Translate this page into:
A cross-sectional study of variations in the biophysical parameters of skin among healthy volunteers
2 Department of Dermatology, SMBT Medical College, Nashik, Maharashtra, India
3 Department of Dermatology, American International Institute of Medical Sciences, Udaipur, Rajasthan, India
4 Department of Physiology, Government Medical College, Bhavnagar, Gujarat, India
Correspondence Address:
Hita H Mehta
Department of Dermatology, Venereology and Leprosy, Sir T Hospital, Government Medical College, Bhavnagar - 364 002, Gujarat
India
| How to cite this article: Mehta HH, Nikam VV, Jaiswal CR, Mehta HB. A cross-sectional study of variations in the biophysical parameters of skin among healthy volunteers. Indian J Dermatol Venereol Leprol 2018;84:521 |
Abstract
Background: Biophysical parameters of skin such as trans-epidermal water loss (TEWL), hydration, elasticity, pH, and sebum reflects it functional integrity. Advances in technology have made it possible to measure these parameters by non-invasive methods. These parameters are useful for the prediction of disease and its prognosis. It also helps in developing new skin care products according to various skin types, and to evaluate, modify, or compare the effects of existing products.
Aim: The aim of the study was to measure, evaluate, and analyze variations in biophysical parameters at pre-selected skin sites in healthy Indian volunteers, across different age groups and gender.
Methods: The study was conducted among 500 healthy Indian volunteers, between 5 and 70 years of age, in the outpatient department of dermatology at Sir T. Hospital, Bhavnagar. Biophysical parameters such as TEWL, hydration, elasticity, and sebum content was measured on four pre-selected body sites by a Dermalab instrument (Cortex Technology, Denmark). The skin pH was measured with a sensitive pH probe (BEPL 2100).
Results: All parameters were higher in males compared to females, except for sebum content, which was equal in both genders. Transepidermal water loss and hydration was lower in middle and older age groups. The skin pH showed no statistically significant difference with age. Sebum content was higher in middle and older age groups. The nose had the highest sebum content across all age groups. The forehead showed higher median values of TEWL and hydration compared to other sites. Though elasticity has highest value on forearm, only leg region showed statistically significant value.
Limitations: The present study was confined to a single geographical area, so the effect of environment changes could not be judged accurately. Seasonal variations were not studied as it was a cross-sectional study.
Conclusion: Skin properties vary with age, gender, and location on the body. This knowledge will help to create a database of these parameters in the Indian population. It would assist in the diagnosis of various clinical conditions and monitor therapeutic response.
Introduction
The skin is the largest organ of the body. Cutaneous microanatomy and physiology are complex and varies with age and gender. The biophysical properties of the skin are a reflection of skin function. The significance and awareness regarding monitoring of biophysical parameters of the skin is on the rise over the last decade.[1]
Biophysical parameters of the skin, such as trans-epidermal water loss (TEWL), hydration, elasticity, sebum and pH may vary with respect to different geographical areas, race, age group, gender and occupation. Study of these parameters in different groups may be helpful to develop new skin care products as well as to modify existing products as per the need of a particular population. There are few studies conducted and published showing variation in biophysical parameters of the skin across various age groups, gender and races.[2] The aim of this study was to determine the normal range of these biophysical parameters among people residing in the western region of India. This study will contribute in establishing a database for our country.
Methods
After receiving institutional review board approval, 500 healthy people of different age groups and gender were randomly enrolled into the study after informed consent. It included healthy relatives of patients, paramedical staff, colleagues and medical students from the dermatology department and Sir T. Hospital, Government Medical College, Bhavnagar. It was conducted between May 2013 and August 2014. We selected healthy volunteers with no dermatoses or any major systemic disorders that could alter the biophysical parameters. We calculated the sample size using open Epi software, version 3.01. We evaluated the study variations in five biophysical parameters (TEWL, hydration, elasticity, pH, and sebum) in both genders across four age groups: 5–20 years (childhood and adolescence-group 1), 21–35 years (young adult-group 2), 36–50 years (middle age-group 3) and 51–70 years (elderly-group 4). The selected sites were scalp, forehead, right forearm and right leg for the first four parameters. For sebum, the sites selected were the scalp, forehead, nose and left nasolabial fold. Measurements were recorded after allowing the person to rest for fifteen minutes. The room temperature and relative humidity were maintained strictly between 20°C–27°C and 10%–60%, respectively. Dermalab USB instrument with preinstalled software (Cortex technology, Denmark) was used to measure the parameters. Three different probes were used to measure the TEWL, hydration and elasticity. A sebum strip was used to measure the sebum content.
TEWL
Water loss is based on the principle of Nilsson's vapour pressure gradient method. Two sets of temperature/humidity sensors are mounted in a measurement chamber at different heights above the skin surface. The measurement chamber is open in order to allow the skin to breathe freely and the evaporation rate follows the Fick's law of diffusion [Normal range: 0–250 g/m 2/h, Resolution: 0.1 g/m 2/h].
Hydration
The principle of hydration module is measuring the conducting properties of upper layers of skin when subjected to alternating voltage. The pin-probe has 8 contact electrodes with spring loaded action, which initiates measurement when pressed against the skin. It indicates water binding capacity of stratum corneum [Normal range: 0–9999 microsiemens, Resolution: 1 microsiemens]
Elasticity
The elasticity screen works on the principle of stress applied by vacuum. The probe has a suction pump which will start lifting the skin to a maximum extension of 2.5 mm. The negative pressure is then relieved and the elevated skin retracts. Two internal detectors in the probe are positioned 1.5 mm apart and are triggered as the skin retracts thereby measuring the time needed for a retraction of 1.5 mm [Normal range: 1.5 mm elevation, Vacuum: 0–75 kPa, Accuracy: 2%, score: 0–99].
Sebum
A microporous polymer film with range of 0–100%, resolution 0.1% and accuracy 5% was used as a collecting material. The strip was calibrated before applying it to the skin. The amount of sebum was measured in tape reader module which provides a slot for insertion of the strip in use. The result is based on the principle of change in translucency of the film [Normal reading 0–99 where 99 signifies very oily skin].
pH
The pH meter measures pH of skin with the help of sensitive electrodes from four sites.
Statistical analysis
All the data was subjected to a normality test and found to be non-Gaussian in distribution. As the median and interquartile ranges are less affected with extreme values, they were used in our study as central tendency and dispersion from central tendency. Hence, non-parametric tests were used to calculate and analyze the data. Mann–Whitney test was used for the comparison of gender parameters. Kruskal–Wallis test with Dunn's test was used for multiple comparisons between different age groups. All statistical calculations were done with graph pad InStat software (version 3.06). P value <0.05 was considered statistically significant. To study correlation between TEWL and hydration, Spearman's rank correlation coefficient test was used.
Results
The demographic distribution is presented in [Table - 1]. All parameters were compared between gender indifferent age groups, across different skin sites [Table - 2]a,[Table - 2]b,[Table - 2]c,[Table - 2]d,[Table - 2]e and [Figure - 1]a,[Figure - 1]b,[Figure - 1]c,[Figure - 1]d,[Figure - 1]e. The median values in the interquartile range (IQR) for all parameters were significantly higher in males, while it was equal in both genders for sebum [Table - 3].
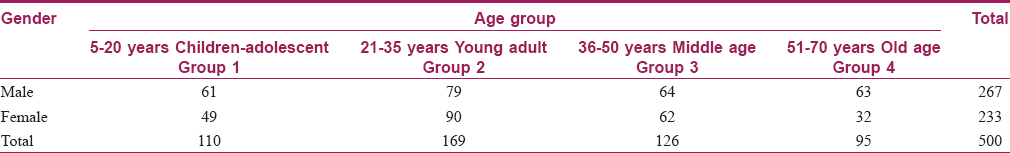
 |
| Figure 1 |
TEWL
The median values were significantly higher in males of all age groups, except the old age group (group 4). The difference was greater in the scalp region. Readings were comparatively higher in age groups 1 and 2 than age groups 3 and 4. This difference was statistically significant in all regions except the forearm. The median values were highest over the forehead, followed by scalp, forearm and leg [Table - 2]a and [Table - 3], [Figure - 1]a.
Hydration
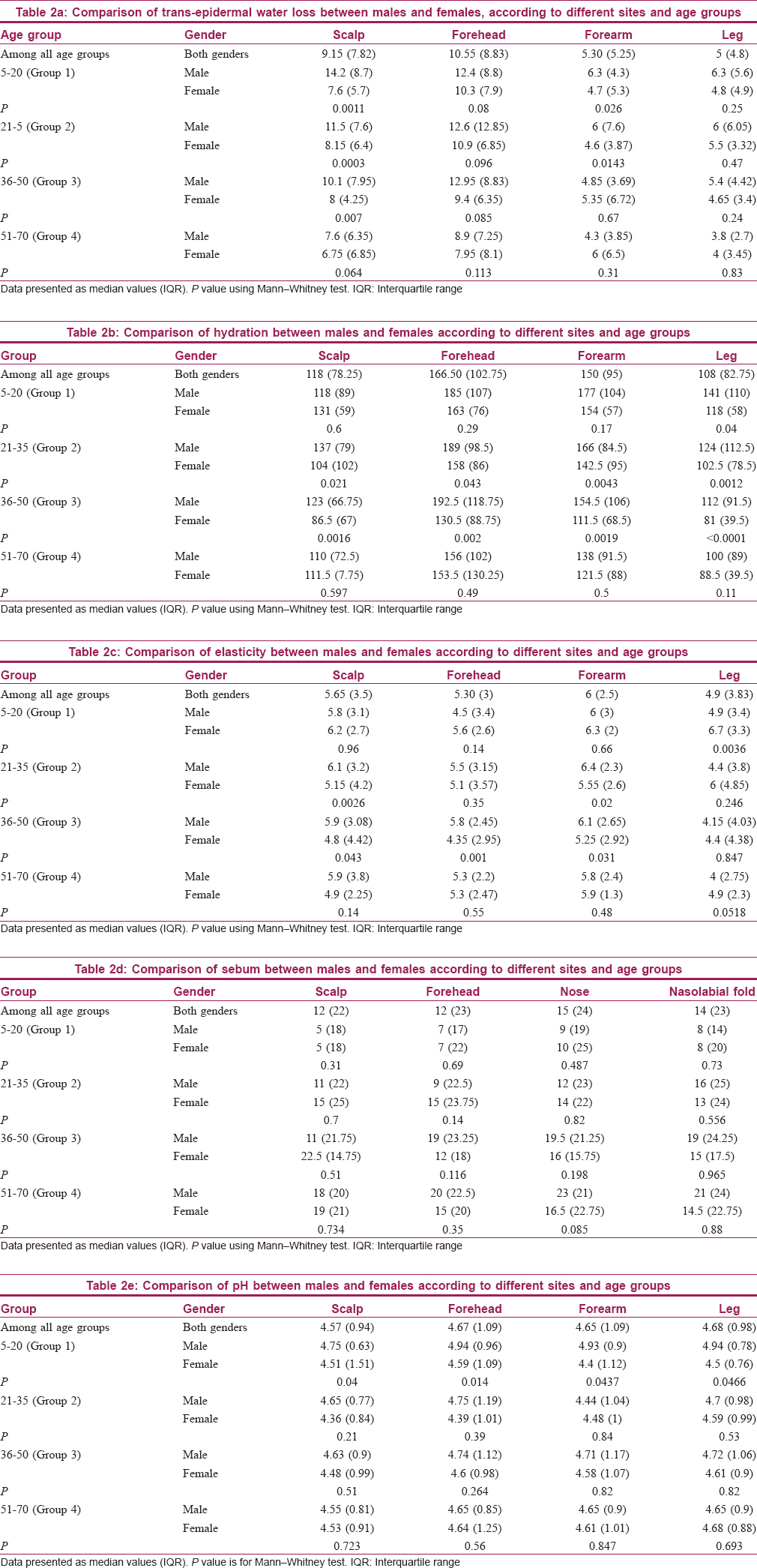
The median values were higher in males at all sites and across all age groups. The difference was significant only in young adult and middle age groups (groups 2 and 3). The median value was found to be highest over the forehead region, and least over the leg area. Among age groups, it was higher in younger age groups (1 and 2) than older age groups (3 and 4), especially over the forearm and leg region. Significant correlation was observed between TEWL and hydration on each site using Spearman correlation coefficient test (scalp P < 0.0001, forehead P = 0.0017, forearm P < 0.0001, leg P = 0.0002) [Table - 2]b and [Table - 3], [Figure - 1]b.
Elasticity
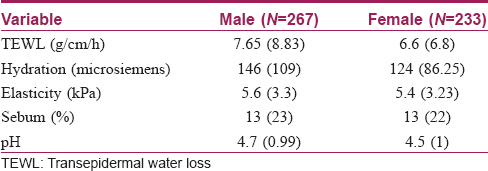
The median values were higher in males compared to females with statistically significant differences in case of the leg region. Females showed a higher median value in group 1. The median value was highest over the forearm and least over the leg. Among the different age groups, the median values were highest in childhood and adolescence (group 1) [Table - 2]c and [Table - 3], [Figure - 1]c.
Sebum
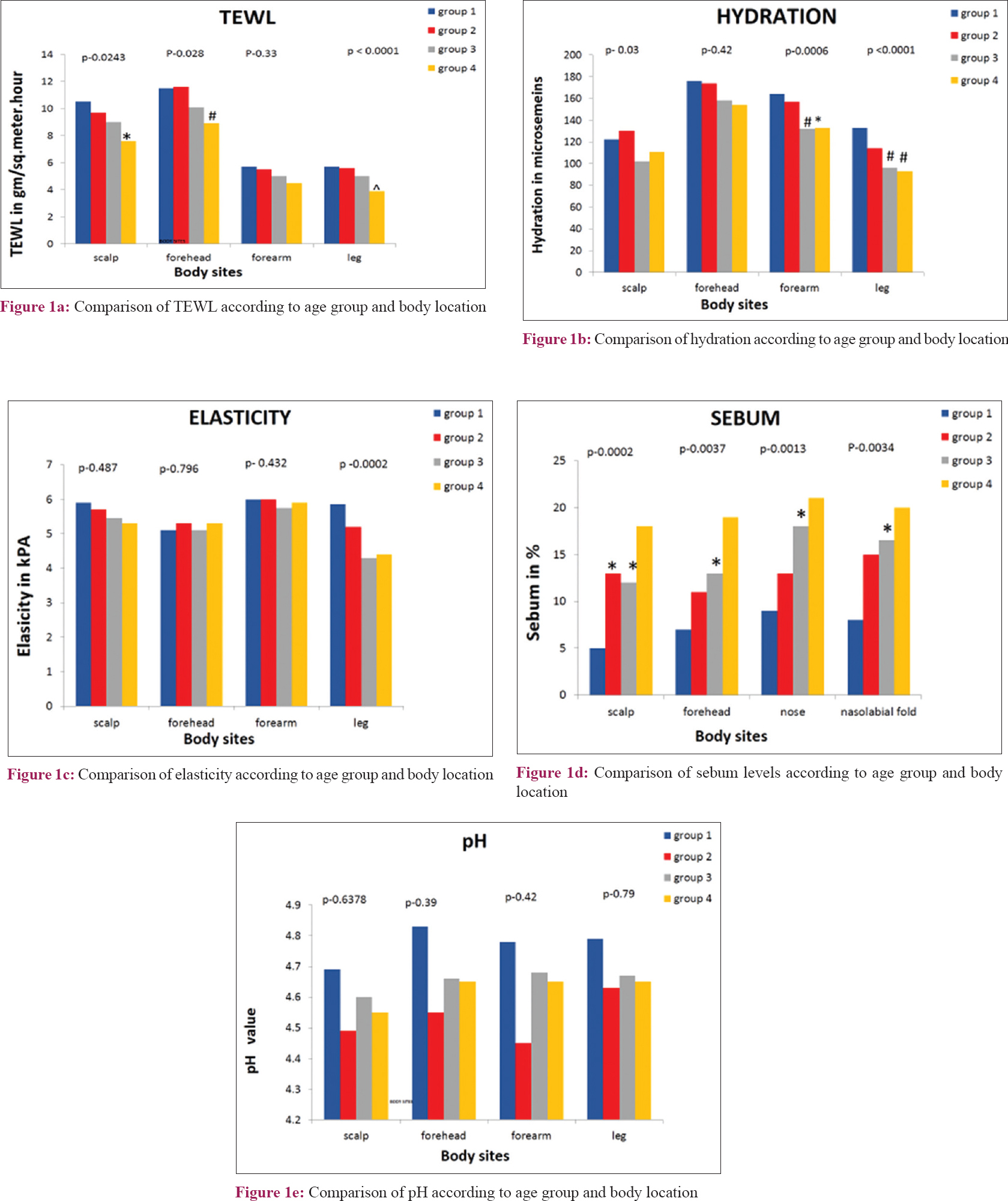
The gender differences in sebum values were not statistically significant. It was found that the median values in groups 1 and 2 were higher in females while they were higher in males of age groups 3 and 4. The nose and nasolabial folds showed higher values of sebum compared to the scalp and forehead. With regards to age group, the median values were higher in age groups 3 and 4, and lower in age groups 1 and 2 [Table - 2]d and [Table - 3], [Figure - 1]d.
pH
Higher median values were recorded in males at all skin sites and found to be statistically significant. Overall median values were higher over the leg and forehead regions but not statistically significant. Among all age groups, adolescent age (group 1) showed the highest values [Table - 2]e and [Table - 3], [Figure - 1]e.
Discussion
In our study, a comprehensive analysis of variations in different biophysical skin parameters was done with respect to site, gender, and age group. Trans-epidermal water loss and skin hydration reflect skin barrier function. These are influenced by variations in the thickness of stratum corneum, sebum secretion, cutaneous perfusion, core body temperature, skin blood flow, environmental conditions and many other factors.[1]
TEWL
Areas like the scalp and forehead are exposed to the environment, and showed higher values for TEWL in all groups and both genders. Compared to the extremities, it was found to be higher over the scalp and forehead and statistically significant (P< 0.05) [Figure - 1]a. The overall TEWL reading was higher in males compared to females [Table - 3]. Higher values in males correlate with their outdoor working habits. Other studies also noticed higher values in males.[2] However, someobserved that TEWL in males is lower than females up to 50 years of age, after which there is no difference.[3] Many other studies did not observe much difference in TEWL between genders.[4] The influence of ageing on skin barrier function is widely accepted but has not yet been conclusively evaluated.[5],[6],[7],[8] Some of the published studies observed decreased TEWL with age. We observed highest median values in the young adult age group and decreased values in the middle and older age group [Table - 2]a and [Figure - 1]a. Studies have found that TEWL was lower in younger and old age subjects but the difference was not significant.[2]
Hydration
Stratum corneum hydration plays a vital role in skin function such as regulation of epidermal proliferation, differentiation and inflammation. Studies haveobserved slightly higher hydration in females.[2],[3] In our study, males were found to have higher hydration among all age groups [Table - 3]. Some studies have observed no gender differences in hydration, while some have reported no correlation of age with hydration.[4],[5] We noticed a decrease in hydration in the middle and old age groups [Table - 2]b and [Figure - 1]b. In a large study of Chinese population, higher hydration was recorded in the people of middle age group.[9] Most of the studies revealed significantly decreased hydration with age, whereas a few have demonstrated no significant differences.[2],[3],[4],[5],[9] We noticed higher hydration over the forehead compared to forearm. This finding was also supported by a few studies.[10],[11] A significant correlation was established between TEWL and skin hydration by using conductance method.[12] In our study, non-parametric correlation coefficients were calculated for hydration and TEWL. We were able to show a statistically significant positive correlation between these two parameters on all four sites.
Elasticity
Skin elasticity represents the functioning of an intact deep dermal and collagen structure. In our study, no statistically significant differences between genders were found. However, males of the outdoor working group in young adult and middle age groups (groups 2 and 3) had relatively more elasticity at most of the skin sites, compared to females [Table - 3] and [Table - 2]c. Some studies reported that females had higher elasticity than the males while othersobserved no such correlation between elasticity and gender.[2],[13] Many studies showed that elasticity decreases with age and our findings were in concordance with earlier studies.[2],[10],[13]
Sebum
Some studies havereported no significant differences in sebum content across age groups.[2],[10] But in our study, we found higher sebum values in age groups 3 and 4 [Table - 2]d and [Figure - 1]d. A large study on Chinese population reported that sebum content peaks at the age of 40 in females and at 50 in males. They also noted higher values of sebum in older age (51–70) groups compared to younger age groups over the forearm in both genders.[9] In other study also older age groups showed higher mean values of sebum than younger age group over the forehead, nose and perioral area.[5] Many authors have noticed that lipid content of skin decreases with age.[3],[14] But a few others have observed no significant difference of sebum values between young and old age groups.[6] This difference between studies may be due to environmental factors which affect sebum production considerably. One study group reported that skin sebum content decreases in menopausal women.[15] Our study found highest sebum values in females of the middle age group especially over the scalp. We found that nose and nasolabial folds had higher sebum content in all age groups, similar to that of a few other studies which found highest sebum values over the nasolabial folds [Figure - 1]d.[2],[5] Our study did not find much variation in sebum content between males and females [Table - 3]. A similar observation has been reported in the Iranian population, while others have reported males to have higher sebum content.[2],[3],[9]
pH value
The pH of skin has a role in antimicrobial defense activity, regulation of epidermal enzyme activity and buffering capacity. In our study, the median pH values were found to be a little higher in males compared to females, in concordance with another study [Table - 3] and [Table - 2]e.[4] A study has reported more acidic pH over the forehead than over the cheeks.[11] This may be due to the fact that sun exposed skin has more melanocytes, pigmentation, and the dendrites of melanocytes tend to be more acidic, as proven by physiological studies.[11],[16] Other studies havereported no significant differences in skin surface pH in either gender, body sites and age groups.[5],[11] But they reported similarity regarding the increase in pH with age. Interestingly, a studyreported an increase in pH with age in males, but decrease in females.[3] In our study, we noted no significant age related changes between subjects for any age group or skin site [Figure - 1]e.
Conclusion
It is difficult to compare our study with other published studies because of ethnic and individual variations, variation in selected sites, age groups and sample size and difference in the types of instrument used. Thus, each study serves as its own control. The present study has been confined to a single geographical area so the effect of environment could not be judged satisfactorily. Multicentric studies may be helpful for better valuations of these parameters in the Indian population. The data in this study will contribute as a baseline to future studies in the Indian population. Their importance can also be studied with respect to diagnosis of various clinical conditions and to monitor therapeutic response. The results of this study provide an important step towards better understanding of gender and age specific skin problems. It also helps to formulate treatment strategies towards specific age groups and gender.
Acknowledgement
We are thankful to Dr. Manish Barvaliya, Assistant Professor, Pharmacology, Government Medical College, Bhavnagar, for his help in the statistical and scientific review of this manuscript.
Financial support and sponsorship
This study is supported by the Indian Association of Dermatology, Venereology and Leprosy through its L'Oreal grant 2013. We are thankful to IADVL for providing us a grant to conduct this study.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kamell JM, Maibach HI. A quantitative approach to anatomy and physiology of aging skin: Barrier, dermal structure and perfusion. In: Baran R, Maibach HI, editors. Text Book of Cosmetology. 4th ed. UK: Informa Health Care; 2010. p. 14-7.
[Google Scholar]
|
| 2. |
Firooz A, Sadr B, Babakoohi S, Sarraf-Yazdy M, Fanian F, Kazerouni-Timsar A, et al. Variation of biophysical parameters of the skin with age, gender, and body region. ScientificWorldJournal 2012;2012:386936.
[Google Scholar]
|
| 3. |
Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women:In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci 2013;35:477-83.
[Google Scholar]
|
| 4. |
Ehlers C, Ivens UI, Møller ML, Senderovitz T, Serup J. Females have lower skin surface pH than men. A study on the surface of gender, forearm site variation, right/left difference and time of the day on the skin surface pH. Skin Res Technol 2001;7:90-4.
[Google Scholar]
|
| 5. |
Marrakchi S, Maibach HI. Biophysical parameters of skin: Map of human face, regional, and age-related differences. Contact Dermatitis 2007;57:28-34.
[Google Scholar]
|
| 6. |
Wilhelm KP, Cua AB, Maibach HI. Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch Dermatol 1991;127:1806-9.
[Google Scholar]
|
| 7. |
Shriner DL, Maibach HI. Regional variation of nonimmunologic contact urticaria. Functional map of the human face. Skin Pharmacol 1996;9:312-21.
[Google Scholar]
|
| 8. |
Tagami H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci 2008;30:413-34.
[Google Scholar]
|
| 9. |
Man MQ, Xin SJ, Song SP, Cho SY, Zhang XJ, Tu CX, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol 2009;22:190-9.
[Google Scholar]
|
| 10. |
Wendling PA, Dell'Acqua G. Skin biophysical properties of a population living in Valais, Switzerland. Skin Res Technol 2003;9:331-8.
[Google Scholar]
|
| 11. |
Lee MR, Nam GW, Jung YC, Park SY, Han JY, Cho JC, et al. Comparison of the skin biophysical parameters of Southeast Asia females: Forehead-cheek and ethnic groups. J Eur Acad Dermatol Venereol 2013;27:1521-6.
[Google Scholar]
|
| 12. |
Mohamad M, Masbbri AR, Matjafri MZ. Non Invasive Measurement of Skin Hydration and Transepidermal Water Loss in Normal Skin. 2012 IEEE Colloquim on Humanities, Science & Engineering (CHUSER); 2012. Available from: http://wwwieeexplore.ieee.org/xpl. [Last accessed on 2013 May 27].
[Google Scholar]
|
| 13. |
Ishikawa T, Ishikawa O, Miyachi Y. Measurement of skin elastic properties with a new suction device (I): Relationship to age, sex and the degree of obesity in normal individuals. J Dermatol 1995;22:713-7.
[Google Scholar]
|
| 14. |
Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: The effect of ageing and the seasons. Arch Dermatol Res 1996;288:765-70.
[Google Scholar]
|
| 15. |
Ohta H, Makita K, Kawashima T, Kinoshita S, Takenouchi M, Nozawa S, et al. Relationship between dermato-physiological changes and hormonal status in pre-, peri-, and postmenopausal women. Maturitas 1998;30:55-62.
[Google Scholar]
|
| 16. |
Gunathilake R1, Schurer NY, Shoo BA, Celli A, Hachem JP, Crumrine D, Sirimanna G, Feingold KR, Mauro TM, Elias PM. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol 2009;129:1719-29.
[Google Scholar]
|
Fulltext Views
5,105
PDF downloads
2,160





