Translate this page into:
Injection-site reaction to ixekizumab histologically mimicking lupus tumidus: Report of two cases
2 Department of Pathology, "12 de Octubre" University Hospital, I+12 Research Institute, Complutense University, Madrid, Spain
Correspondence Address:
Marta Prieto-Barrios
12 de Octubre" University Hospital, Av. de Córdoba s/n, 28041, Madrid
Spain
| How to cite this article: Prieto-Barrios M, Rodriguez-Peralto J, Vico-Alonso C, Velasco-Tamariz V, Calleja-Algarra A, Ortiz-Romero PL, Rivera-Diaz R. Injection-site reaction to ixekizumab histologically mimicking lupus tumidus: Report of two cases. Indian J Dermatol Venereol Leprol 2018;84:610-613 |
Sir,
Ixekizumab is a humanized immunoglobulin G4 monoclonal antibody with antiinterleukin 17A activity, which has been recently approved for the treatment of moderate-to-severe psoriasis. This agent has become a safe and efficacious therapeutic tool for these patients. The most common adverse events are upper tract respiratory infections, headache, arthralgia and injection-site reactions.[1] We present two cases of injection-site reaction histopathologically mimicking cutaneous lupus erythematosus.
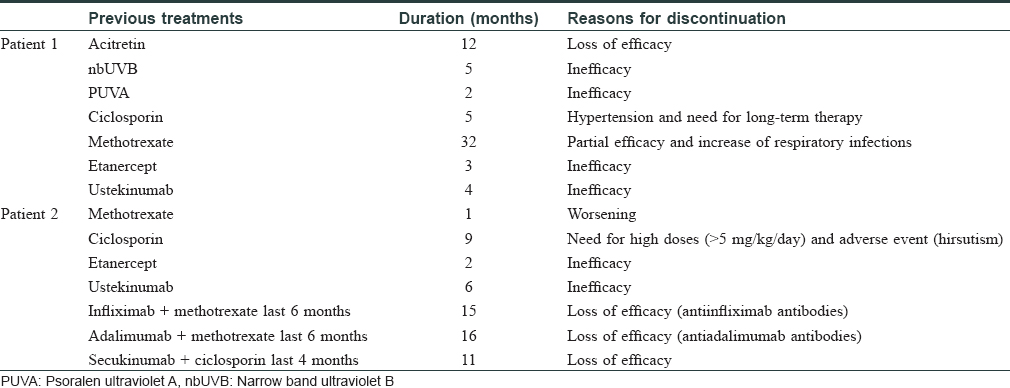
The first patient was a 55-year-old man with long-standing psoriasis treated with multiple medications, including etanercept for 3 months and ustekinumab for 5 months [Table - 1]. His baseline psoriasis area and severity index score was 14; ixekizumab was administered as two initial 80 mg injections, followed by one every 2 weeks. Forty-eight hours after the second dose, the patient reported a painful, well-defined warm and indurated plaque, about 10 cm in diameter on the left abdomen over the injection site. [Figure - 1]a. He had neither fever nor systemic symptoms. He had also developed a smaller and less painful edematous plaque on the abdomen after the first dose, which resolved spontaneously by 4 days.
 |
| Figure 1: |
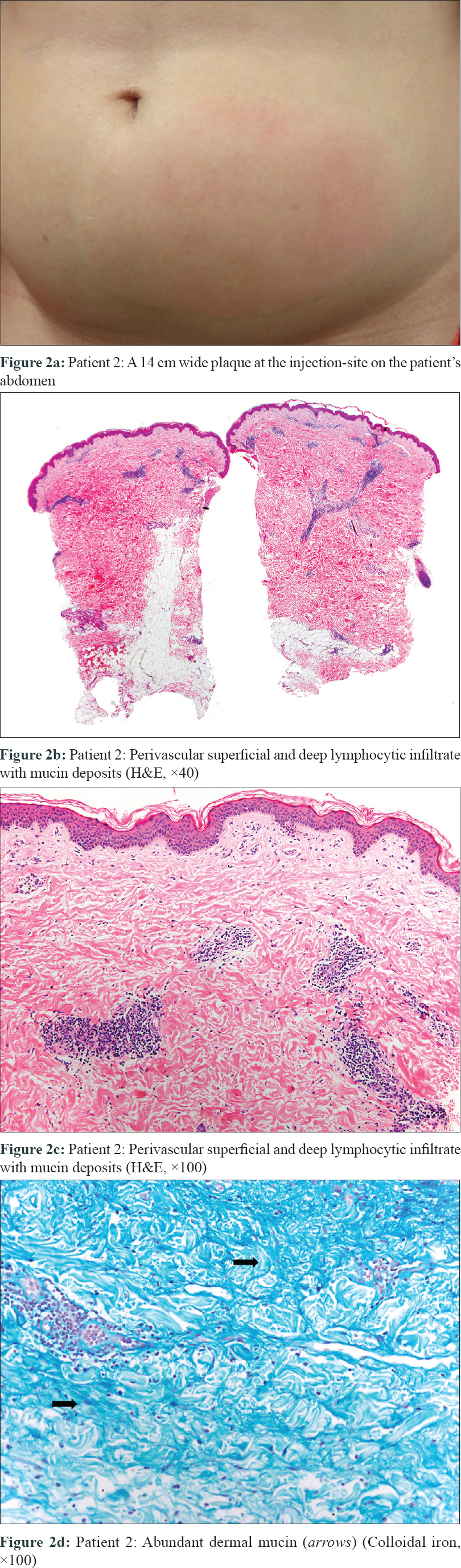
The second patient was a 25-year-old woman with severe psoriasis since infancy. She had received etanercept, ustekinumab, infliximab, adalimumab and secukinumab without any appreciable response [Table - 1]. Her psoriasis area and severity index score was 8.8 prior to initiation of treatment. Twenty-four hours after the first dose of ixekizumab, she presented with a painful, warm erythematous and edematous plaque on the injection site, about 14 cm in diameter [Figure - 2]a. She revealed the development of slight erythema over secukinumab injection site in the past, but this reaction was uneventful. No other dermatological or systemic complaints were obtained. In both cases, cutaneous biopsies revealed a profuse perivascular and perifollicular lymphocytic infiltrate [Figures 1b, c and 2b, c], with abundant mucin deposit [Figure - 1]d and [Figure - 2]d. No changes were found in the epidermal layer or the panniculus. Direct immunofluorescence was negative. From the histopathological point of view, the initial differential diagnosis included other conditions with predominantly lymphocytic infiltrate, like urticarial reaction, toxicoderma, viral exanthema or insect bite. However, the presence of mucin and the clinicopathological correlation suggested a reaction mimicking lupus tumidus at the injection site of ixekizumab. Renal function test, antinuclear antibody profile, and C3, C4 levels were normal at all times. Mantoux test and perinuclear antineutrophil cytoplasmic antibody were negative. Lesions lasted approximately 8 days in both cases and resolved without any sequelae. Both patients were studied by the allergology department and showed similar results. Intradermal tests with ixekizumab (0.8 and 0.08 mg/ml) were positive in immediate and late reading. Intraepidermal and epicutaneous tests with ixekizumab were negative. The first patient was switched to apremilast, whereas the second, considering the lack of options, was subjected to a desensitization process. She kept receiving the drug in a more diluted concentration and several injections per dose. This allowed her to continue treatment with no further reactions.
 |
| Figure 2: |
Although injection-site reactions are some of the commonest ixekizumab induced side-effects, they are generally mild and discontinuation is not needed.[1],[2] During UNCOVER trials, almost 7.7-10% of patients developed injection-site reaction, although statistically non-significant compared to placebo.[3] Conversely, in SPIRIT-P1 trial, safety evaluation proved a significant difference in injection-site reaction (12.1–15.7% ixekizumab vs. 0% placebo).[1] Reich et al. observed an overall frequency of erythema and pain at the injection site in 2.7 and 1.6%, patients respectively.[2] Approximately, half of patients reported a single event and frequency of injection-site reaction markedly decreased after the second week of treatment.[1],[2] The average resolution time was 2 days.[2] These reactions seem dose-dependent as there is a predilection for low-weight patients and those who are receiving higher dosage (80 mg every 2 weeks instead of every 4 weeks).[2] There is no evidence of circulating antidrug antibodies.[2] Our patients' allergy test results are consistent with an immunoglobulin E-mediated mechanism; however, in injection-site reactions, the related type of hypersensitivity remains unclear. None of these injection-site reactions have been described as type 1 hypersensitivity and although severe anaphylactic reactions have been reported after ixekizumab, the latter are considered to be a different entity.[4] The formulation of ixekizumab contains sodium citrate, citric acid, sodium chloride, polysorbate 80 and water, excipients which are normally used for other drugs, and not thought to be responsible for these reactions.[2] After literature review, we were unable to find any previous report depicting the histopathology of an injection-site reaction to ixekizumab, nor did we find any case of injection-site reaction mimicking lupus to other biologic agents. Injection-site reactions have been reported in almost 37% and 12% patients receiving etanercept and adalimumab respectively, while ustekinumab shows negilible incidence (<1% of patients). Zeltser et al. described 21 cases of etanercept-induced injection-site reaction, showing perivascular lymphocytic infiltrate and eosinophils, but mucin was not present in any of them.[5] To our knowledge, injection-site reaction mimicking lupus has only been reported with interferon till date. Arrue et al. described five patients with lupus like injection-site reactions after intramuscular interferon;[6] based on their histopathological resemblance with lupus tumidus, showing a dense lymphocytic infiltrate with perivascular and perifollicular distribution, along with abundant mucin deposit and occasional areas of basal cell degeneration. In those cases, there was no autoimmune association or other signs of systemic lupus erythematous. Treatment suspension was not needed.[6] They proposed that interferon could stimulate fibroblasts to secrete excess mucin. While interleukin-17 has been associated with fibroblastic stimulation and production of proinflammatory factors, an antiinterleukin-17 drug is supposed to be devoid of such effect. Lately, lupus erythematous has been linked to the Th17/interleukin-17/interleukin-23 axis, with the observation of higher levels of interleukin-17 in patients with lupus erythematous, which correlate with disease activity. In spite of the similarity, we do not think that these are real lupoid lesions but a peculiar type of injection-site reaction which histopathologically mimics lupus tumidus. The fact that there are no published cases of ixekizumab-induced systemic or cutaneous lupus erythematous, together with the self-limited course of the reaction and the absence of other signs of disease support this contention.
In conclusion, we communicate two cases of injection-site reaction secondary to ixekizumab which mimic lupus tumidus. The histopathology of ixekizumab-induced injection-site reaction has not been reported till now, so further studies are needed to corroborate our findings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Raquel Rivera-Diaz has acted as a consultant, investigator and/or speaker for AbbVie, Almirall, Celgene Corporation, Eli Lilly, GSK, Janssen-Cilag, LEO Pharma, MSD, Novartis, Pfizer. The rest of the authors have no conflicts of interest to declare.
| 1. |
Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: Etanercept comparisons and integrated data. J Am Acad Dermatol 2017;76:432-40.e17.
[Google Scholar]
|
| 2. |
Reich K, Leonardi C, Ohtsuki M, Gooderham M, Pangallo B, Xu WI, et al. Safety and Tolerability of Ixekizumab: Integrated Analysis of Injection-Site Reactions in Patients with Moderate-to-Severe Psoriasis Treated with Ixekizumab Compared with Placebo or Etanercept from Three Phase 3 Trials. In Vienna, Austria; 2016.
[Google Scholar]
|
| 3. |
Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet 2015;386:541-51.
[Google Scholar]
|
| 4. |
Henderson Berg MH, Carrasco D. Injection site reactions to biologic agents used in psoriasis and psoriatic arthritis. J Drugs Dermatol 2017;16:695-8.
[Google Scholar]
|
| 5. |
Zeltser R, Valle L, Tanck C, Holyst MM, Ritchlin C, Gaspari AA, et al. Clinical, histological, and immunophenotypic characteristics of injection site reactions associated with etanercept: A recombinant tumor necrosis factor alpha receptor: Fc fusion protein. Arch Dermatol 2001;137:893-9.
[Google Scholar]
|
| 6. |
Arrue I, Saiz A, Ortiz-Romero PL, Rodríguez-Peralto JL. Lupus-like reaction to interferon at the injection site: Report of five cases. J Cutan Pathol 2007;34 Suppl 1:18-21.
[Google Scholar]
|
Fulltext Views
3,522
PDF downloads
1,843





