Translate this page into:
Cutaneous toxicity of a new BRAF inhibitor, LGX818 (encorafenib)
Correspondence Address:
Leire Loidi Pascual
Department of Dermatology, Complejo Hospitalario de Navarra, C/Irunlarrea, 31008 Pamplona, Navarra
Spain
| How to cite this article: Pascual LL, Muruzàbal RS, Gigli ML, Bayona JI. Cutaneous toxicity of a new BRAF inhibitor, LGX818 (encorafenib). Indian J Dermatol Venereol Leprol 2017;83:102-104 |
Sir,
BRAF geneis mutated in almost 50% of metastatic biopsy specimens, with V600E as the most common mutation (73–91%) followed by V600K (7–20%) and, less commonly, V600D and L597R mutations.[1] It results in the constitutive activation of the MAPK pathway. Cutaneous toxicity of LGX818 or encorafenib is not well-known, as there are just a few case reports of it in the current literature.
We present a 78-year-old woman with cutaneous malignant melanoma on abdomen in Stage IV. She was being treated with a new BRAF-inhibitor drug (LGX818 or encorafenib). After 2 months, she presented with multiple erythematous and hyperkeratotic papules localized to the trunk and neck, and palmoplantar hyperkeratosis [Figure - 1]. Punch biopsy of some of the lesions was performed. Histopathology revealed suprabasal acantholysis with dyskeratosis in all specimens and wart-like lesions [Figure - 2]. These findings were compatible with the diagnosis of Grover-like eruption, palmoplantar hyperkeratosis and verrucal keratosis secondary to encorafenib. This diagnosis was made based on the recent development of skin lesions after the introduction of the suspicious drug. Moreover, similar lesions had been reported in the literature with other BRAF inhibitors. After a few months, the patient died because of progression of her disease.
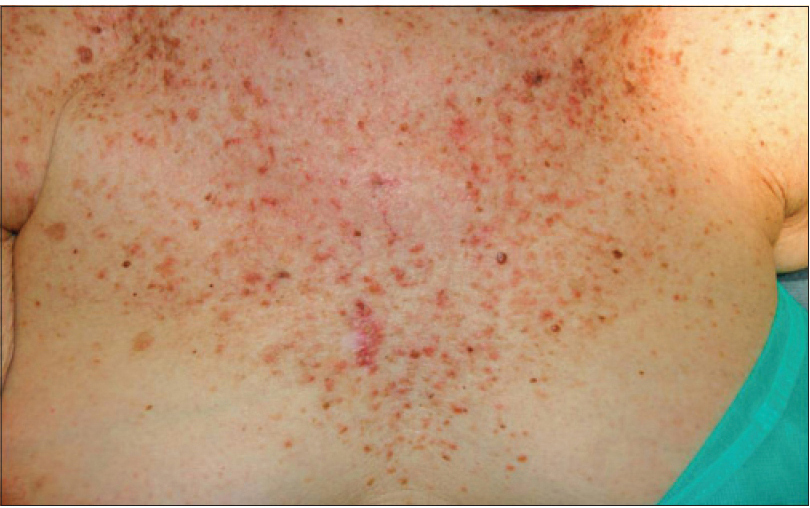 |
| Figure 1a: Multiple erythematous and hyperkeratotic papules localized to the trunk and neck |
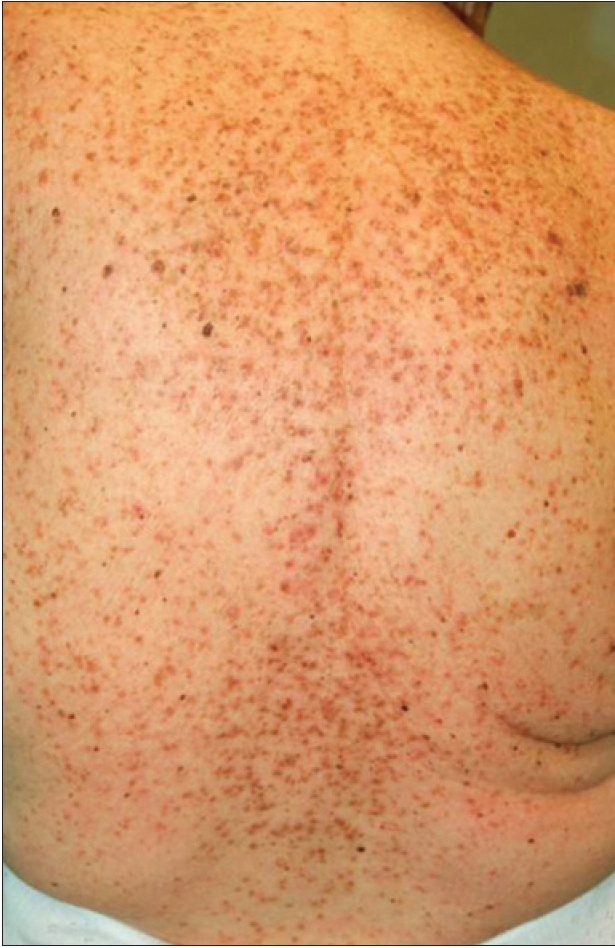 |
| Figure 1b: Multiple erythematous and hyperkeratotic papules localized to the trunk and neck |
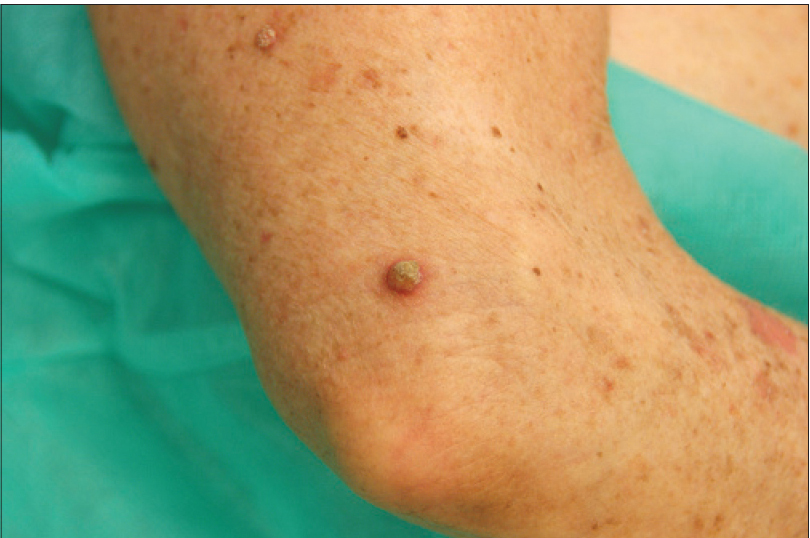 |
| Figure 1c: Erythematous and hyperkeratotic papule on the arm |
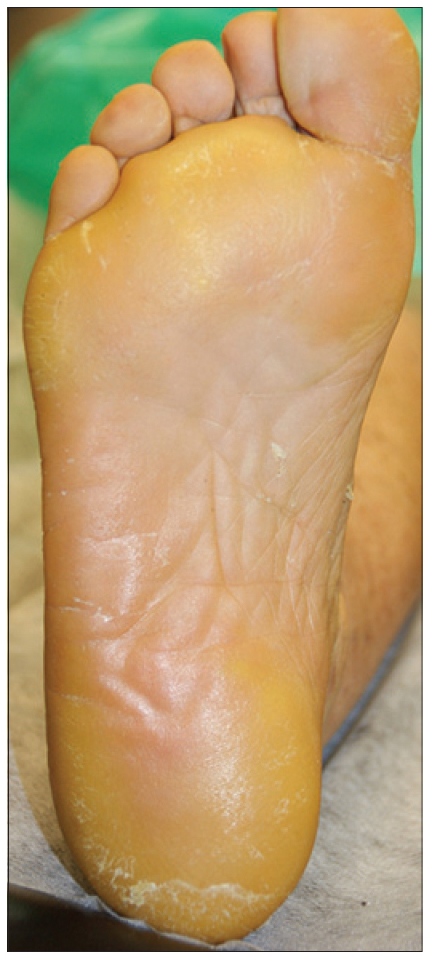 |
| Figure 1d: Plantar hyperkeratosis |
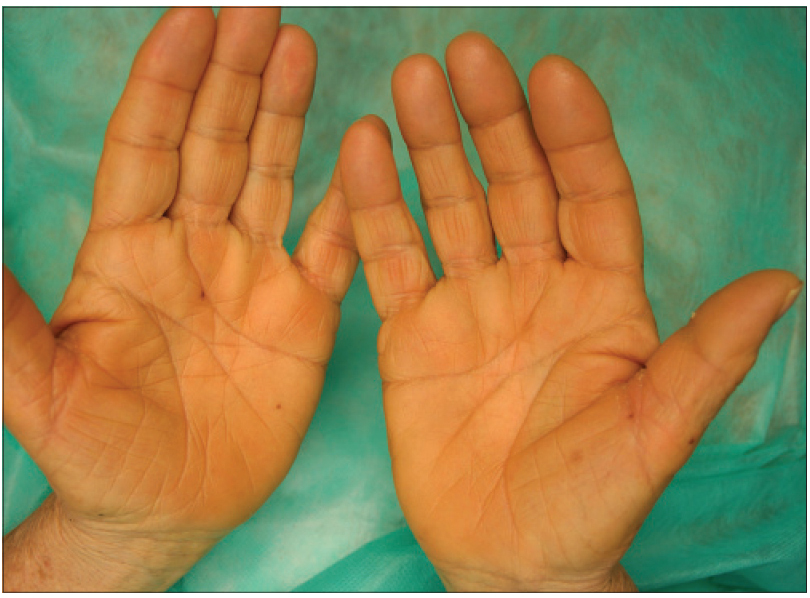 |
| Figure 1e: Palmar hyperkeratosis |
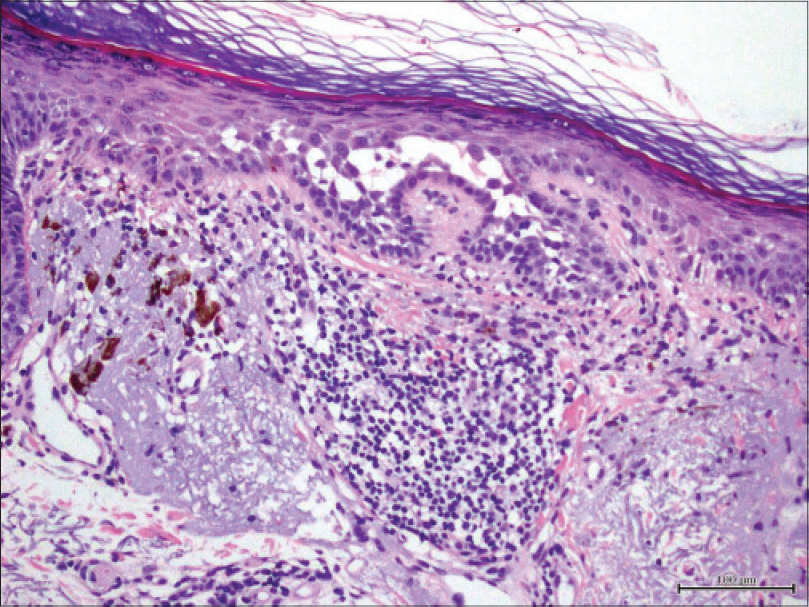 |
| Figure 2: Histopathology revealed suprabasal acantholysis with dyskeratosis (H and E, ×200) |
In general, BRAF inhibitors are well-tolerated drugs but cutaneous toxicity is common because of paradoxical activation of the MAPK pathway in wild-type BRAFcells. A spectrum of cutaneous toxicities has widely been reported with both vemurafenib and dabrafenib but encorafenib skin toxicity is not well-known.[2] The former include both benign and malignant lesions such as cutaneous squamous cell carcinoma, verrucal keratosis and plantar hyperkeratosis, Grover disease, hair follicle changes, panniculitis and photosensitivity.
Verrucal keratosis is a term used to describe a keratotic lesion induced by the new generation type 1 RAF inhibitors. These lesions present as hyperkeratotic papules similar to viral warts with papillomatosis, hyperkeratosis and acanthosis in the biopsy. The difference is that there are no koilocytes or large keratinohyalin granules in the pathological study.
Plantar hyperkeratosis presents with lesions predominantly at points of friction and blisters are infrequent. Hands are rarely involved. Nevertheless, we saw these types of lesions in our patient.
Grover disease is an acantholytic disorder that presents as several scattered erythematous papules. It predominantly affects trunk and upper arms and sometimes it is pruriginous. The typical finding in histology is the acantholytic dyskeratosis of the epidermis. It has been reported in up to 27% of patients receiving dabrafenib and vemurafenib.[2]
LGX818 (encorafenib) is a highly potent BRAF inhibitor under development for BRAF-mutated advanced melanoma. Initial results of a phase Ib study were reported in the 2013 ASCO annual meeting.[3] Its toxicity profile appears to be similar to those of vemurafenib and dabrafenib, with nausea, vomiting and headache being the most common adverse events. There were no cutaneous side effects in this report. After that, Anforth et al.[4] reported a case of bilateral plantar hyperkeratosis, verrucal keratosis, Grover's disease on chest and abdomen and eruptive nevi on the back, chest and legs in a 61-year-old man with metastatic melanoma, after 2 months of taking this drug. This case was similar to our patient. In addition, a 43-year-old man with metastatic melanoma receiving treatment with the combination of binimetinib and encorafenib who developed an erythema nodosum-like panniculitis has been reported.[5]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Hall RD, Kudchadkar RR. BRAF mutations: Signaling, epidemiology, and clinical experience in multiple malignancies. Cancer Control 2014;21:221-30.
[Google Scholar]
|
| 2. |
Anforth R, Fernandez-Peñas P, Long GV. Cutaneous toxicities of RAF inhibitors. Lancet Oncol 2013;14:e11-8.
[Google Scholar]
|
| 3. |
Dummer R, Robert C, Nyakas M, McArthur GA, Kudchadkar RR, Gomez-Roca C, et al. Initial results from a phase I, open-label, dose escalation study of the oral BRAF inhibitor LGX818 in patients with BRAF V600 mutant advanced or metastatic melanoma. J Clin Oncol. 2013;:suppl. [Abstr 9028].
[Google Scholar]
|
| 4. |
Anforth RM, Carlos GR, Scolyer RA, Chou S, Fernandez-Peñas P. Eruptive naevi in a patient treated with LGX818 for BRAF mutant metastatic melanoma. Melanoma Res 2015;25:91-4.
[Google Scholar]
|
| 5. |
Galliker NA, Murer C, Kamarashev J, Dummer R, Goldinger SM. Clinical observation of panniculitis in two patients with BRAF-mutated metastatic melanoma treated with a combination of a BRAF inhibitor and a MEK inhibitor. Eur J Dermatol 2015;25:177-80.
[Google Scholar]
|
Fulltext Views
3,671
PDF downloads
3,125





