Translate this page into:
Linear atrophoderma of Moulin: A rare entity
Correspondence Address:
Bhagyashri Ambarnath Abak
Department of Dermatology, Seth G. S. Medical College and KEM Hospital, Acharya Donde Marg, Parel, Mumbai - 400 012, Maharashtra
India
| How to cite this article: Kharkar VD, Abak BA, Mahajan SA. Linear atrophoderma of Moulin: A rare entity. Indian J Dermatol Venereol Leprol 2018;84:591-594 |
Sir,
Linear atrophoderma of Moulin is a rare skin condition characterized by unilateral, hyperpigmented and atrophic band-like skin lesions following the lines of Blaschko. Usually, the disease begins in childhood or adolescence on the trunk or limbs, with no preceding inflammation or subsequent sclerodermatous changes. It then progresses for a few months in a blaschkoid pattern and then persists as an atrophic plaque. We could find only 34 cases of linear atrophoderma of Moulin previously reported in the English language literature.[1]
A 17-year-old male presented to the dermatology department of Seth G. S. Medical College and KEM Hospital, Mumbai, Maharashtra, India, with a 3-year history of progressive depressed linear lesions along the Blaschko's lines. It was associated with occasional pruritus. Lesions initially started over the abdomen, near the umbilicus and progressed gradually towards the right side of the back. It was not preceded by any symptoms or other lesions. Cutaneous examination revealed two parallel bands of atrophic, linear hyperpigmented plaques along Blaschko's lines extending from the umbilicus to the right side of back [Figure - 1]. There were no signs of inflammation or induration. Rest of the physical examination was unremarkable. There was no history of similar lesions in the family members. Linear scleroderma, atrophoderma of Moulin, atrophoderma of Pasini and Pierini were considered in differential diagnosis.
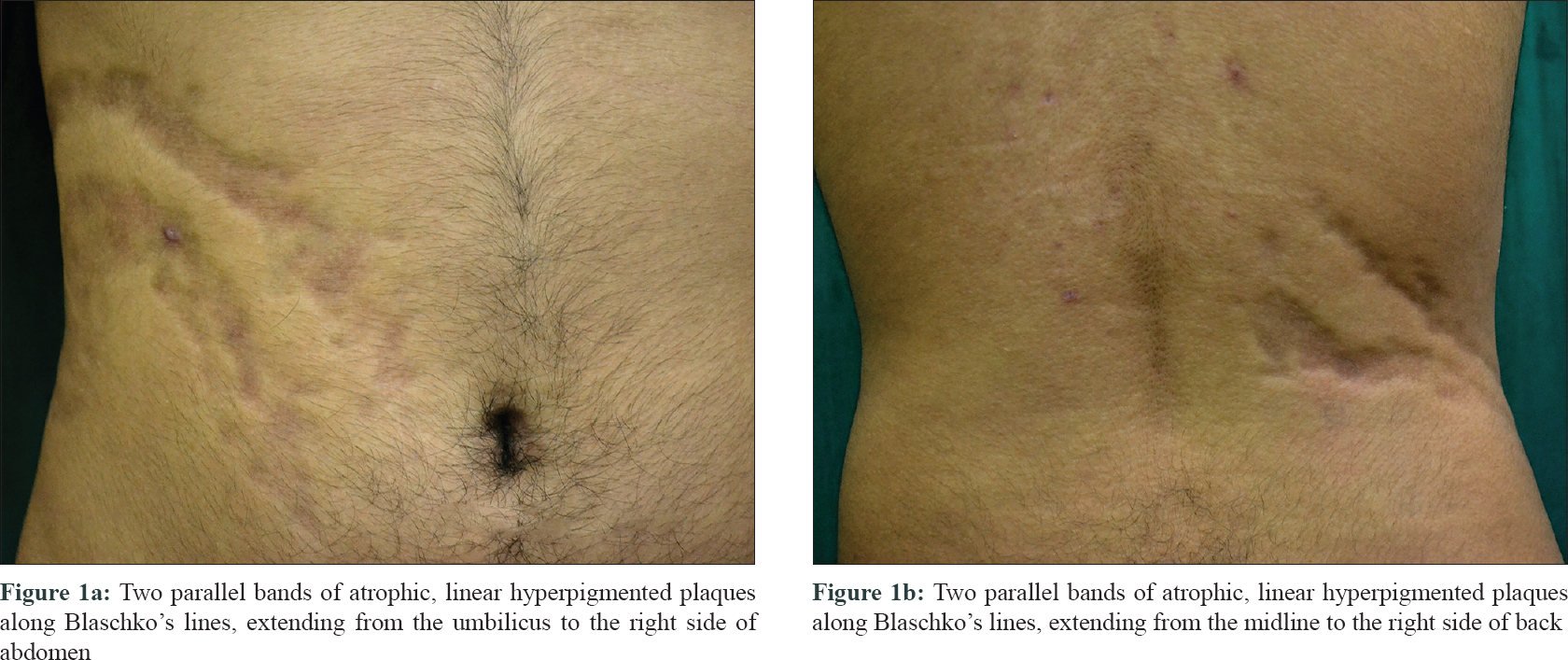 |
| Figure 1: |
Skin punch biopsy was done from the center of the atrophic plaque over the abdomen. Histopathological examination revealed mild hyperkeratosis and acanthosis with prominent melanin deposition in the basal layer. Moderately dense perivascular lymphohistiocytic infiltrate was also noted without any signs of sclerosis [Figure - 2]a and [Figure - 2]b. Special staining for elastin did not reveal any abnormality [Figure - 3].
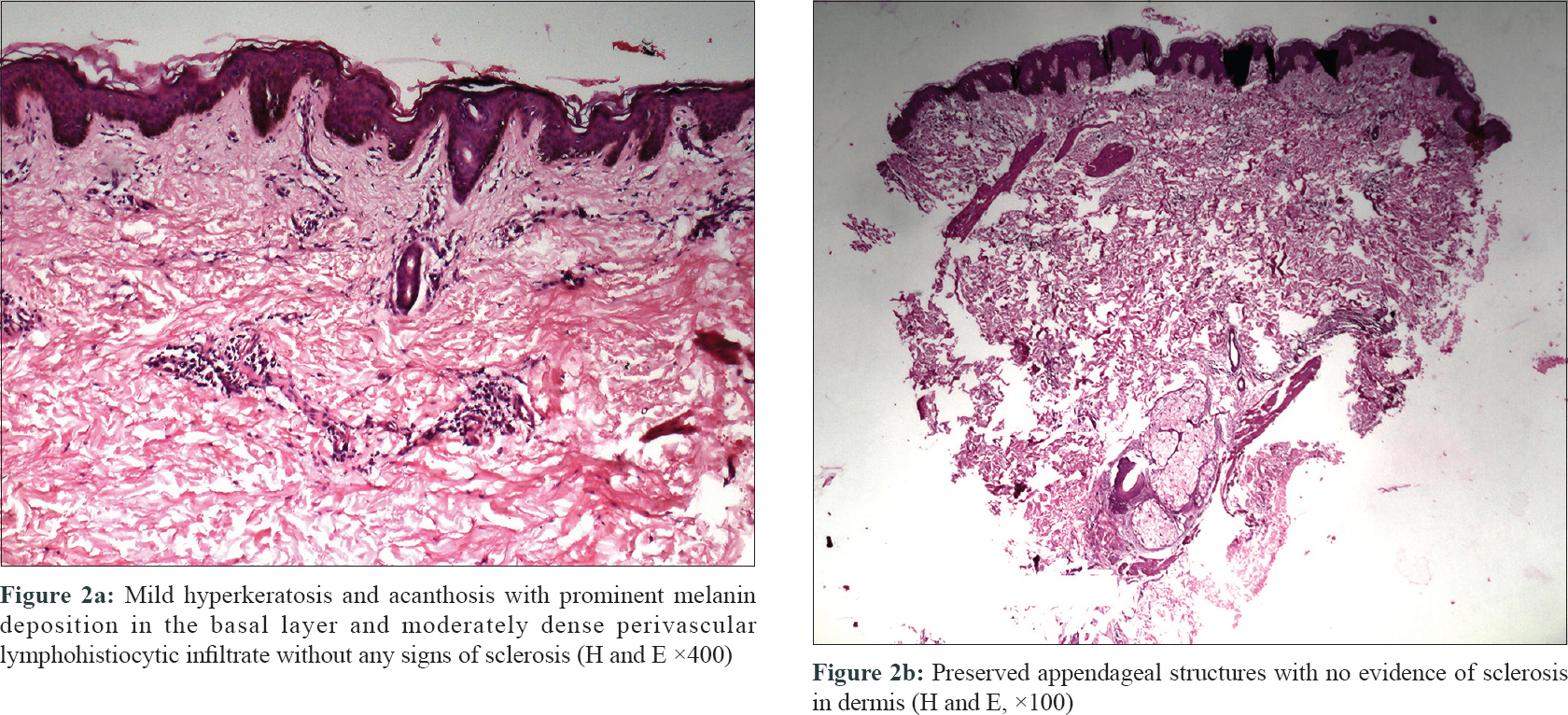 |
| Figure 2: |
 |
| Figure 3: Verhoeff's Van Gieson stain normal (×400) |
On lab investigations, antinuclear antibody was positive. However, due to financial constraints, complete antinuclear antibody profile could not be done. Chest X-ray revealed prominent bronchovascular markings, haziness and fibrotic changes in bilateral lower zone and enlargement of hilar lymph nodes. High-resolution computed tomography was within normal limits and revealed no significant abnormality.
Correlating the clinical picture with histological examination, a diagnosis of linear atrophoderma of Moulin was made. The patient was treated with intralesional platelet-rich plasma injections. After four sessions, there was partial improvement in terms of reduction in the depth of the plaque. However, the texture and the color remained the same.
Moulin first described linear atrophoderma of Moulin in 1992, as an acquired unilateral hyperpigmented atrophic band along Blaschko's lines.[2]
Browne and Fisherreported a case with antecedent inflammation in a 16-year-old male and proposed that linear atrophoderma of Moulin may occasionally be preceded by an inflammatory stage that evolves into hyperpigmentation and atrophy.[3]
Therefore they suggested that there are two variants of the same disease: an inflammatory and a non-inflammatory linear atrophoderma of Moulin. Later, Utikal et al. described two patients with prominent telangiectatic erythema within lesions of linear atrophoderma and argued that these cases may represent a novel variant of linear atrophoderma of Moulin.
Histologically, linear atrophoderma of Moulin is characterized by hyperpigmentation of the basal cells along with slight thickening of the collagen fibers in the dermis and a sparse perivascular lymphocytic infiltrate. There may be some acanthosis, epidermal atrophy and decreased or fragmented elastic tissue. No clear signs of dermal atrophy are seen. Rather, atrophic appearance on clinical examination is secondary to reduction in subcutaneous tissue, which has been demonstrated by ultrasound imaging.[4]
Yan et al. have reported association of linear atrophoderma of Moulin with serum immunological markers, including positive antinuclear antibody, ribonucleoprotein, immunoglobulin M and anti-Sm antibody. The authors also identified unexpected connective tissue disease-like changes on histological analysis in this patient. Therefore, they speculated that the pathogenesis of linear atrophoderma of Moulin is associated with autoimmunity or that it is a variant connective tissue disease.[5]
The differential diagnoses include atrophoderma of Pasini and Pierini, linear scleroderma and focal dermal hypoplasia. Atrophoderma of Pasini and Pierini resembles linear atrophoderma of Moulin in all aspects except that it does not follow a blaschkoid pattern. Histopathologically, linear scleroderma reveals dense dermal sclerosis with marked appendageal atrophy and replacement of the adipose tissue by collagen. Focal dermal hypoplasia is characterized by absence of dermal collagen and accompanying appearance of adipose tissue in dermis. Linear atrophoderma of Moulin is differentiated from the above conditions by absence of dermal sclerosis and marked subcutaneous atrophy with preservation of dermal appendages. The elastic tissue stain does not reveal any abnormality.[6]
Our case matches the characteristic clinical and pathological features seen with linear atrophoderma of Moulin; i.e., without antecedent inflammation and with positive antinuclear antibody.
There is no proven effective treatment for linear atrophoderma of Moulin. Topical steroids and high-dose penicillin have been reported to be ineffective. Artola et al. described a patient whose lesions stabilized with oral potassium aminobenzoate 12 g/day. Wongkietkachornet al. reported a case with partial response to topical calcipotriol.[6] Zaouak et al. reported a case successfully treated with methotrexate.[7]
Platelet-rich plasma contains platelets as well as specific growth factors, like transforming growth factor beta, platelet-derived growth factor, insulin-like growth factor, vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-2. Platelet-derived growth factor, endothelial growth factor and fibroblast growth factor-2 have been shown to stimulate proliferation of osteoblastic progenitors as well as affect the mitogenesis of mesenchymal stem cells. The atrophic appearance of linear atrophoderma of Moulin is secondary to reduction in the subcutaneous tissue and therefore, the growth factors in platelet-rich plasma may help in enhancing the subcutaneous tissue. Also, as there is no proven effective treatment for linear atrophoderma of Moulin and platelet-rich plasma therapy is a familiar form of treatment with an excellent safety profile, we decided to give it a trial.
Herein, we report a case of classic linear atrophoderma of Moulin with partial response to intralesional platelet-rich plasma therapy. However, further larger clinical studies are required to establish the effectiveness of platelet-rich plasma in the management of linear atrophoderma of Moulin.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Patrizi A, Venturi M, Neri I, Pazzaglia M, Passarini B. Atrophoderma following lines of Blaschko: An interesting diagnostic dilemma. Linear atrophoderma of Moulin, blaschko linear atrophoderma of Pasini and Pierini or linear morphea. Clin Dermatol 2013;1:29-36.
[Google Scholar]
|
| 2. |
Moulin G, Hill MP, Guillaud V, Barrut D, Chevallier J, Thomas L, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol 1992;119:729-36.
[Google Scholar]
|
| 3. |
Browne C, Fisher BK. Atrophoderma of Moulin with preceding inflammation. Int J Dermatol 2000;39:850-2.
[Google Scholar]
|
| 4. |
Norisugi O, Makino T, Hara H, Matsui K, Furuichi M, Shimizu T, et al. Evaluation of skin atrophy associated with linear atrophoderma of Moulin by ultrasound imaging. J Am Acad Dermatol 2011;65:232-3.
[Google Scholar]
|
| 5. |
Yan W, Wang S, Liu HJ, Wang L, Li W, Ran YP, et al. Linear atrophoderma of Moulin: A disease related to immunity or a kind of connective tissue disease? Australas J Dermatol 2017;58:e126-8.
[Google Scholar]
|
| 6. |
Wongkietkachorn K, Intarasupht J, Srisuttiyakorn C, Aunhachoke K, Nakakes A, Niumpradit N, et al. Linear atrophoderma of Moulin: A case report and review of the literature. Case Rep Dermatol 2013;5:11-4.
[Google Scholar]
|
| 7. |
Zaouak A, Ghorbel HH, Benmously-Mlika R, Koubaa W, Badri T, Fenniche S, et al. Acase of linear atrophoderma of Moulin successfully treated with methotrexate. Dermatol Ther 2014;27:153-5.
[Google Scholar]
|
Fulltext Views
8,552
PDF downloads
2,356





