Translate this page into:
End of the road for terbinafine? Results of a pragmatic prospective cohort study of 500 patients
Correspondence Address:
Sanjay Singh
Department of Dermatology and Venereology, Institute of Medical Sciences, Banaras Hindu University, Varanasi - 221 005, Uttar Pradesh
India
| How to cite this article: Singh S, Shukla P. End of the road for terbinafine? Results of a pragmatic prospective cohort study of 500 patients. Indian J Dermatol Venereol Leprol 2018;84:554-557 |
Abstract
Background: There is a general impression among dermatologists in India that terbinafine has been losing its effectiveness in dermatophytoses over the past few years, but there are no recent data to support this.
Aims: To determine the effectiveness of terbinafine in tinea corporis, tinea cruris and tinea faciei with a pragmatic prospective cohort study.
Methods: A sample size of 361 patients was calculated taking a 5% margin of error and a 95% confidence level. Five hundred patients with tinea corporis, tinea cruris and tinea faciei confirmed by potassium hydroxide microscopy received oral terbinafine (5mg/kg/day) and topical terbinafine 1% applied twice daily for 4 weeks. Patients were evaluated at 2 and 4 weeks. Cure was defined as total clearance of lesions and negative microscopy.
Results: Patients who came for follow-up at 2 and 4 weeks numbered 357 and 362 respectively. Ten patients were cured at 2 weeks (cure rate 2%, 95% confidence interval 1.0–3.7%, intention-to-treat analysis) and 153 patients were cured at 4 weeks (cure rate 30.6%, 95% confidence interval 26.7–34.8%).
Limitations: Culture and antifungal susceptibility testing were not performed since this was a pragmatic study. There was also no follow up after completion of treatment to check for relapses, but the poor response makes this less relevant.
Conclusion: The effectiveness of terbinafine in dermatophytosis was abysmal in this study.
Introduction
The current scenario of dermatophytosis in India, the challenges it entails and the urgency of the problem have been discussed recently in this journal.[1] The need for evidence-based guidelines and their implementation to counter the problem was stressed. Similar concerns have been raised elsewhere too.[2],[3] A general impression among dermatologists in India is that terbinafine, once considered a very effective treatment,[4] is fast losing its efficacy in dermatophytic infections, but there are no published data to support this. In this study, we sought to determine whether this perception of a lack of effectiveness of terbinafine holds true in practice.
Methods
Setting
The study was conducted at the Sir Sunderlal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi.
The hospital caters to all patients seeking its services and not only referred patients; the latter only constitute a small proportion of all patients seen. Being the only big hospital in the region, it also caters to patients coming from neighboringstates.
The study (registration number CTRI/2017/05/008687) was approved by the Institutional Ethics Committee. Patients were enrolled between February 2016 and September 2016.
Sample size
Due to the absence of data from recent studies, we did a mid-study sample size calculation (https://select-statistics.co.uk/) after analyzing the results of the first 100 patients, of whom 38 were cured in 4 weeks. Taking a 5% margin of error and a 95% confidence level, the sample size was determined to be 361 patients. After taking into account the possibility of attrition, we enrolled 500 patients.
Selection criteria, enrollment and follow up
Consecutive patients with suspected tinea corporis, tinea cruris and tinea faciei attending the dermatology outpatient services on two particular days of the week (Monday and Wednesday, as per the OPD schedule of the first author) were enrolled. Patients who had any other type of tinea were not enrolled. Inclusion criteria were: (a) age between 4 years and 80 years, (b) witnessed written informed consent, given by the patient or parents in case of minors and (c) demonstration of fungal elements on potassium hydroxide (KOH) microscopy of skin scrapings. Patients were excluded in case of any of the following: (a) pregnancy, (b) lactation, (c) expressed inability to come for follow-up visits at 2 and 4 weeks, (d) use of terbinafine (oral or topical) in the past 1 month, (e) hepatic and/or renal disease revealed by history and (f) past history of drug reaction to terbinafine (oral or topical). None of the patients gave a history of HIV infection or immunosuppressive treatment. Eleven patients had diabetes and were on treatment for the same.
If a patient had multiple lesions, one lesion which had maximum erythema and scaling was identified as the index lesion, from which scrapings were taken for a KOH mount at every visit. The patients were examined by both authors at each visit. KOH mounts were made by the second author (PS) in all patients and were examined microscopically every time by both authors (SS and PS). Each patient had a case report form (CRF) in which the details were recorded. Location of the index lesion was recorded in the CRF.
Treatment
Enrolled patients were prescribed oral terbinafine in the dose of 5 mg/kg/day, with the dose calculated to the nearest 62.5 mg (half of the 125 mg tablet) as rounding off to a smaller dose was not possible. In addition, terbinafine cream 1% was prescribed to be applied twice daily on the lesions for the duration of the study. This being a pragmatic trial, the medicines were purchased by the patients, who were reminded at each visit to take the prescribed treatment as per instructions, but compliance was not assessed objectively. Patients were asked to not use any other treatment besides the one prescribed. No other advice was given. Family history of similar illness was not recorded. Patients were examined at 2 and 4 weeks, and skin scrapings from index lesions were taken for repeat potassium hydroxide examinations. Cure was defined to have been achieved when both of the following criteria were fulfilled: (a) complete disappearance of all lesions and (b) negative KOH microscopy from the index lesion site. Patients who did not achieve a cure after 4 weeks of treatment were prescribed oral itraconazole in the dose of 5 mg/kg/day for 1month.
Outcome measure and statistical analysis
The main outcome measures were the numbers of patients cured at 2 and 4 weeks. For the baseline data, mean and standard deviation were calculated for data with normal distribution and median and range for data with non-normal distribution. Proportion of cure and its 95% confidence interval were calculated.
Results
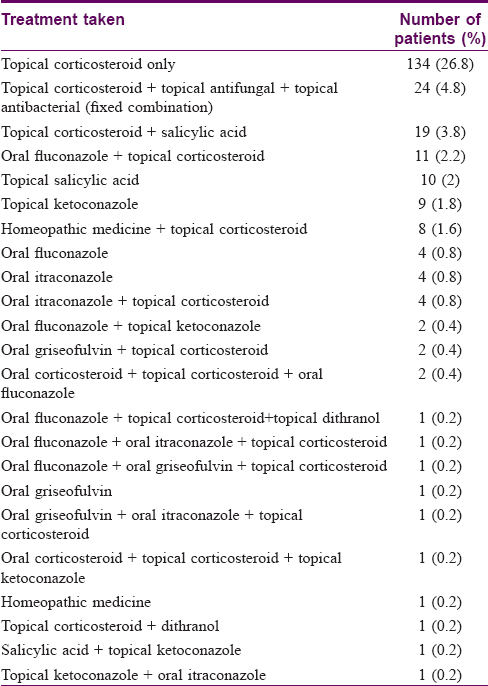
A total of 500 patients with KOH mounts positive for dermatophytes (presence of septate branching hyphae) were enrolled in the study [Table - 1]. Twenty three patients (4.6%) were less than 14 years of age. A majority (188 patients, 37.6%) of the patients had tinea cruris et corporis at presentation, followed by tinea cruris et corporis et faciei (122, 24.4%) [Table - 1]. Two hundred and forty-three of the total patients enrolled (48.6%) had already taken some form of oral and/or topical treatment. The most commonly used treatment was found to be topical corticosteroid alone (131 patients, 26.2%). Other medicines used were fixed combinations of topical corticosteroid, antifungal and antibacterial (26, 5.2%), topical corticosteroid and salicylic acid combinations (18, 3.6%), and oral fluconazole with topical corticosteroid (13, 2.6%), among others [Table - 2]. Two hundred and ten patients (42%) had used topical corticosteroid in some form.

Out of the 500 patients, 357 came for follow-up at 2 weeks, of whom 10 (2%, 95% confidence interval 1.0-3.7%, intention-to-treat analysis [ITT]) had clinical and microscopic cure, which corresponded in all patients. For the follow-up at 4 weeks, 362 patients reported and 153 (30.6%, 95% confidence interval 26.7-34.8%, ITT) of these had achieved cure (inclusive of the 10 patients who had achieved cure at 2 weeks) [Table - 3]. Clinical cure was accompanied by microscopic cure in all patients. Details of these 153 patients are as follows: age (mean ± SD), 30.6 ± 15.0 years; 112 males and 41 females; 4 of these patients had tinea faciei.

None of the patients who came for follow-up visits reported any adverse events.
Discussion
Though Indian studies on the efficacy of terbinafine in tinea corporis and tinea cruris performed in the recent past [Table - 4] show acceptable cure rates, our data indicate that the drug may no longer be very effective. Since our patients were consecutively selected from those attending the dermatology outpatient services and since our hospital caters to all patients seeking its services including those from neighbouring states, we feel that our results may have some applicability to the larger population. Fifty four patients included in the present study were from states other than Uttar Pradesh.
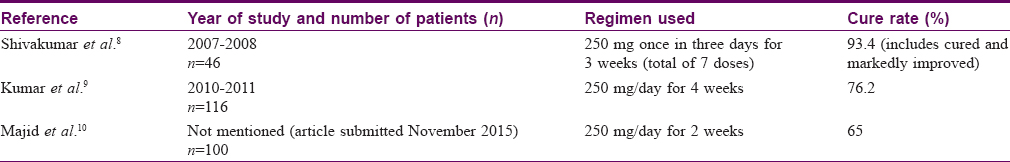
The regimen of terbinafine used in the present study is longer than those recommended by standard textbooks, which recommend a fixed dose of 250 mg per day to be given for 10 days to 4 weeks.[5],[6],[7] We used a flexible dose of 5 mg/kg/day for 4 weeks. Weight-adjusted dosing is better than giving a fixed dose to all patients irrespective of body weight. Despite these modifications, our intention-to-treat analysis showed cure rates of 2% at 2 weeks and 30.6% at 4 weeks. The reasons for such low cure rates of a drug which was once very effective, including possible role of incorrect use of topical corticosteroids, need to be investigated to prevent the emergence of a similar situation with other drugs.
We did not do fungal cultures and antifungal susceptibility testing and this may be considered a limitation of the present study. However, owing to the logistical difficulties these tests are almost never performed when treating a patient with dermatophytosis. Mycology laboratory facilities are not usually available to practicing dermatologists. Further, fungus culture takes up to 4-6 weeks and for susceptibility testing subculture takes another week, followed by susceptibility testing which takes 1 more week, which makes such testing impractical for the treatment of a patient. In this pragmatic study, however, our aim was to determine the effectiveness of terbinafine in a real-world clinical setting.
We did not follow-up patients after completion of treatment to determine the relapse rate. However, the value of follow-up with the low cure rates we obtained is debatable. Other limitations of the study are lack of compliance data and follow up rate at 4 weeks being 72.4%, which may be considered low. However, we performed intention-to-treat analysis to take care of the attrition bias.
To summarize, the present study found that the effectiveness of terbinafine in the treatment of tinea corporis, tinea cruris and tinea faciei is 2% at 2 weeks and 30.6% at 4 weeks. Our findings are in line with the general perception among dermatologists that terbinafine is now not as effective as in the past in the treatment of dermatophytosis. New antifungal drugs, preferably of new classes, are perhaps needed.
Financial support and sponsorship
Institute of Medical Sciences, Banaras Hindu University.
Conflicts of interest
There are no conflicts of interest.
[8-10]
| 1. |
Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol 2017;83:281-4.
[Google Scholar]
|
| 2. |
Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol 2017;62:227-36.
[Google Scholar]
|
| 3. |
Dogra S, Uprety S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J 2016;7:73-6.
[Google Scholar]
|
| 4. |
Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J 2016;7:77-86.
[Google Scholar]
|
| 5. |
Hay RJ, Ashbee HR. Fungal infections. In: Griffiths CE, Barker J, Bleiker J, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Chichester: Wiley Blackwell; 2016. p. 32-7.
[Google Scholar]
|
| 6. |
Schieke SM, Garg A. Superficial fungal infection. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York: McGraw-Hill; 2012. p. 2295.
[Google Scholar]
|
| 7. |
Shenoy MM, Shenoy SM. Superficial fungal infections. In: Sacchidanand S, Oberai C, Inamadar AC, editors. IADVL Textbook of Dermatology. 4th ed. Mumbai: Bhalani; 2015. p. 467-8.
[Google Scholar]
|
| 8. |
Shivakumar V, Okade R, Rajkumar V, Sajitha K, Prasad SR. Intermittent pulse-dosed terbinafine in the treatment of tinea corporis and/or tinea cruris. Indian J Dermatol 2011;56:121-2.
[Google Scholar]
|
| 9. |
Kumar A, Budania N, Sharma P, Singh M. A comparative study of efficacy of terbinafine and fluconazole in patients of tinea corporis. Int J Pharm Med Bio Sci 2013;2:92-6.
[Google Scholar]
|
| 10. |
Majid I, Sheikh G, Kanth F, Hakak R. Relapse after oral terbinafine therapy in dermatophytosis: A Clinical and mycological study. Indian J Dermatol 2016;61:529-33.
[Google Scholar]
|
Fulltext Views
4,226
PDF downloads
2,145





