Translate this page into:
Safety of important dermatological drugs (retinoids, immune suppressants, anti androgens and thalidomide) in reproductively active males with respect to pregnancy outcome: A brief review of literature
2 Department of Dermatology, KPC Medical College and Hospital, Kolkata, West Bengal, India
3 Department of Dermatology, ESI-PGIMSR and ESIC Medical College, Kolkata, West Bengal, India
4 Department of Dermatology, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India
5 Department of Dermatology, CGHS, Kolkata, West Bengal, India
Correspondence Address:
Anupam Das
Department of Dermatology, KPC Medical College and Hospital, Kolkata, West Bengal
India
| How to cite this article: Kumar P, Das A, Lal NR, Jain S, Ghosh A. Safety of important dermatological drugs (retinoids, immune suppressants, anti androgens and thalidomide) in reproductively active males with respect to pregnancy outcome: A brief review of literature. Indian J Dermatol Venereol Leprol 2018;84:539-546 |
Abstract
Paternally transmitted damage to offspring is recognized as a complex issue. Each parent contributes 23 chromosomes to a child; hence, it is necessary to know the effects of both maternal and paternal pre-and peri-conceptional exposure to drugs on pregnancy outcome. While there are many studies on the effects of maternal drug exposure on pregnancy outcome, literature on paternal exposure is scarce. Of late however, paternal exposure has been receiving increasing attention. We present a brief review on the safety of commonly used drugs in dermatology, focused on retinoids, immune suppressants, anti androgens and thalidomide.
Introduction
According to the Organization of Teratology Information Specialists, paternal exposure refers to anything the father of the baby is exposed to before or during his partner's pregnancy. This includes alcohol, tobacco and other drugs, chemotherapy or radiation treatments, work place exposures and prescription or over-the-counter medicines.[1] Paternal exposure can be pre-, peri-and post-conceptional and can result in an inability to conceive, spontaneous abortions, stillbirths, congenital malformations at birth and conditions detected only months to years after birth. The major mechanisms of male reproductive toxicity are nongenetic (e.g., due to the presence of a drug in seminal fluid leading to negative effects on sperms or its absorption through the vaginal mucosa), genetic (e.g., gene mutation or chromosomal abnormality of sperm DNA) and epigenetic (e.g., involving changes in gene expression without a change in nucleotide sequence). Therapeutic drugs may need to be continued for a long time in males of reproductive age, and as humans continue to have intercourse during pregnancy, the fetus may be exposed to drugs in semen at various critical stages of its development. Hence, it is imperative to consider the safety of drugs in reproductively active males with respect to pregnancy outcomes.
Retinoids
Isotretinoin, acitretin and bexarotene are commonly used systemic retinoids. Though there is much concern over the teratogenic potential of retinoids, data regarding safety of their usage in reproductively active males is limited.
Acitretin has not been found to have any genotoxic/mutagenic effects in animal or molecular studies.[2] It does not cause any alteration in sperm parameters.[3],[4],[5] Isotretinoinin high doses has been found to adversely affect spermatogenes is in some animal studies.[6],[7] However, human studies have shown beneficial effects on spermatogenesis.[8],[9] Isotretinoin and bexarotene were non mutagenic in various animal tests.[10],[11] It appears that retinoic acid has some role in normal spermatogenesis.[12] A study on 24 men who underwent testicular biopsy found that the intra testicular levels of 13-cis-retinoic acid were significantly low in 7 men with abnormal semen analysis compared to 17 others who had a normal semen analysis.[13]
The maximal non teratogenic dose of acitretin in rabbits (the species most sensitive to its teratogenic effects) is 0.2 mg/kg/day.[14] Determination of the maximal non teratogenic dose of retinoids in humans would be unethical. Humans have been retrospectively found to be most sensitive to the teratogenic potential of isotretinoin (maximal nonteratogenic dose – 0.4-1.0 mg/kg).[15] Various studies which looked at drug levels in the ejaculate of men on a retinoid for ≥1 month have concluded that the risk of teratogenicity is negligible, even presuming 100% vaginal absorption of the drug.[5],[12],[15] Considering 5 ml of ejaculate and 5L blood volume of distribution in a recipient female, the amount of retinoid that reaches the circulation transvaginally in the female is approximately 1/100,000th the oral dose taken by the male. This is considered negligible compared to the physiological levels of endogenous retinoid and much less than the maximal non-teratogenic dose.[4]
Post-marketing surveillance reported 13 pregnancies with known pregnancy outcomes where the father was taking acitretin.[5] Only one fetus had malformations, which were not consistent with retinoid embryopathy. Five mothers delivered healthy neonates. There were six spontaneous abortions, which is not significantly higher than the 30% general incidence of spontaneous abortions.[16] Post-marketing surveillance for isotretinoin included four events with isolated defects compatible with features of a retinoid-exposed fetus; however, two of these reports were incomplete and two cases had other possible explanations.[10] In summary, men on either acitretin or isotretinoin can plan fatherhood. Limited data is available through post-marketing surveillance for bexarotene, hence the manufacturer recommends the use of condoms for men for at least one month after the last dose.[11]
Corticosteroids
Leydig cells in the seminiferous tubules produce testosterone upon stimulation by luteinizing hormone, and are influenced by glucocorticoid levels in body.[17] Treatment with systemic corticosteroids has an inhibitory effect on luteinizing hormone secretion, as documented in animal studies. Martin and Tremblay in their study on mice found that dexamethasone inhibits the cyclic - adenosine monophosphate (cAMP)-mediated stimulation of steroidogenic genes, thus decreasing testosterone production by Leydig cells.[18] Similar findings have been reported by Bambino and Hsueh.[19] Further, it has been noted that the extent of reduction in testosterone levels varied with steroid used. Reduction was the most with triamcinolone, followed by dexamethasone and cortisol; corticosterone had the least effect on testosterone levels.[19] Other than suppressing luteinizing hormone[20],[21],[22] and testosterone production,[17],[23] corticosteroids induce apoptosis of Leydig cells and spermatogonia.[24],[25]
Contradictory results were documented by Mogilner et al. who found that dexamethasone inhibits germ cell apoptosis, thereby improving oligospermia.[26] Low-dose corticosteroids are found to be useful in the treatment of infertility due to antisperm antibodies.[27]
Systemic steroids are known to be non teratogenic with maternal exposure, but whether paternal exposure around the time of conception is teratogenic to the fetus is not known.[28] Conclusive evidence of its effects on male fertility is also lacking. As of now, prospective fathers can only be counseled that there is not enough data to make a recommendation regarding planning pregnancy, but termination of therapy is not recommended.
Methotrexate
Methotrexate inhibits the multiplication of rapidly dividing cells including spermatozoa, and its use causes concern among patients and treating physicians regarding its effects on male fertility and teratogenicity.
Where male fertility while on methotrexate is concerned, current data are conflicting [Table - 1]. Methotrexate may lead to oligospermia and even azoospermia. However, some of these patients were simultaneously receiving alkylating agents, which could be responsible for the detrimental effects on the quality and quantity of spermatozoa.[40] The mechanism of methotrexate leading to deleterious effects on spermatozoa is debated, but the most widely accepted hypothesis is that methotrexate inhibits spermiogenesis (transformation of spermatids into mature sperm).[40] Thus, azoospermia is possible during methotrexate therapy and males may be unable to conceive with their partner.
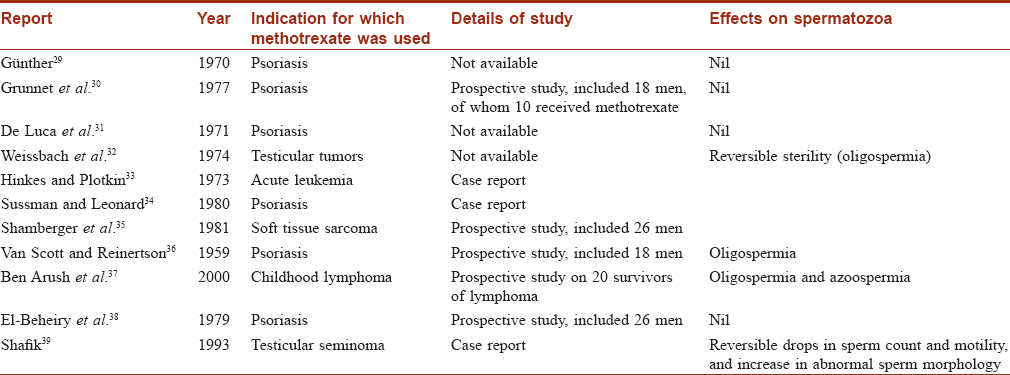
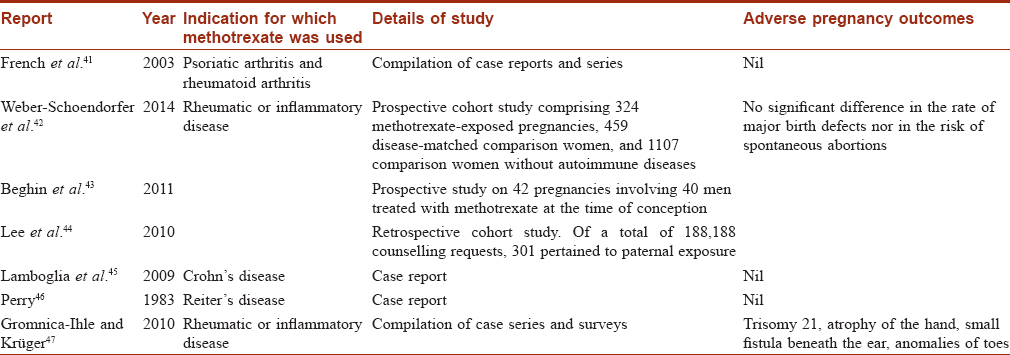
The next major concern is pregnancy outcomes in couples where the male partner was exposed to methotrexate during conception. Again, the outcome reported in different studies are conflicting as summarized in [Table - 2].[41],[42],[43],[44],[45],[46],[47] Earlier, at the Psoriasis Foundation Consensus Conference (2009), it was concluded that conception be avoided during methotrexate therapy and after completion of therapy for at least 3 months in males.[48] However, recent studies by Beghin et al. and Weber-Schoendorfer et al. found no evidence of increased risk of adverse pregnancy outcome after paternal low-dose methotrexate therapy (≤15 mg per week).[43] Based on current evidence, the authors concluded that paternal methotrexate exposure during conception does not pose any serious hazards to the offspring.
Cyclosporine
Cyclosporine, a calcineurin inhibitor, has immunomodulatory effects on T cells. Dermatologic indications includes psoriasis, atopic dermatitis, chronic idiopathic urticaria, pyoderma gangrenosum and Behçet'sdisease.[49]
A meta-analysis performed to determine whether cyclosporine exposure during pregnancy is associated with an increased risk of congenital malformations, preterm delivery or low birth weight, found that it does not appear to be a major human teratogen.[50] However, it may be associated with increased rates of prematurity, and more research is needed to evaluate whether cyclosporine increases teratogenic risk.
In men, doses greater than 2 mg/kg/day have been implicated in asthenoteratospermia.[51] No significant difference was seen in men receiving lower doses compared to the control group. Therefore, authors did not recommend discontinuation of the drug (especially at lower doses) if pregnancy is desired. Haberman et al. evaluated fertility among 9 young men on cyclosporine A following renal transplantation. Most parameters of semen analysis and testicular hormones were normal in 8 patients. Attempts at conception were successful in three out of four cases.
Though cyclosporine does not seem to adversely affect fertility in men,[52] animal studies have documented contradictory results; hence, some caution is warranted. In animal studies, 1–2mg/kg/day of cyclosporine has been associated with reduced weight of accessory sex organs, decreased testicular and epididymal sperm counts and decreased sperm motility (by 50%) and hence, decreased fertility (by 60%).[53] Another study has reported that the toxic effect of cyclosporine on spermiogenesis in rats is by it directly impairing spermiogenic cell development and by impeding Sertoli cell function.[54]
Very little amount (5–20%) of cyclosporine cross placenta and the current evidence does not support its teratogenic potential in humans.[52],[53] However, reports on the effects on fetus where the father was on long-term cyclosporine are not available.
Azathioprine
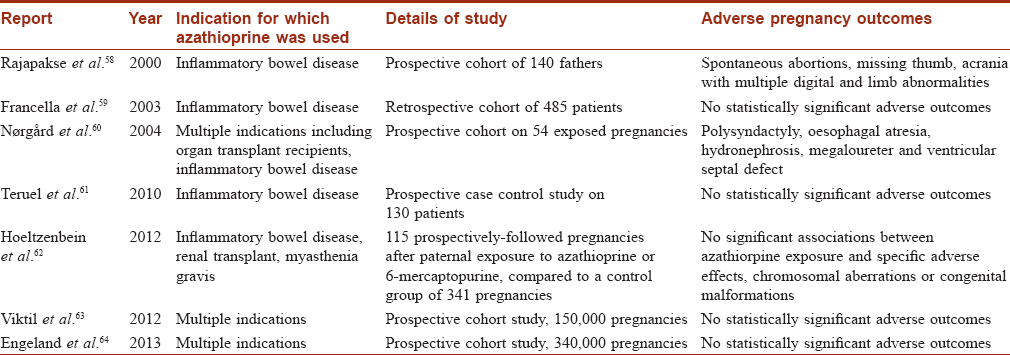
Azathioprine, a purine analogue, reduces T and B cell response and thereby inhibits the synthesis of antibodies. Studies of the effects of azathioprine on male fertility are summarised in [Table - 3].

Ligumsky et al. conducted a study in male mice and found that azathioprine led to an increased risk of resorption of embryos. This was attributed to occult sperm damage.[55]
There is a rising concern that exposure of future fathers to azathioprine might adversely affect pregnancy outcome. There are reports of deleterious outcomes of pregnancy,[58],[60] but recent reports suggest that no specific adverse effects are observed after paternal treatment with azathioprine.[59],[61],[62],[63],[64] [Table - 4].
Instances of elective termination of pregnancy after paternal exposure to azathioprine during conception are known.[62] This may be attributed to the apprehension of having a baby with congenital malformations. However, current evidence suggests that conception planning need not be delayed when the future father is on unavoidable azathioprine therapy.[59],[61],[62],[63],[64] In addition, there is no need to abort the fetus if a chance pregnancy occurs, though in such circumstances, high-frequency serial ultrasound must be carried out to look for any malformations or defects.[62]
Cyclophosphamide
Cyclophosphamide is the most researched chemotherapeutic agent because of its severe effect on human fertility (pregnancy category D).[65] Cyclophosphamide is a pro drug, and its teratogenic effects are mediated through its breakdown products phosphoramide mustard and acrolein.[66] Kirshon et al. have reported multiple anomalies, including absent thumbs, cleft palate, low-set ears and multiple eye abnormalities in a neonate born to a mother treated with cyclophosphamide for lupus.[67] In men, cyclophosphamide has been known to induce infertility at cumulative doses above 7500 mg/m2. It causes depletion and aplasia of germinal epithelial cells in the testes, resulting in severe oligozoospermia or azoospermia within 90–120 days of treatment, with poor long-term recovery. Arnon et al. reported that men who regained spermatogenesis did so after an average interval of 31 months.[65] Gaffan et al., in patients of germ cell tumor, reported an insignificant difference in the doses of cyclophosphamide received in the fertile and infertile group (1500 mg m−2 in the fertile and 1750 mg m−2 in the infertile).[68]
Animal studies have reported that pre conceptional paternal exposure to cyclophosphamide leads to increases in embryo loss, malformations and behavioral deficits in offspring which are transmissible to subsequent generations.[69] In a study by Hales et al., the progeny of male rats treated with 5.1 mg/kg/day of cyclophosphamide resulted in an F2 generation (second filial generation- the offspring of the interbreeding F1 generations) with significantly decreased mean fetal weight, omphalocele, generalized edema, syndactyly, gigantism and dwarfism.[70]
Because of these known adverse effects of cyclophosphamide on male fertility and pregnancy outcome, it is advisable for male patients to avoid fathering a child during treatment and for 3 months after treatment with this drug.[71]
Mycophenolate
Exposure to mycophenolic acid products in early pregnancy in female transplant recipients is associated with an increased risk of miscarriage (32–45%) and multiple craniofacial congenital malformations (mycophenolate mofetil-associated embryopathy EMFO tetrad: Ear, Mouth, Fingers, Ocular/Organ malformation, according to The European Network of Teratology Information Services).[72] Hence in 2006, the U.S Food and Drug Administration changed the pregnancy category of mycophenolic acid from category C to D.[73]
Despite a large number of patients being treated with mycophenolic acid products, little clinical data are available concerning its effects on male fertility.[72] Kim et al. have reported that there is no evidence of mycophenolic acid impacting male patients' fertility or contributing to birth defects in their offspring. In an animal study, mycophenolate mofetil had no effect on fertility in male rats at a dose equivalent to 200 mg/day in humans, while fetal malformations were noted in female rats at less than a quarter of this dose.[74] Of note, no ill effects on the subsequent generations were observed.[74]
Two registry studies of pregnancies with paternal exposure to mycophenolate derivatives have not identified an increased incidence of fetal malformations compared to the general population (3.1% vs. 3%, 2.1% vs 1.9%).[75],[76]
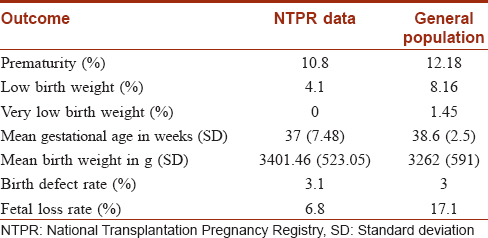
National Transplantation Pregnancy Registry (NTPR) data demonstrate that pregnancies fathered by transplant recipients with exposure to mycophenolic acid have outcomes, complication rates and malformations similar to those in the general population. [Table - 5] compares NTPR outcome data with data for the general population.[76]
In May 2016, the Medicines and Healthcare products Regulatory agency, United Kingdom, gave recommendations for men taking mycophenolate derivatives, that sexually active men exposed to these agents should use condoms during treatment and for 90 days after discontinuation. It also stated that, “female partners of male patients treated with mycophenolate mofetil or mycophenolic acid should use highly effective contraception during treatment and for 90 days after the last dose. However, recommendations do not appear to have high-level scientific evidence, so we may keep our minds open.[75]
Finasteride
Finasteride is a potent type II 5α-reductase inhibitor used in the treatment of androgenetic alopecia and benign prostatic hyperplasia. Its frequent use in reproductively active males raises concerns about potential teratogenicity. Finasteride has been detected in semen. In a study on 35 males taking 1 mg oral finasteride daily for 6 weeks, mean semen finasteride level was 0.26 ng/ml while the highest level achieved was 1.52 ng/ml. Assuming a 5 ml ejaculate volume, the female partner would be exposed to a maximum of 7.6 ng finasteride/day, which is considered insignificant for causing developmental anomalies.[77] In experimental studies, exposures in male monkeys up to 800 ng/day did not result in any developmental anomalies in their off spring. However, administering higher doses (2mg/kg/day, equivalent to 100 times the human dose of 1 mg/day) to pregnant monkeys resulted in anomalies of external genitalia in male fetus. No other anomalies and no effects on female fetuses were observed.[77]
The effect of finasteride on male fertility is controversial and studies have shown conflicting results. In a multicentric study, 1 mg/day of finasteride for 48 weeks did not have any significant effect on sperm concentration, total sperm per ejaculate, sperm motility or sperm morphology.[78] However, a study by Amory et al. documented compromise in all semen parameters (except sperm morphology) in patients taking finasteride (5 mg/day) and dutasteride (0.5 mg/day) for 1 year.[79] Inability to conceive while the male partner is on finasteride and conception following discontinuation of finasteride have been documented.[80] Thus, finasteride at 1 mg/day may not impair male fertility, especially if it is used for short periods. However, female partners of males with pre-existing subnormal fertility may not be able to conceive while their partners are on finasteride.[80] Hence, it appears prudent to get a baseline semen analysis before starting finasteride and to discontinue finasteride in males with oligospermia, as well as in instances of failed attempts at conception.
Some recent reports have noted an increased sperm DNA fragmentation index with chronic use of finastride (1 mg/day).[80],[81] This impairment in sperm integrity might result in an inability to conceive and in spontaneous abortions. Improvement in the sperm DNA fragmentation index and successful conception have been observed months after discontinuation of finasteride.
Spironolactone
Spironolactone is an aldosterone receptor antagonist with anti-androgenic actions. Common dermatological indications include female pattern hair loss, hirsutism and acne. Gynecomastia, abnormal menstrual cycles and impotence are frequently noted with prolonged use, which limit its long-term use. In males, it is primarily used for non-dermatological indications though it had been used to treat rosacea in males.[82] Spironolactone at a dose of 400 mg/day impairs spermatogenesis by decreasing testosterone levels[83] However, a study in male rats showed that there were no changes in sperm motility or fertility despite decreases in sperm concentration.[84] More recently, mineralocorticoid receptors have been identified in human sperms.[85] However, their functional importance and the effects of blockade of these receptors by spironolactone are not known.
Mineralocorticoid blockade in pregnant rats has been shown to inhibit fetal organogenesis in (descending order) hind limbs > forelimbs >optic stalk > brain > olfactory pits >otic vesicles.[86] Though human data are not available, this raises concerns about the use of spironolactone in males who are planning to have a child.
Thalidomide
Thalidomide consists of a single central asymmetric carbon atom with a right glutarimide ring and a left pthalidimide ring.[87],[88],[89] Sedative effects are mediated by the glutarimide ring whereas the pthalidimide ring leads to teratogenic effects.[90] It was introduced in Europe and Canada in the late 1950s as a non barbiturate sedative hypnotic and antiemetic for treating morning sickness during pregnancy, but by 1962, it was withdrawn from the market due to its teratogenic effects.[91] However, there is a dearth of literature on the effects of thalidomide on paternity and male fertility.
Cecilia Lutwak-Mannin (1964) found some deleterious effects on the progeny of male rabbits fed thalidomide.[92] Teo et al. in an animal study found that sperm count, motility and density were not influenced by thalidomide (up to 500 mg/kg) after 8 weeks of dosing.[93] A double-blind, placebo-controlled study performed by Teo et al. on HIV-seropositive patients found a significant correlation between plasma and semen thalidomide levels and significantly greater semen levels at higher doses (>100 mg/day). Because the threshold dose for birth defects due to thalidomide exposure is not known, they advised barrier contraception for male patients.[94] Another study using liquid chromatography-tandem mass spectrometric assays of human semen and plasma, confirmed the presence of the drug in human semen; the concentration achieved in semen is similar to that in plasma.[95]
Because evidence regarding the effects of thalidomide on male fertility and its transmission to future generations are lacking or contradictory, to avoid a repetition of the 1960s tragedy, its marketing and use has been restricted through the mandatory System for Thalidomide Education and Prescribing Safety program that regulates prescribing, dispensing and dosing of thalidomide in United States of America.[87] It is recommended to use barrier contraceptives during thalidomide treatment and for 1 week after stopping treatment.[96]
To summarize, currently available data suggests that the use of retinoids (except bexarotene), methotrexate or azathioprine by prospective fathers is safe. On the other hand, literature does not support the use of cyclophosphamide, mycophenolate mofetil or spironolactone by prospective fathers. However, data for avoidance of mycophenolate mofetil is not sufficiently robust. Among the drugs reviewed above, data for cyclosporine A, corticosteroids, finasteride and thalidomide is not sufficient enough to draw a conclusive opinion regarding its use in prospective fathers.
The role of male partners in mediating drug-induced adverse outcomes of pregnancy is being increasingly recognized. Because the father contributes half the genome in the progeny, it appears prudent that we analyze the effects of drug use by prospective fathers critically. At present, only drug use by females is being tested for teratogenic effects. There is therefore a need to design robust studies evaluating all drugs for potential male-mediated teratogenic effects. Treating dermatologists need to exercise caution and limit the use of drugs by prospective fathers and/or advise contraception till conclusive data regarding the safety of drugs with respect to progeny become available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Organization of Teratology Information Specialists. Paternal Exposures and Pregnancy; 2002. Available from: http://www.otispregnancy.org/. [Last accessed on 2016 Oct 14].
[Google Scholar]
|
| 2. |
Hummler H, Bürgin H, Gocke E. Can retinoid therapy of the father cause malformation in his progeny? Retinoids Today Tomorrow 1989;16:6-9.
[Google Scholar]
|
| 3. |
Sengör B, Bayramgürler D, Müezzinoglu B, Altintas L, Bilen N, Apaydin R, et al. Effects of acitretin on spermatogenesis of rats. J Eur Acad Dermatol Venereol 2006;20:689-92.
[Google Scholar]
|
| 4. |
Geiger JM, Walker M. Is there a reproductive safety risk in male patients treated with acitretin (neotigason/soriatane)? Dermatology 2002;205:105-7.
[Google Scholar]
|
| 5. |
Parsch EM, Ruzicka T, Przybilla B, Schill WB. Andrological investigations in men treated with acitretin (Ro 10-1670). Andrologia 1990;22:479-82.
[Google Scholar]
|
| 6. |
Kamm JJ. Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids. J Am Acad Dermatol 1982;6:652-9.
[Google Scholar]
|
| 7. |
Stinson SF, Reznik-Schüller H, Reznik G, Donahoe R. Atrophy induced in the tubules of the testes of Syrian hamsters by two retinoids. Toxicology 1980;17:343-53.
[Google Scholar]
|
| 8. |
Torok L, Kasa M. Spermatological and endocrinological examinations connected with isotretinoin treatment. In: Saurat JH, editor. Retinoids: new Trends in Research and Therapy. Basel: Karger; 1985. p. 407-10.
[Google Scholar]
|
| 9. |
Török L, Kádár L, Kása M. Spermatological investigations in patients treated with etretinate and isotretinoin. Andrologia 1987;19:629-33.
[Google Scholar]
|
| 10. |
Isotretinoin (Accutane) productmonograph. Hoffmann-La Roche Limited. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018662s060lbl.pdf. [Last accessed on 2018 Jan 23].
[Google Scholar]
|
| 11. |
Bexarotene. TARGRETIN® Capsules Package Insert. Woodcliff Lake, New Jersey: Eisai Inc.; 2007. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018662s060lbl.pdf. [Last accessed on 2018 Jan 23].
[Google Scholar]
|
| 12. |
Chung SS, Wang X, Wolgemuth DJ. Expression of retinoic acid receptor alpha in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development 2009;136:2091-100.
[Google Scholar]
|
| 13. |
Nya-Ngatchou JJ, Arnold SL, Walsh TJ, Muller CH, Page ST, Isoherranen N, et al. Intratesticular 13-cis retinoic acid is lower in men with abnormal semen analyses: A pilot study. Andrology 2013;1:325-31.
[Google Scholar]
|
| 14. |
Kistler A, Hummler H. Teratogenesis and reproductive safety evaluation of the retinoid etretin (Ro 10-1670). Arch Toxicol 1985;58:50-6.
[Google Scholar]
|
| 15. |
Schmitt-Hoffmann AH, Roos B, Sauer J, Brown T, Weidekamm E, Meyer I, et al. Low levels of alitretinoin in seminal fluids after repeated oral doses in healthy men. Clin Exp Dermatol 2011;36 Suppl 2:12-7.
[Google Scholar]
|
| 16. |
Modvig J, Schmidt L, Damsgaard MT. Measurement of total risk of spontaneous abortion: The virtue of conditional risk estimation. Am J Epidemiol 1990;132:1021-38.
[Google Scholar]
|
| 17. |
Schultz R, Isola J, Parvinen M, Honkaniemi J, Wikström AC, Gustafsson JA, et al. Localization of the glucocorticoid receptor in testis and accessory sexual organs of male rat. Mol Cell Endocrinol 1993;95:115-20.
[Google Scholar]
|
| 18. |
Martin LJ, Tremblay JJ. Glucocorticoids antagonize cAMP-induced star transcription in leydig cells through the orphan nuclear receptor NR4A1. J Mol Endocrinol 2008;41:165-75.
[Google Scholar]
|
| 19. |
Bambino TH, Hsueh AJ. Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesisin vivo and in vitro. Endocrinology 1981;108:2142-8.
[Google Scholar]
|
| 20. |
Kamel F, Kubajak CL. Modulation of gonadotropin secretion by corticosterone: Interaction with gonadal steroids and mechanism of action. Endocrinology 1987;121:561-8.
[Google Scholar]
|
| 21. |
Briski KP, Sylvester PW. Acute inhibition of pituitary LH release in the male rat by the glucocorticoid agonist decadron phosphate. Neuroendocrinology 1991;54:313-20.
[Google Scholar]
|
| 22. |
Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macacamulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod 1985;33:423-31.
[Google Scholar]
|
| 23. |
Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol 2010;35:109-25.
[Google Scholar]
|
| 24. |
Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP, et al. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology 2002;143:130-8.
[Google Scholar]
|
| 25. |
Yazawa H, Sasagawa I, Nakada T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum Reprod 2000;15:1917-20.
[Google Scholar]
|
| 26. |
Mogilner JG, Elenberg Y, Lurie M, Shiloni E, Coran AG, Sukhotnik I, et al. Effect of dexamethasone on germ cell apoptosis in the contralateral testis after testicular ischemia-reperfusion injury in the rat. FertilSteril 2006;85 Suppl 1:1111-7.
[Google Scholar]
|
| 27. |
Omu AE, al-Qattan F, Abdul Hamada B. Effect of low dose continuous corticosteroid therapy in men with anti sperm antibodies on spermatozoal quality and conception rate. Eur J Obstet Gynecol Reprod Biol 1996;69:129-34.
[Google Scholar]
|
| 28. |
Lockshin MD, Sammaritano LR. Corticosteroids during pregnancy. Scand J Rheumatol Suppl 1998;107:136-8.
[Google Scholar]
|
| 29. |
Günther E. Andrologic examinations in the antimetabolite therapy of psoriasis. DermatolMonatsschr 1970;156:498-502.
[Google Scholar]
|
| 30. |
Grunnet E, Nyfors A, Hansen KB. Studies of human semen in topical corticosteroid-treated and in methotrexate-treated psoriatics. Dermatologica 1977;154:78-84.
[Google Scholar]
|
| 31. |
De Luca M, Ciampo E, Rossi A. Study of the seminal fluid in subjects treated with methotrexate. G Ital Dermatol Minerva Dermatol 1971;46:247-9.
[Google Scholar]
|
| 32. |
Weissbach L, Lange CE, Rodermund OE, Zwicker H, Gropp A, Pothmann W, et al. Fertility disturbances following treatment of patients with testicular tumors (author's transl). Urologe A 1974;13:80-5.
[Google Scholar]
|
| 33. |
Hinkes E, Plotkin D. Reversible drug-induced sterility in a patient with acute leukemia. JAMA 1973;223:1490-1.
[Google Scholar]
|
| 34. |
Sussman A, Leonard JM. Psoriasis, methotrexate, and oligospermia. Arch Dermatol 1980;116:215-7.
[Google Scholar]
|
| 35. |
Shamberger RC, Sherins RJ, Rosenberg SA. The effects of postoperative adjuvant chemotherapy and radiotherapy on testicular function in men undergoing treatment for soft tissue sarcoma. Cancer 1981;47:2368-74.
[Google Scholar]
|
| 36. |
Van Scott EJ, Reinertson RP. Morphologic and physiologic effects of chemotherapeutic agents in psoriasis. J Invest Dermatol 1959;33:357-69.
[Google Scholar]
|
| 37. |
Ben Arush MW, Solt I, Lightman A, Linn S, Kuten A. Male gonadal function in survivors of childhood Hodgkin and non-Hodgkin lymphoma. Pediatr Hematol Oncol 2000;17:239-45.
[Google Scholar]
|
| 38. |
El-Beheiry A, El-Mansy E, Kamel N, Salama N. Methotrexate and fertility in men. Arch Androl 1979;3:177-9.
[Google Scholar]
|
| 39. |
Shafik A. Intratunical injection of methotrexate for the treatment of seminoma of the testicle. Anticancer Drugs 1993;4:193-5.
[Google Scholar]
|
| 40. |
Morris LF, Harrod MJ, Menter MA, Silverman AK. Methotrexate and reproduction in men: Case report and recommendations. J Am Acad Dermatol 1993;29:913-6.
[Google Scholar]
|
| 41. |
French AE, Koren G; Motherisk Team. Effect of methotrexate on male fertility. Can Fam Physician 2003;49:577-8.
[Google Scholar]
|
| 42. |
Weber-Schoendorfer C, Chambers C, Wacker E, Beghin D, Bernard N; Network of French Pharmacovigilance Centers. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: A prospective multicenter cohort study. Arthritis Rheumatol 2014;66:1101-10.
[Google Scholar]
|
| 43. |
Beghin D, Cournot MP, Vauzelle C, Elefant E. Paternal exposure to methotrexate and pregnancy outcomes. J Rheumatol 2011;38:628-32.
[Google Scholar]
|
| 44. |
Lee CY, Jin C, Mata AM, Tanaka T, Einarson A, Koren G, et al. Apilot study of paternal drug exposure: The Motherisk experience. Reprod Toxicol 2010;29:353-60.
[Google Scholar]
|
| 45. |
Lamboglia F, D'Incã R, Oliva L, Bertomoro P, Sturniolo GC. Patient with severe Crohn's disease became a father while on methotrexate and infliximab therapy. Inflamm Bowel Dis 2009;15:648-9.
[Google Scholar]
|
| 46. |
Perry WH. Methotrexate and teratogenesis. Arch Dermatol 1983;119:874-5.
[Google Scholar]
|
| 47. |
Gromnica-Ihle E, Krüger K. Use of methotrexate in young patients with respect to the reproductive system. Clin Exp Rheumatol 2010;28:S80-4.
[Google Scholar]
|
| 48. |
Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am AcadDermatol 2009;60:824-37.
[Google Scholar]
|
| 49. |
Kutlubay Z, Erdogan BÇ, Engin B, Serdaroglu S. Cyclosporine in dermatology. Skinmed 2016;14:105-9.
[Google Scholar]
|
| 50. |
Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: A meta-analysis. Transplantation 2001;71:1051-5.
[Google Scholar]
|
| 51. |
Available from: https://www.ojrd.biomedcentral.com/articles/10.1186/s13023-015-0332-8. [Last accessed on 2016 Oct 25].
[Google Scholar]
|
| 52. |
Haberman J, Karwa G, Greenstein SM, Soberman R, Glicklich D, Tellis V, et al. Male fertility in cyclosporine-treated renal transplant patients. J Urol 1991;145:294-6.
[Google Scholar]
|
| 53. |
Seethalakshmi L, Flores C, Carboni AA, Bala R, Diamond DA, Menon M, et al. Cyclosporine: Its effects on testicular function and fertility in the prepubertal rat. J Androl 1990;11:17-24.
[Google Scholar]
|
| 54. |
Masuda H, Fujihira S, Ueno H, Kagawa M, Katsuoka Y, Mori H, et al. Ultra structural study on cytotoxic effects of cyclosporine A in spermiogenesis in rats. Med Electron Microsc 2003;36:183-91.
[Google Scholar]
|
| 55. |
Ligumsky M, Badaan S, Lewis H, Meirow D. Effects of 6-mercaptopurine treatment on sperm production and reproductive performance: A study in male mice. Scand J Gastroenterol 2005;40:444-9.
[Google Scholar]
|
| 56. |
Dejaco C, Mittermaier C, Reinisch W, Gasche C, Waldhoer T, Strohmer H, et al. Azathioprine treatment and male fertility in inflammatory bowel disease. Gastroenterology 2001;121:1048-53.
[Google Scholar]
|
| 57. |
Xu LG, Wang HW, Peng WL, Jin LM, Zhu XF, Xu HM, et al. Marital status and fertility of 185 male renal transplant recipients in China. J Androl 2008;29:618-21.
[Google Scholar]
|
| 58. |
Rajapakse RO, Korelitz BI, Zlatanic J, Baiocco PJ, Gleim GW. Outcome of pregnancies when fathers are treated with 6-mercaptopurine for inflammatory bowel disease. Am J Gastroenterol 2000;95:684-8.
[Google Scholar]
|
| 59. |
Francella A, Dyan A, Bodian C, Rubin P, Chapman M, Present DH, et al. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: A retrospective cohort study. Gastroenterology 2003;124:9-17.
[Google Scholar]
|
| 60. |
Nørgård B, Pedersen L, Jacobsen J, Rasmussen SN, Sørensen HT. The risk of congenital abnormalities in children fathered by men treated with azathioprine or mercaptopurine before conception. Aliment Pharmacol Ther 2004;19:679-85.
[Google Scholar]
|
| 61. |
Teruel C, López-San Román A, Bermejo F, Taxonera C, Pérez-Calle JL, Gisbert JP, et al. Outcomes of pregnancies fathered by inflammatory bowel disease patients exposed to thiopurines. Am J Gastroenterol 2010;105:2003-8.
[Google Scholar]
|
| 62. |
Hoeltzenbein M, Weber-Schoendorfer C, Borisch C, Allignol A, Meister R, Schaefer C, et al. Pregnancy outcome after paternal exposure to azathioprine/6-mercaptopurine. Reprod Toxicol 2012;34:364-9.
[Google Scholar]
|
| 63. |
Viktil KK, Engeland A, Furu K. Outcomes after anti-rheumatic drug use before and during pregnancy: A cohort study among 150,000 pregnant women and expectant fathers. Scand J Rheumatol 2012;41:196-201.
[Google Scholar]
|
| 64. |
Engeland A, Bjørge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, et al. Effects of pre conceptional paternal drug exposure on birth outcomes: Cohort study of 340 000 pregnancies using Norwegian population-based databases. Br J Clin Pharmacol 2013;75:1134-41.
[Google Scholar]
|
| 65. |
Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update 2001;7:394-403.
[Google Scholar]
|
| 66. |
Mirkes PE. Cyclophosphamide teratogenesis: A review. Teratog Carcinog Mutagen 1985;5:75-88.
[Google Scholar]
|
| 67. |
Kirshon B, Wasserstrum N, Willis R, Herman GE, McCabe ER. Teratogenic effects of first-trimester cyclophosphamide therapy. Obstet Gynecol 1988;72:462-4.
[Google Scholar]
|
| 68. |
Gaffan J, Holden L, Newlands ES, Short D, Fuller S, Begent RH, et al. Infertility rates following POMB/ACE chemotherapy for male and female germ cell tumours – A retrospective long-term follow-up study. Br J Cancer 2003;89:1849-54.
[Google Scholar]
|
| 69. |
Barton TS, Robaire B, Hales BF. Epigenetic programming in the pre implantation rat embryo is disrupted by chronic paternal cyclophosphamide exposure. Proc Natl Acad Sci U S A 2005;102:7865-70.
[Google Scholar]
|
| 70. |
Hales BF, Crosman K, Robaire B. Increased post implantation loss and malformations among the F2 progeny of male rats chronically treated with cyclophosphamide. Teratology 1992;45:671-8.
[Google Scholar]
|
| 71. |
Available from: http://www.patient.info/in/medicine/cyclophosphamide. [Last accessed on 2016 Oct 22].
[Google Scholar]
|
| 72. |
Leroy C, Rigot JM, Leroy M, Decanter C, Le Mapihan K, Parent AS, et al. Immunosuppressive drugs and fertility. Orphanet J Rare Dis 2015;10:136.
[Google Scholar]
|
| 73. |
Coscia LA, Armenti DP, King RW, Sifontis NM, Constantinescu S, Moritz MJ, et al. Update on the teratogenicity of maternal mycophenolate mofetil. J Pediatr Genet 2015;4:42-55.
[Google Scholar]
|
| 74. |
Kim M, Rostas S, Gabardi S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am J Transplant 2013;13:1383-9.
[Google Scholar]
|
| 75. |
Morken N-H, Diaz-Garcia C, Raisaeter AV et al. Obstetric and Neonatal Outcome of Pregnancies Fathered by Males on Immunosuppression After Solid Organ Transplantation. Am J Transplantation 2015; 15:1666-1673
[Google Scholar]
|
| 76. |
Jones A, Clary MJ, McDermott E, Coscia LA, Constantinescu S, Moritz MJ, et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant 2013;23:153-7.
[Google Scholar]
|
| 77. |
Pole M, Koren G. Finasteride. Does it affect spermatogenesis and pregnancy? Can Fam Physician 2001;47:2469-70.
[Google Scholar]
|
| 78. |
Overstreet JW, Fuh VL, Gould J, Howards SS, Lieber MM, Hellstrom W, et al. Chronic treatment with finasteride daily does not affect spermatogenesis or semen production in young men. J Urol 1999;162:1295-300.
[Google Scholar]
|
| 79. |
Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab 2007;92:1659-65.
[Google Scholar]
|
| 80. |
Ricci G, Martinelli M, Luppi S, Lo Bello L, De Santis M, Skerk K, et al. Finasteride and fertility: Case report and review of the literature. J Drugs Dermatol 2012;11:1511-3.
[Google Scholar]
|
| 81. |
Tu HY, Zini A. Finasteride-induced secondary infertility associated with sperm DNA damage. Fertil Steril. 2011;95(6):2125.e13-4. Ref 81
[Google Scholar]
|
| 82. |
Aizawa H, Niimura M. Oral spironolactone therapy in male patients with rosacea. J Dermatol. 1992 May; 19(5):293-7.
[Google Scholar]
|
| 83. |
Millsop JW, Heller MM, Eliason MJ, Murase JE. Dermatological medication effects on male fertility. Dermatol Ther 2013;26:337-46.
[Google Scholar]
|
| 84. |
Wong PY, Lee WM. Effects of spironolactone (aldosterone antagonist) on electrolyte and water content of the cauda epididymidis and fertility of male rats. Biol Reprod 1982;27:771-7.
[Google Scholar]
|
| 85. |
Fiore C, Sticchi D, Pellati D, Forzan S, Bonanni G, Bertoldo A, et al. Identification of the mineralocorticoid receptor in human spermatozoa. Int J Mol Med 2006;18:649-52.
[Google Scholar]
|
| 86. |
Mirshahi M, Ayani E, Nicolas C, Golestaneh N, Ferrari P, Valamanesh F, et al. The blockade of mineralocorticoid hormone signaling provokes dramatic teratogenesis in cultured rat embryos. Int J Toxicol 2002;21:191-9.
[Google Scholar]
|
| 87. |
Radomsky CL, Levine N. Thalidomide. Dermatol Clin 2001;19:87-103.
[Google Scholar]
|
| 88. |
Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering thalidomide: A review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol 1996;35:969-79.
[Google Scholar]
|
| 89. |
Perri AJ 3rd, Hsu S. A review of thalidomide's history and current dermatological applications. Dermatol Online J 2003;9:5.
[Google Scholar]
|
| 90. |
Shanbhag PS, Viswanath V, Torsekar RG. Thalidomide: Current status. Indian J Dermatol Venereol Leprol 2006;72:75-80.
[Google Scholar]
|
| 91. |
Lenz W. A short history of thalidomide embryopathy. Teratology 1988;38:203-15.
[Google Scholar]
|
| 92. |
Lutwak-Mann C. Observations on progeny of thalidomide-treated male rabbits. Br Med J 1964;1:1090-1.
[Google Scholar]
|
| 93. |
Teo SK, Denny KH, Stirling DI, Thomas SD, Morseth SL, Hoberman AM, et al. Effects of thalidomide on reproductive function and early embryonic development in male and female new Zealand white rabbits. Birth Defects Res B Dev Reprod Toxicol 2004;71:1-6.
[Google Scholar]
|
| 94. |
Teo SK, Harden JL, Burke AB, Noormohamed FH, Youle M, Johnson MA, et al. Thalidomide is distributed into human semen after oral dosing. Drug Metab Dispos 2001;29:1355-7.
[Google Scholar]
|
| 95. |
Teo SK, Chandula RS, Harden JL, Stirling DI, Thomas SD. Sensitive and rapid method for the determination of thalidomide in human plasma and semen using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2002;767:145-51.
[Google Scholar]
|
| 96. |
Available from: https://ec.europa.eu/health/documents/community-register/2010/2010122094568/anx_94568_en.pdf [Last accessed on 2016 Dec 19].
[Google Scholar]
|
Fulltext Views
10,907
PDF downloads
3,249





