out iron deficiency. A level of serum ferritin between 15 and 70 ng/mL with normal hemoglobin levels is the gray zone, for which one should apply clinical judgment and delve deeper.
Take home message
Whenever ordering for serum ferritin, one must also order for the hemoglobin level and an independent acute phase reactant such as the erythrocyte sedimentation rate or c-reactive protein. In case the erythrocyte sedimentation rate/c-reactive protein is normal, 40 ng/ml should be treated as the cut-off for iron deficiency. In case the erythrocyte sedimentation rate/c-reactive protein is elevated, 70 ng/ml should be treated as the cut-off, and iron supplementation may be considered even in the absence of low Hb. Mean corpuscular volume should be the guide in such situations to assess whether the case represents iron deficiency or iron depletion.
How important is fasting in lipid profile examination
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles.
Traditionally, this has been defined as an 8- to 12-hour period without food.[38] Studies investigating the effect of meals on laboratory values have found that triglycerides are consistently elevated postprandially up to a maximum of 12 hours.[39] The effect of the fasting state on total cholesterol, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.[40]
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force supports this practice. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing low-density lipid cholesterol levels.[23]
Take home message
The clinical relevance of postprandial vs fasting lipid levels is equivocal.
Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment.[41]
The Road Ahead
As this editorial was getting prepared, on 15 May 2018, the World Health Organization (WHO) announced the publication of the first edition of its Model List of Essential In Vitro Diagnostics.[42] In its news release, WHO cited the fact that as of today many people are unable to get tested for diseases because they cannot access diagnostic services, and many are incorrectly diagnosed. As a result, they do not receive the treatment they need and, in some cases, may actually receive the wrong treatment.[42] The first Essential Diagnostics List was published to address this gap that exists in getting an accurate diagnosis and an effective treatment.
The list concentrates on in-vitro tests, i.e. tests of human specimens such as blood and urine. It contains 113 tests, 58 of which are listed for detection and diagnosis of a wide range of common conditions, providing an essential package that can form the basis for screening and management of patients. The remaining 55 tests are designed for the detection, diagnosis and monitoring of “priority” diseases such as HIV, tuberculosis, malaria, hepatitis B and C, human papillomavirus and syphilis.[43] This is the first step taken on a global scale to introduce the concept of essential diagnostics, much like what its precursor, the Model List of Essential Medicines, did for the global health policy. According to the WHO, the items included in the Essential Medicines List are “drugs that satisfy the health care needs of the population [and]. are intended to be available at all times.... at a price the individual and community can afford.”[44] In other words, these are the medications that can satisfy the basic health needs of a population served by physicians steeped in the principles of rational therapy. The Essential Diagnostics List seeks to extend the same principles of rationality to the field of laboratory tests.
This is a welcome move and should provide a fillip to all physicians, dermatologists included, towards rational utilization of in vitro tests in their diagnostic protocol.
| 1. |
Gregorius A, Spiewak R. The principles of rational selection of diagnostic tests in allergology. Alerg Astma Immunol 2013;18: 221-30.
[Google Scholar]
|
| 2. |
Ottomano C. Errors in medicine and errors in laboratory medicine: What is the difference? Blood Transfus 2010;8:79-81.
[Google Scholar]
|
| 3. |
Wallach JB. Introduction to normal values (reference ranges). In: Interpretation of Diagnostic Tests. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2007. p. 3-7.
[Google Scholar]
|
| 4. |
Axt-Adam P, van der Wouden JC, van der Does E. Influencing behavior of physicians ordering laboratory tests: A literature study. Med Care 1993;31: 784-94.
[Google Scholar]
|
| 5. |
van Walraven C, Naylor CD. Do we know what inappropriate laboratory utilization is? A systematic review of laboratory clinical audits. JAMA 1998;280: 550-8.
[Google Scholar]
|
| 6. |
Oliveira AM, Oliveira MV, Souza CL. Prevalence of unnecessary laboratory tests and related avoidable costs in Intensive Care Unit. J Bras Patol Med Lab 2014; 50: 410-6.
[Google Scholar]
|
| 7. |
Prinsloo EA, Dimpe MW, Maphakisa MV, Matika MD, Shabalala SL, Joubert G. Doctors' use of laboratory tests in the diagnosis and treatment of patients. South Afr J Epidemiol Infect 2010; 25: 16-20.
[Google Scholar]
|
| 8. |
Carter JY, Lema OE, Wangai MW, Munafu CG, Rees PH, Nyamongo JA, et al. Laboratory testing improves diagnosis and treatment outcomes in primary health care facilities. Afr J Lab Med 2012;1:8.
[Google Scholar]
|
| 9. |
McAlister FA, Straus SE, Guyatt GH, Haynes RB. Users' guides to the medical literature: XX. Integrating research evidence with the care of the individual patient. Evidence-based medicine working group. JAMA 2000; 283: 2829-36.
[Google Scholar]
|
| 10. |
Bornstein BH, Emler AC. Rationality in medical decision making: A review of the literature on doctors' decision-making biases. J Eval Clin Pract 2001; 7: 97-107.
[Google Scholar]
|
| 11. |
Shin WU, Baek YS, Kim TJ, Oh CH, Kim J. Laboratory tests and compliance of dermatologic outpatients. F1000Res 2013; 2: 206.
[Google Scholar]
|
| 12. |
Bandyopadhyay D. Antinuclear Antibody Tests: Rationale and Clinical Utilities. IADVL Dermagyan Lecture Series; April, 2018. Available from: https://www.iadvl.mediknit.org/antinuclear-antibody-tests-rationale-and-clinical-utilities-ae08d55f. [Last accessed on 2018 May 17].
[Google Scholar]
|
| 13. |
Wians FH. Clinical laboratory tests: Which, why, and what do the results mean? Lab Med 2009; 40: 105-13.
[Google Scholar]
|
| 14. |
Conway R, Carey JJ. Risk of liver disease in methotrexate treated patients. World J Hepatol 2017; 9: 1092-100.
[Google Scholar]
|
| 15. |
Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: Integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E initiative. Ann Rheum Dis 2009; 68: 1086-93.
[Google Scholar]
|
| 16. |
Ledingham J, Gullick N, Irving K, Gorodkin R, Aris M, Burke J, et al. BSR/BHPR non-biologic DMARD Guidelines 2016. Available from: http://www.rheumatology.org.uk/includes/documents/cm_docs/2016/f/full_dmards_guideline_and_the_executive_summary.pdf.
[Google Scholar]
|
| 17. |
Smeenk RJ. Antinuclear antibodies: Cause of disease or caused by disease? Rheumatology (Oxford) 2000; 39: 581-4.
[Google Scholar]
|
| 18. |
Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997; 40: 1601-11.
[Google Scholar]
|
| 19. |
Malaviya AN, Singh RR, Singh YN, Kapoor SK, Kumar A. Prevalence of systemic lupus erythematosus in India. Lupus 1993; 2: 115-8.
[Google Scholar]
|
| 20. |
Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med 2013;126:342-8.
[Google Scholar]
|
| 21. |
Kumar Y, Bhatia A, Minz RW. Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: A journey revisited. Diagn Pathol 2009; 4: 1.
[Google Scholar]
|
| 22. |
Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: An update. Am Fam Physician 2012; 86: 244-51.
[Google Scholar]
|
| 23. |
Tessier J, Downen M, Engel-Brower J, Naevem L, Sayler M, Hornig K, et al. Pitfalls & pearls for 8 common lab tests. J Fam Pract 2014; 63: 198-205.
[Google Scholar]
|
| 24. |
Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila) 2009; 47: 286-91.
[Google Scholar]
|
| 25. |
Volpé R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci 1997; 34: 405-38.
[Google Scholar]
|
| 26. |
Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18: 988-1028.
[Google Scholar]
|
| 27. |
Helfand M; U.S. Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2004; 140: 128-41.
[Google Scholar]
|
| 28. |
Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes 2009; 1: 9-17.
[Google Scholar]
|
| 29. |
Franco RS. The measurement and importance of red cell survival. Am J Hematol 2009; 84:109-14.
[Google Scholar]
|
| 30. |
Tarim O, Küçükerdoğan A, Günay U, Eralp O, Ercan I. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int 1999; 41: 357-62.
[Google Scholar]
|
| 31. |
Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care 2010; 33: 780-5.
[Google Scholar]
|
| 32. |
Dasher K, Worthington MT. Iron: Not too much and not too little. Pract Gastroenterol 2009; 16: 19-21, 24-6.
[Google Scholar]
|
| 33. |
Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C, et al. Laboratory diagnosis of iron-deficiency anemia: An overview. J Gen Intern Med 1992; 7: 145-53.
[Google Scholar]
|
| 34. |
Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venereol Leprol 2013; 79: 591-603.
[Google Scholar]
|
| 35. |
Headington JT. Telogen effluvium. New concepts and review. Arch Dermatol 1993; 129: 356-63.
[Google Scholar]
|
| 36. |
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011-23.
[Google Scholar]
|
| 37. |
Trost LB, Bergfeld WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol 2006; 54: 824-44.
[Google Scholar]
|
| 38. |
Turgeon ML. Linne & Ringsrud's Clinical Laboratory Science. 5th ed. Saint Louis: Mosby; 2007. p. 50.
[Google Scholar]
|
| 39. |
Watts GF, Cohn JS. Whither the lipid profile: Feast, famine, or no free lunch? Clin Chem 2011; 57: 363-5.
[Google Scholar]
|
| 40. |
Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008; 118: 993-1001.
[Google Scholar]
|
| 41. |
Cohn JS, McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem 1988; 34: 2456-9.
[Google Scholar]
|
| 42. |
WHO News Release. First-ever WHO List of Essential Diagnostic Tests to Improve Diagnosis and Treatment Outcomes. Geneva; 15 May, 2018. Available from: http://www.who.int/news-room/detail/15-05-2018- first-ever-who-list-of-essential-diagnostic-tests-to-improve-diagnosis-and-treatment-outcomes#. [Last accessed on 2018 16 May].
[Google Scholar]
|
| 43. |
World Health Organization. World Health Organization Model List of Essential in vitro Diagnostics. 1st ed. Geneva: World Health Organization; 2018.
[Google Scholar]
|
| 44. |
Schroeder LF, Guarner J, Elbireer A, Castle PE, Amukele TK. Time for a model list of essential diagnostics. N Engl J Med 2016; 374: 2511-4.
[Google Scholar]
|
Fulltext Views
6,726
PDF downloads
2,296





