Translate this page into:
A study of the efficacy of platelet-rich plasma in the treatment of androgenetic alopecia in males
Corresponding Author:
Shruti Gupta
Nivedita Niwas, 32-UB, Jawahar Nagar, New Delhi - 110 007
India
shruti1308@gmail.com
| How to cite this article: Gupta S, Revathi T N, Sacchidanand S, Nataraj H V. A study of the efficacy of platelet-rich plasma in the treatment of androgenetic alopecia in males. Indian J Dermatol Venereol Leprol 2017;83:412 |
Sir,
Androgenetic alopecia is a type of progressive patterned hair loss, where there is androgen-mediated conversion of susceptible terminal hairs into vellus hairs in genetically predisposed individuals.[1] It is known that transforming growth factor-β, an inhibitory factor secreted by hair follicles, plays an important role in the pathogenesis of androgenic alopecia.[1],[2] The potential of platelet-rich plasma in promoting hair growth is known since 1993.[3] This study was conducted to determine the effect of platelet-rich plasma in the management of androgenetic alopecia, mainly in terms of improvement in hair density and diameter and assess the variation in response among the different grades of androgenetic alopecia.
This was an open-labeled pilot study conducted in the department of dermatology, venereology and leprosy, Bangalore Medical College and Research Institute, Bengaluru. Approval of the Institute Ethics Committee was obtained. A convenience sample of 30 men in the age group of 20–50 years, with androgenetic alopecia Grade III-VII (Hamilton–Norwood classification) were included in the study. Patients with alopecia other than androgenetic alopecia, those on any other treatment modalities for androgenetic alopecia, history of bleeding disorders and active infection at the local site were excluded from the study. After obtaining written informed consent, a detailed history was elicited from each patient and a clinical examination was performed. Hair density and diameter were measured using a CapilliCARE hair and scalp analysis system (trichoscan).[4] Blood investigations were performed to rule out metabolic causes of alopecia.
Platelet-rich plasma was prepared by the double spin method.[5],[6] The scalp was activated by microneedling following which platelet-rich plasma was massaged on the scalp. A total of six sittings were administered in each patient at an interval of 15 days. Hair density and diameter were measured over an area near the vertex, 10 cm from the glabella. Digital photographs were taken before starting the treatment and periodically thereafter. Results were assessed at the end of 6 months on the basis of change in the density and diameter of hair as measured by trichoscan, by an independent observer evaluation of global photographs and patient's self-satisfaction, as evaluated by a questionnaire. Data analysis was done with the help of Epidemiological Information Package (2010) developed by the Centre for Disease Control, Atlanta.
The age of participants ranged from 25 to 35 years with a mean age of 28.3 ± 3.1 years. The number of patients belonging to androgenetic alopecia grades 6, 5A, 5, 4A, 4, 3V and 3 were 11, 7, 3, 2, 2, 3 and 2, respectively. The age at onset of androgenetic alopecia ranged between 20 and 28 years with the mean age being 23.2 ± 2.3 years. The duration of hair loss varied from 1 to 11 years with the mean duration being 5.5 ± 2.6 years. A positive family history was present in 26 (86.7%) of thirty patients.
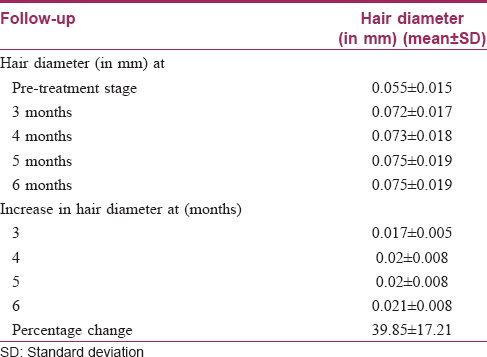
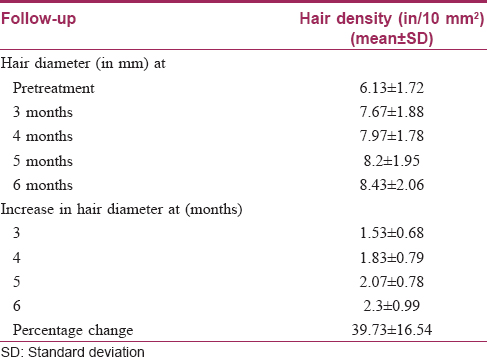
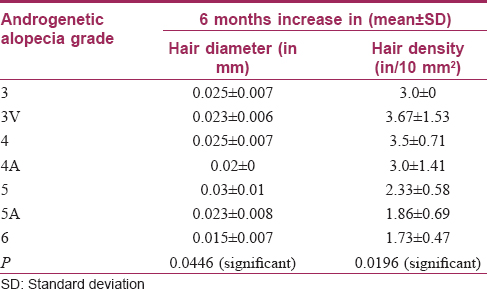
On the basis of trichoscan evaluation, the percentage increase in hair diameter was 39.8 ± 17.2 [Table - 1] and the percentage increase in hair density was 39.7 ± 16.5 [Table - 2]. On the basis of independent observers' evaluation of global photographs, the average improvement was 30.2 ± 12.2%. Ten (33.3%) patients had <25% improvement, 17 (56.7%) had 25–49.9% improvement and 3 (10%) had 50–74.9% improvement [Figure - 1] and [Figure - 2]. On the basis of self-assessment by the patient, the mean percentage improvement was 30 ± 13.1, the range being 10–70. Twenty eight (93.3%) patients reported complete cessation of hair fall as early as at the end of 2 months of treatment. Twenty (66.7%) patients reported an increase in hair growth. Eleven (36.7%) patients also noticed an improvement in hair quality in terms of hair texture. Patients with a lower grade of alopecia responded better to the therapy [Table - 3]. The response to platelet-rich plasma was found to be inversely proportional to the duration of alopecia [Table - 4]. The response also varied significantly with family history [Table - 5]. No adverse effects were noted.


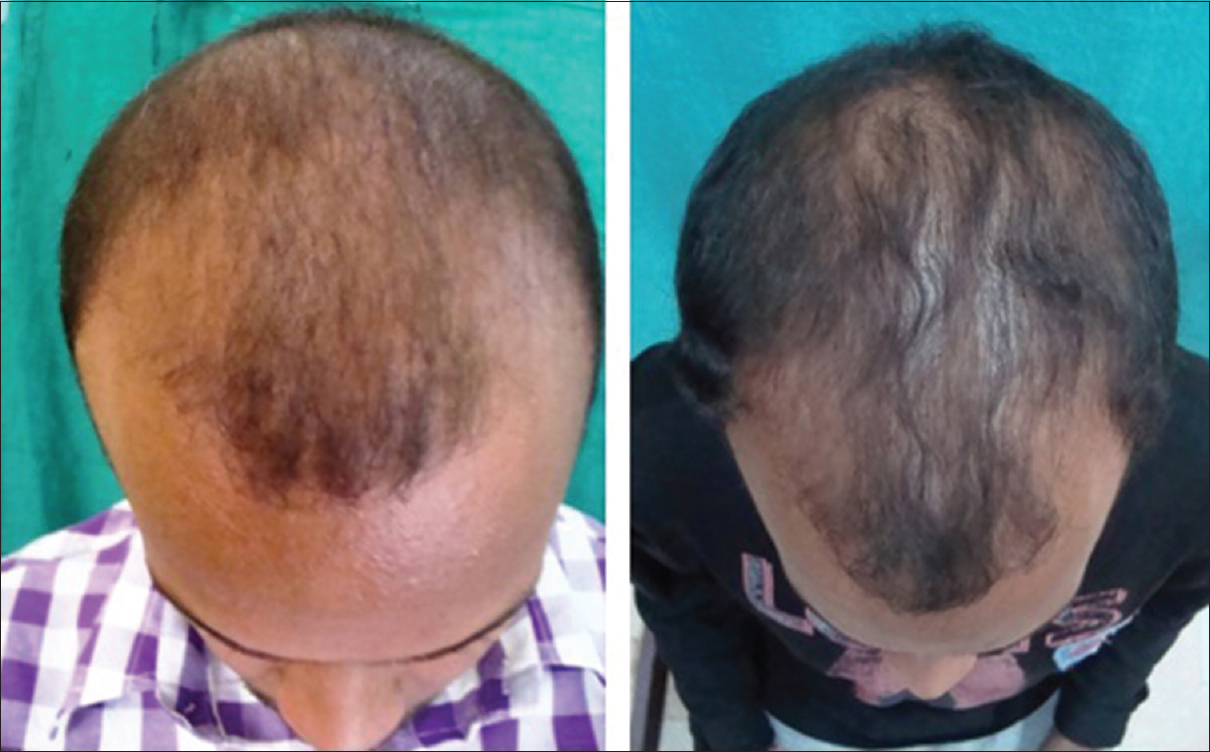 |
| Figure 1: Pre- and post-treatment (at 6 months) photograph of patient 1 |
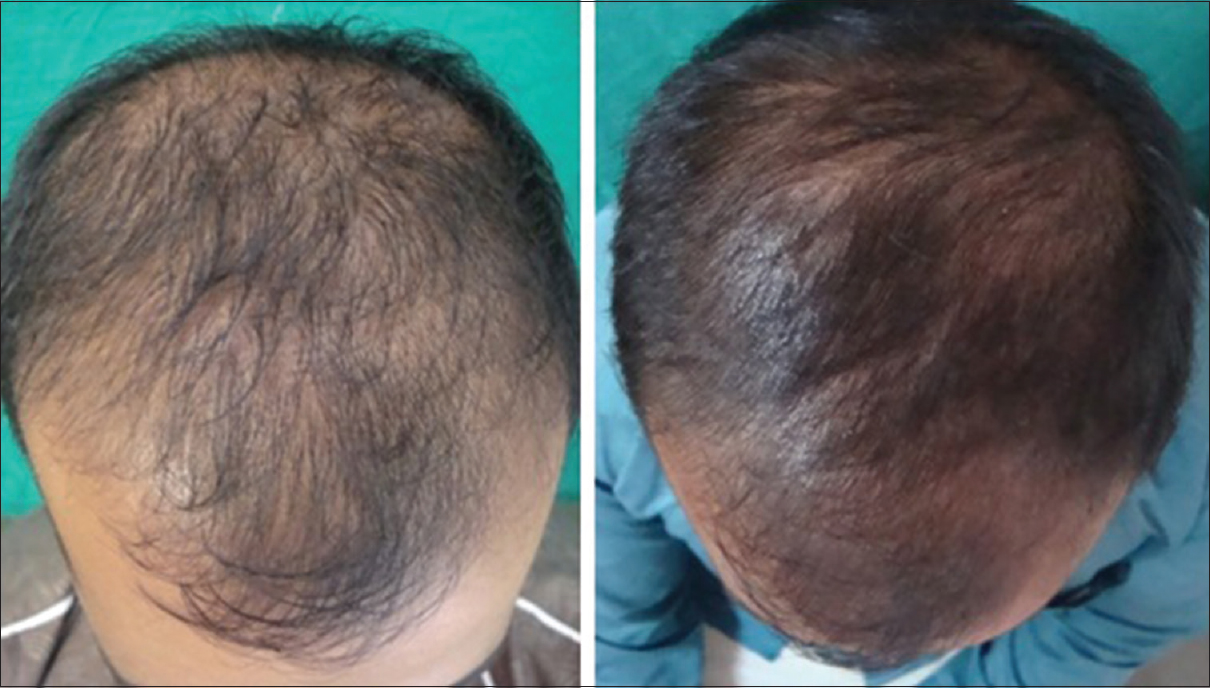 |
| Figure 2: Pre- and post-treatment (at 6 months) photograph of patient 2 |
Androgenetic alopecia is the most common cause of hair loss. Platelet-rich plasma contains a large array of growth factors such as platelet-derived growth factors, vascular endothelial growth factors, epidermal growth factors, fibroblast growth factor-2 and insulin-like growth factors which promote hair growth by inducing the follicular stem cells to shift from a dormant to an active state. Vascular endothelial growth factor-8 and platelet-derived growth factor-4 also facilitate angiogenesis around the hair follicle.[7] Rinaldi et al. found that growth factors from platelet-rich plasma could prevent dermal papilla apoptosis, prolong anagen phase and delay catagen and telogen phase.[8] There is histopathological evidence supporting these findings.[9]
The findings from our study also support the beneficial role of platelet rich plasma in androgenetic alopecia; the improvement in length and density of hair was objectively measured and confirmed [Figure - 3]. Complete cessation of hair fall was seen as early as following 3–4 sittings. Another interesting fact noticed was that the response to platelet-rich plasma depended significantly on the grade of androgenetic alopecia, duration of hair loss and the presence of family history of androgenetic alopecia.
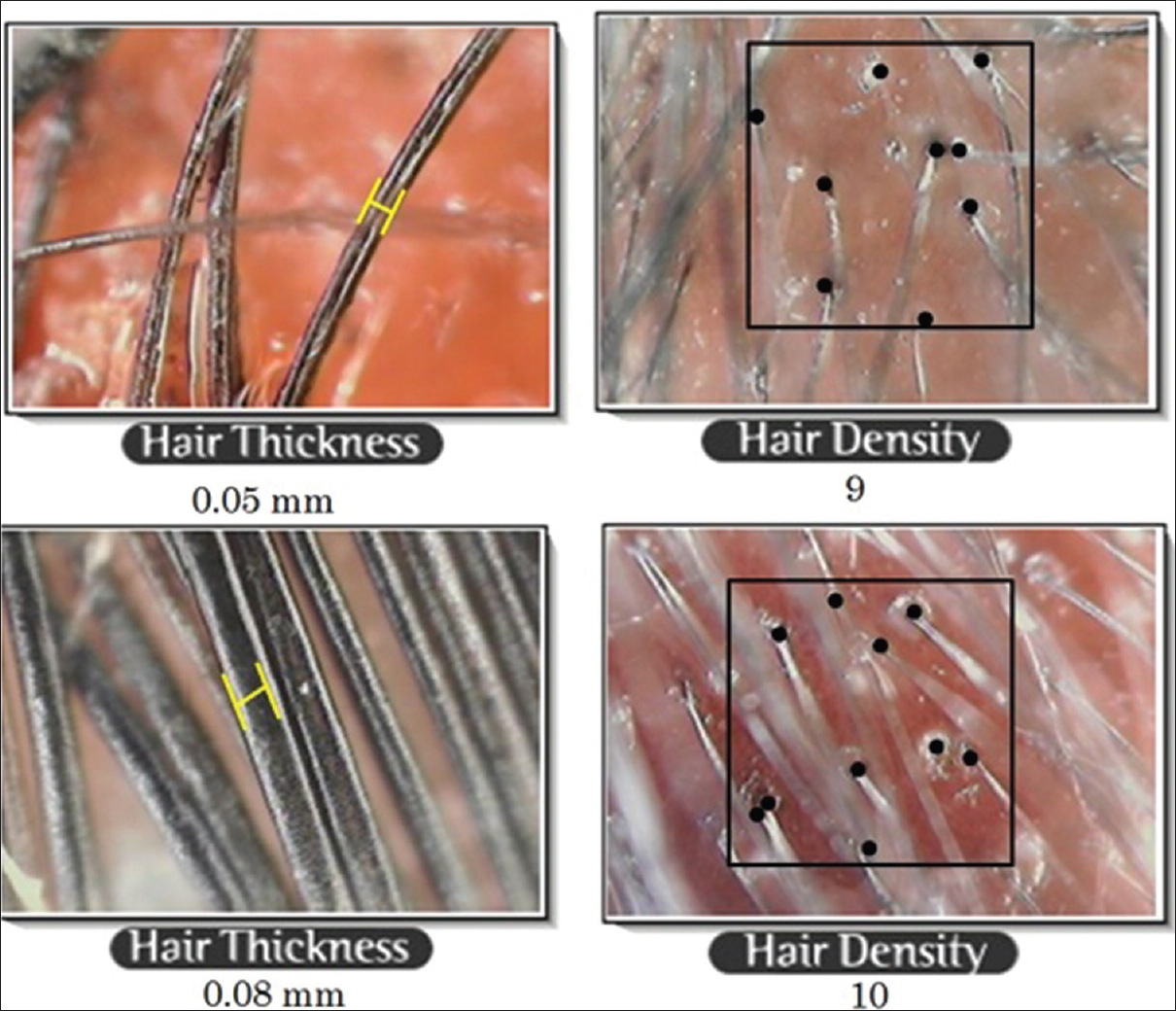 |
| Figure 3: Trichoscan findings showing change in diameter and density of hair |
The major limitation of this study is small sample size. Other limitations are lack of a control group and short follow up period. Controlled, prospective clinical trials are needed to assess the role of this modality in the treatment of androgenetic alopecia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. UK: Blackwell Publishing Ltd.; 2010. p. 66.1-66.100. [Google Scholar] |
| 2. | Paus R, Oslen EA, Messenger AG. Hair growth disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. USA: The McGraw-Hill Companies, Inc.; 2008. p. 766-9. [Google Scholar] |
| 3. | Knighton DR, inventor; Curative Technologies, Inc., assignee. Method for Promoting Hair Growth. United States patent 5178883. 1993 Jan 12. [Google Scholar] |
| 4. | Sarangi K. Androgenic alopecia. In: Savant SS, editor. Textbook of Dermatosurgery and Cosmetology. 2nd ed. India: Association of Scientific Cosmetologists and Dermatosurgeons; 2008. p. 576-8. [Google Scholar] |
| 5. | Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent 2001;10:225-8. [Google Scholar] |
| 6. | Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 2003;18:93-103. [Google Scholar] |
| 7. | Reese RJ. Autologous platelet rich plasma: What do we know? Important concepts relevant to hair restoration surgery. Hair Transplant Forum Int 2010;20:14-7. [Google Scholar] |
| 8. | Rinaldi F, Sorbellini E, Coscera T. The role of platelet rich plasma to control anagen phase: Evaluation in vitro and in vivo in hair transplant and hair treatment. Int J Trichol. 2011;3:S14-5. [Google Scholar] |
| 9. | Takikawa M, Nakamura S, Nakamura S, Ishirara M, Kishimoto S, Sasaki K, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg 2011;37:1721-9. [Google Scholar] |
Fulltext Views
7,396
PDF downloads
3,486





