Translate this page into:
Onychoscopy: A practical guide
Correspondence Address:
Chander Grover
Department of Dermatology and STD, UCMS and GTB Hospital, Dilshad Garden, Delhi
India
| How to cite this article: Grover C, Jakhar D. Onychoscopy: A practical guide. Indian J Dermatol Venereol Leprol 2017;83:536-549 |
Abstract
Onychoscopy refers to the examination of the nail unit using a dermoscope. Since the advent of dermoscopy, attempts have been made to use it for the diagnosis of nail disorders, most commonly pigmented lesions. As of now, onychoscopy has carved out a distinct niche for itself in the diagnostic work up of nail disorders. The nail is capable of mounting only a limited number of reaction patterns to the large number of disorders affecting it. Therefore, simple visual inspection may not be helpful in diagnosing many conditions reliably. Even a nail biopsy may not give a definitive answer every time. Onychoscopy is thus a valuable aid not only in enhancing visible nail features but also in revealing cryptic features of diagnostic value. This review aims to summarize the current level of knowledge about onychoscopic features of various diseases of the nail unit. It also aims to offer practical tips on how to conduct onychoscopy. For the purpose of review, a PubMed search about the indications and results of onychoscopy was done using the keywords “onychoscopy,” “nail fold capillaroscopy,” “dermoscopy nail” and “dermatoscopy nail.” All the articles were retrieved and classified into case reports, reviews and clinical studies. The final data was then analyzed and presented in a narrative fashion.Introduction
Onychoscopy is dermatoscopic examination of nail unit and its components, namely, the proximal nail fold, lateral nail fold, hyponychium, nail plate and bed. The nail matrix can ordinarily be visualized only in larger nails (that too partly) or can be seen intraoperatively. Onychoscopy enables easier and closer observation of nail features not visible to naked eye. Just as dermoscopy is skin surface microscopy, onychoscopy is surface microscopy of nail. However, nail is a more complex structure than skin, therefore onychoscopy differs from skin dermatoscopy in significant ways.
Dermoscopy is a valuable interface between macroscopic dermatology (i.e., clinical features) and microscopic dermatology (i.e., histopathological features). Similarly, onychoscopy is a potential link between naked eye examination (clinical onychology) and nail histopathology, opening up a valuable second front with a potential to prevent biopsy. Nail biopsies are not very commonly done by dermatologists, owing to the need of surgical expertise and a trained pathologist who can interpret nail specimens. Thus, onychoscopy offers a distinct advantage by helping avoid some nail biopsies.
Historical aspects
The term “dermoscopy” was coined by Saphier.[1] In the 1950s, Goldman used dermatoscope to study pigmented skin lesions.[2],[3] In 1971, MacKie outlined its advantages in evaluation of pigmented lesions [4] and Ronger et al. detailed its use in studying nail pigmentation. Since then, lot of research has gone into analyzing its importance in nail disease.[5],[6]
The field of onychoscopy has seen rapid development over the years with most initial studies focusing on nail pigmentation (especially to rule out melanoma).[7] However, of late, distinctive features of other nail diseases have also been evaluated. This review focuses on recent developments in onychoscopy and ways and means to incorporate it in daily practice.
Principles of dermoscopy
Simply put, dermoscopy is examination of the skin/nail with a magnifying device and attached light system. Various light systems, both nonpolarized and polarized, have been used. Polarized light helps overcome the difference in refractive indices of stratum corneum and air, thus preventing surface reflectance. This can also be achieved with the use of interface media or by tangentially tilting the device. For dermoscopy, various interface media have been used; however, for onychoscopy, it is better to use a medium with higher viscosity which stays in contact with nail surface.[8],[9] Water-based gels (e.g., ultrasound gel), antiseptic gels (e.g., alcohol itself or alcohol-based hand sanitizers) or oils (e.g., mineral oil) serve this purpose well. A comparative evaluation concluded that ultrasound gel is preferred medium for dermoscopy in special locations such as mucosae or nails.[8] Lencastre et al. suggested immersion gel as ideal for use with contact dermatoscopes and alcohol or oil for high magnifications achieved by video dermatoscopes.[6]
Onychoscopy
Prerequisites
A thorough knowledge of nail anatomy is an essential prerequisite because specific features in different parts of nail unit determine the final diagnosis. A variety of instruments can be used. Handheld dermatoscopes provide more clarity but lower magnification (×10) and are good enough for evaluating nail features; however, they may not assess vascular architecture reliably. On the other hand, video dermatoscopes are versatile devices capable of providing much higher magnifications (×200) though resolution may be suboptimum. An important feature is image storage and retrieval. This is particularly easy with the universal serial bus-based (USB) video dermatoscopes. Digital images of good resolution can be stored with cameras attached to handheld devices as well. In the authors' experience, USB dermatoscopes are versatile devices offering good adaptability, higher magnification to judge vasculature with easy image capture and storage.
Technique
Onychoscopy should be performed with the digit kept lightly, on a hard, dull working surface, avoiding any undue pressure by the patient or the examiner. For optimum evaluation of vasculature, hand should be at the level of heart and normal ambient temperature should be maintained. Nail plate should be thoroughly cleaned with acetone/spirit to remove debris, dirt or external applications.
First, one should choose where to look as the whole nail unit may not be visible in one field. The instrument needs to be moved back and forth, as well as transversally, to visualize all other parts. Initial examination is a “dry examination” (without any interface medium) though later a “wet examination” (using an interface medium) will be needed. Nail plate changes such as pits, scales or surface irregularities are seen well with “dry examination.” Interface media increase the transparency of plate and penetration of light; hence, nail bed changes are better visualized by “wet examination.” Choosing the appropriate light source is also important. Nonpolarized light ensures better visualization of surface irregularities such as pits and ridges, whereas polarization blunts surface irregularities and enables visualization of nail bed details. Both these examinations need to be performed.
Advantages of onychoscopy
The technique offers many advantages. Mostly, it enhances visible nail features; however, it can also help identify additional unique and fascinating features, not visible to the naked eye. It is practical and noninvasive, still opens up a second microscopic level of inspection. It contributes toward confirmation of diagnosis and assessment of treatment response as well as prognosis.
Procedural constraints
One is likely to face more procedural constraints with onychoscopy as compared to conventional dermatoscopy. It is physically difficult because of the peculiar anatomy, convexity, hardness, size and shape of nail. The entire nail cannot usually be visualized in one field, requiring frequent shifting of focus and angle. Photographic documentation of different parts may not be easy as features at the periphery tend to go out of focus. Nail convexity and hardness prevent complete adherence of the lens surface; hence, contact techniques may face more challenges and noncontact dermatoscopes (USB dermatoscopes) may prove more useful. Noncontact method also helps in visualizing nail fold capillary flow and architecture without pressing or blanching them. It must be kept in mind that onychoscopy cannot completely replace clinical examination.
Onychoscopy of Healthy Nail
The onychoscopic and capillaroscopic parameters for normal nail need to be understood before disease-induced changes can be appreciated.
Normal nail plate appears pale pink in color with a smooth shiny surface [Figure 1a] and [Figure 1b]. Aging nails may show longitudinal striae with a beaded appearance. A ×10–20 magnification is normally enough. Surface abnormalities are best appreciated without polarized light or interface medium [Figure 1a] and [Figure 1b]; however, for evaluating color abnormalities, an interface medium helps [Figure 2a] and [Figure 2b]. Nail plate is examined by keeping the dermatoscope vertically but for examining the distal free edge of nail plate, it needs to be tilted. This part should be examined, especially in longitudinal melanonychia (to assess the level of pigment origin) or in cases with distal onycholysis as detailed below.
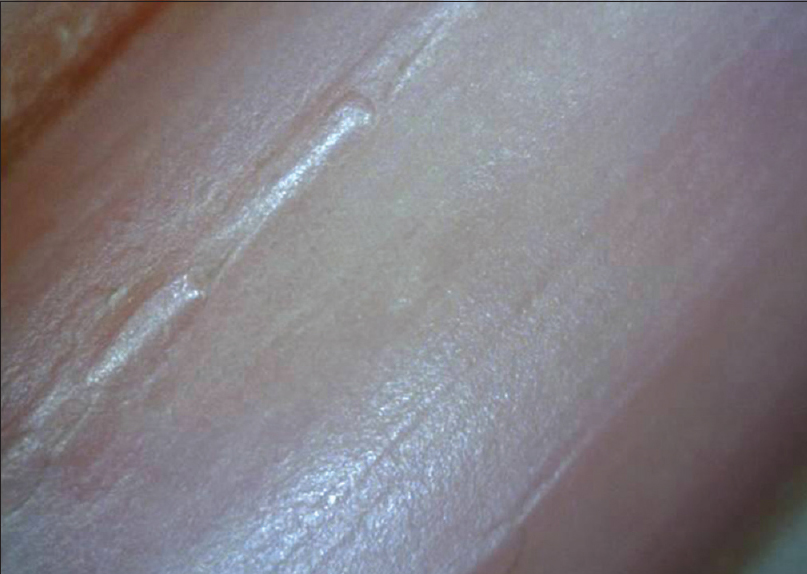 |
| Figure 1a: Examination of nail plate surface at low magnification (20X). Nail plate examined under nonpolarized light shows the surface irregularities in the form of longitudinal ridges and "sausage beads" |
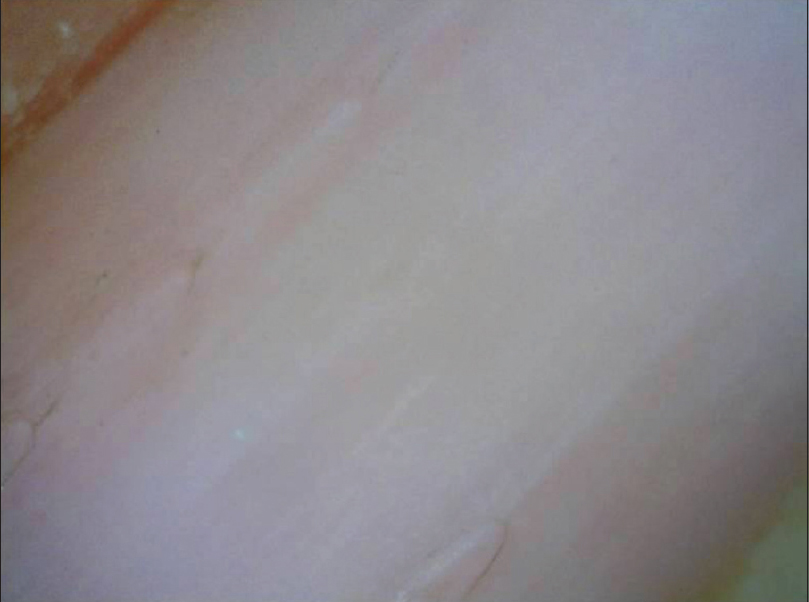 |
| Figure 1b: Examination under the polarized light tends to blunt out the nail plate surface irregularities which can now be seen only faintly (20X) |
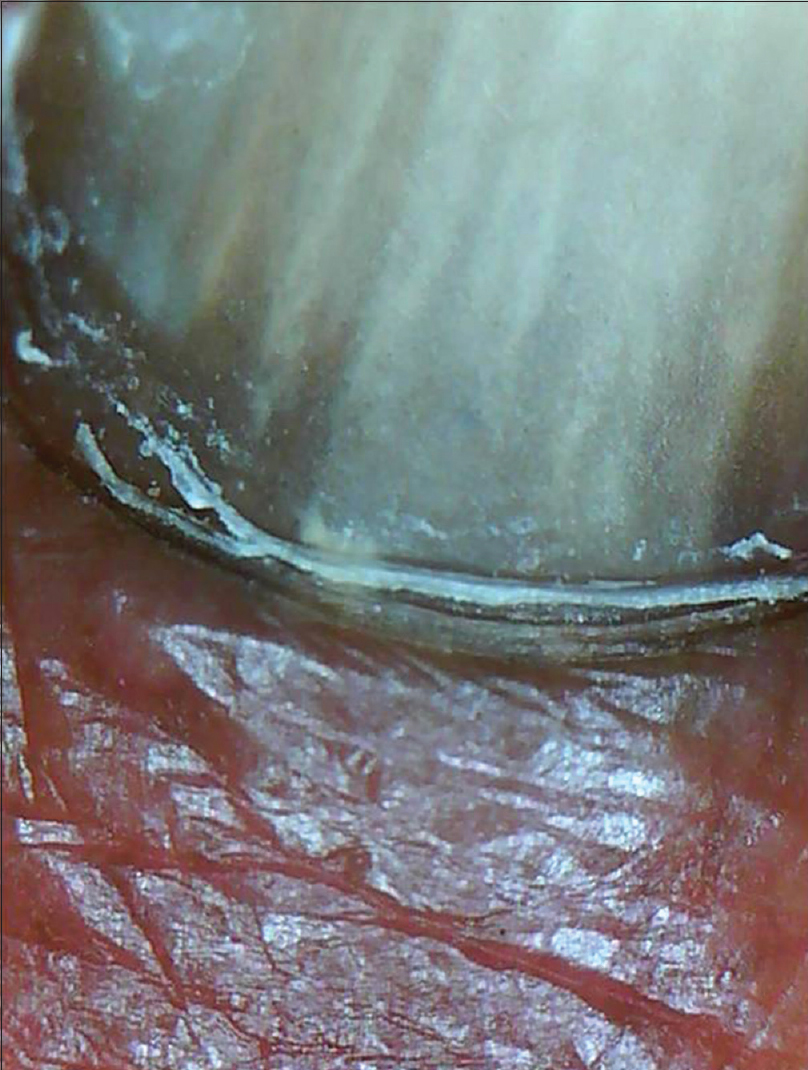 |
| Figure 2a: The color changes within the nail plate examined without an interface medium (50X) |
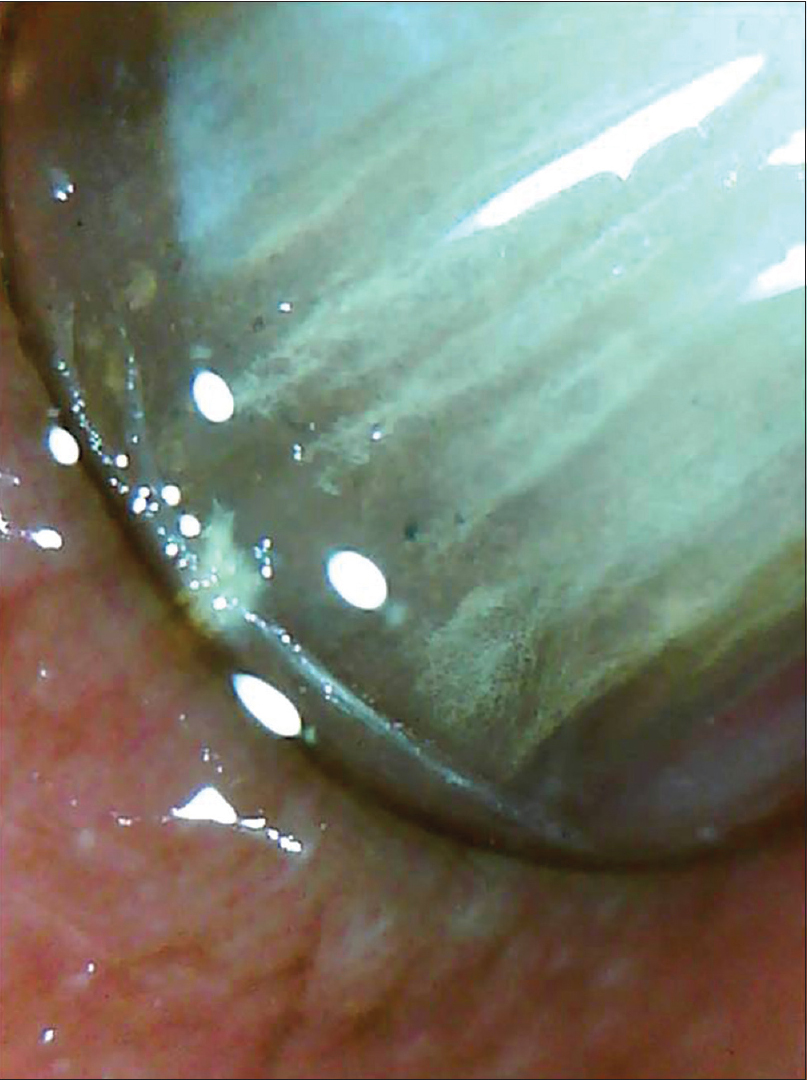 |
| Figure 2b: The changes are appreciably better outlined with the use of mineral oil as an interface medium (50X) |
For examining nail bed, an interface medium is needed to increase the transparency of nail plate. Normal nail bed appears pale pink with distal part showing some dilated capillaries, especially in manual workers. Proximal part may show the distinctly white nail matrix [Figure - 3]a-d.
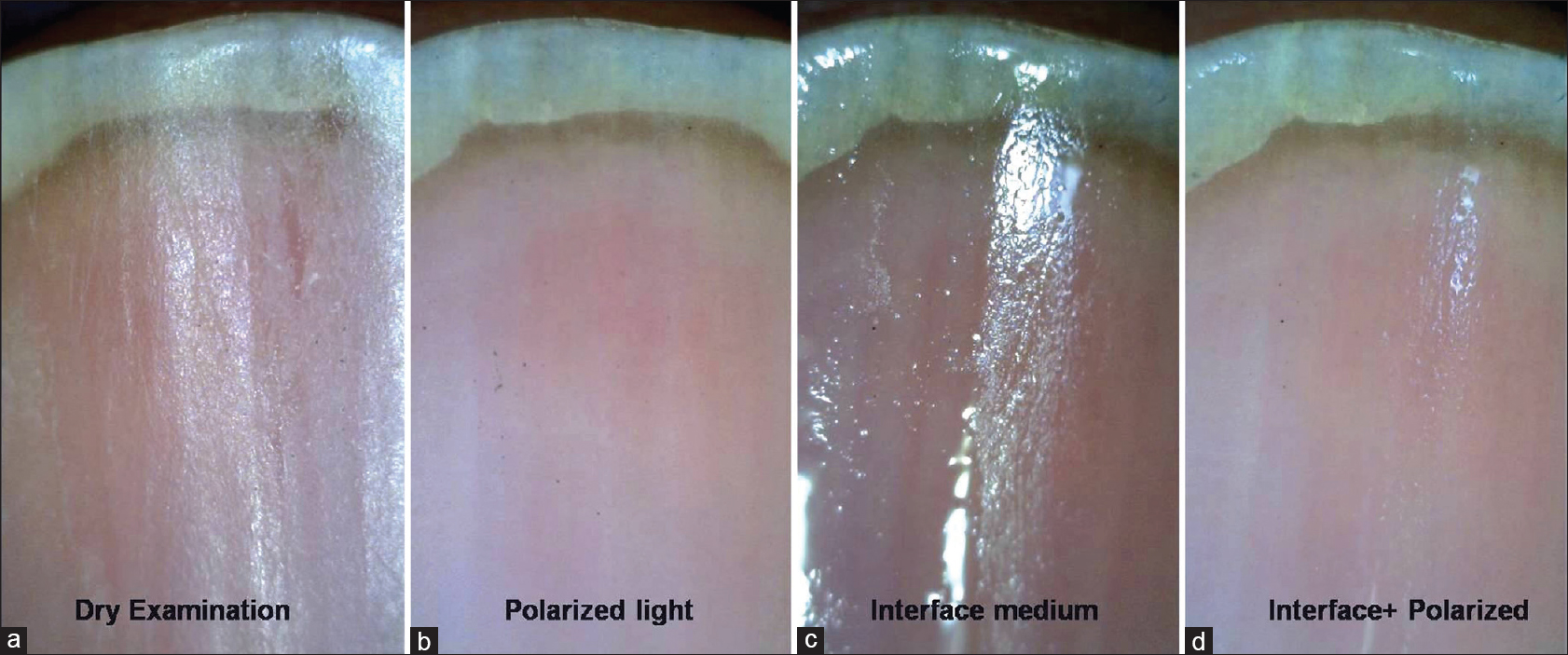 |
| Figure 3: Onychoscopy of Nail bed (50X). When a dry examination is done, it is difficult to visualize the nail bed, rather nail plate surface changes can be appreciated (a). With polarized light, the nail bed details are better visualized (b). With the use of interface medium, nail plate is rendered even more transparent showing the nail bed details (c). Best visualization is with use of interface with polarization (as seen in the last panel) which enhances transparency as well as cuts-off the surface glare (d) |
Examination of the hyponychium requires the dermatoscope to be slanted and this maneuver may need practice. At lower magnification (×10–30), the nail plate structure, digital creases and subungual contents are seen [Figure 4a] and [Figure 4b], whereas at higher magnification (×70–100), the detailed capillary architecture can be visualized.
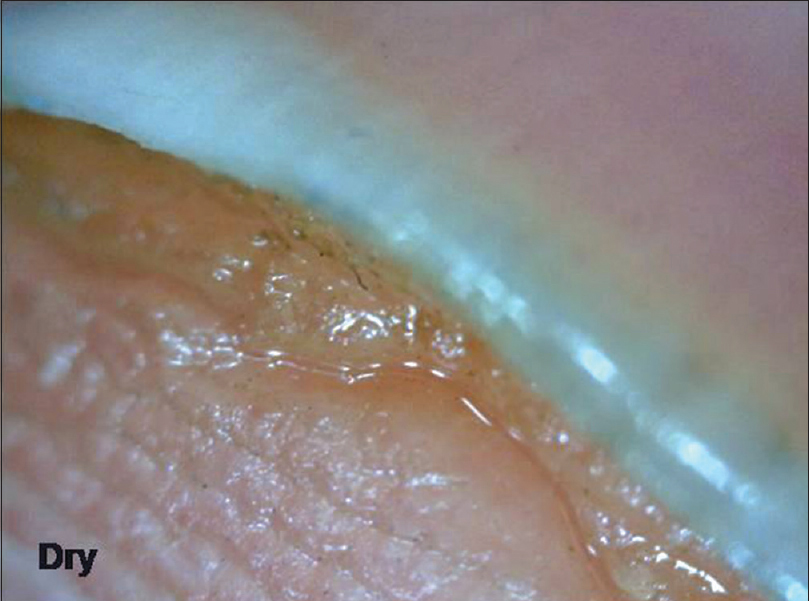 |
| Figure 4a: Hyponychium with a prominent solehorn as seen under polarized light without any interface medium (50X) |
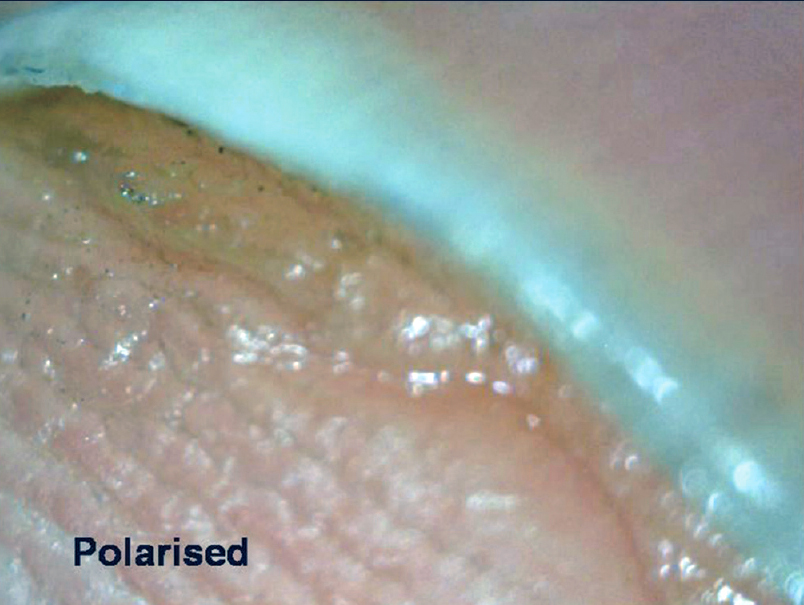 |
| Figure 4b: The same focus being seen with an interface medium under polarized light (50X) |
At low magnification, the proximal nail fold has a smooth, pale pink surface with the cuticle seen as transparent transverse band sealing the plate. At higher magnification, horizontally oriented capillary loops are seen [Figure 5a] and [Figure 5b] which flow parallel to skin surface (each resembling a hairpin) with a uniform morphology and homogeneous alignment. Up to 5% of capillaries may have a tortuous morphology. The nail fold capillary density and architecture are important markers evaluated in various connective tissue disorders (CTD).
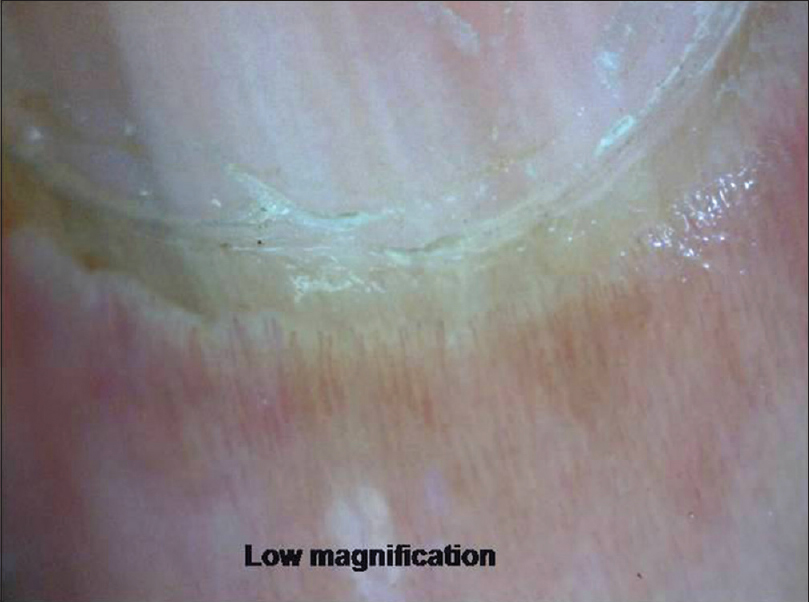 |
| Figure 5a: Examination of proximal nail fold area. At lower magnification (50X), the closely spaced capillary loops are seen |
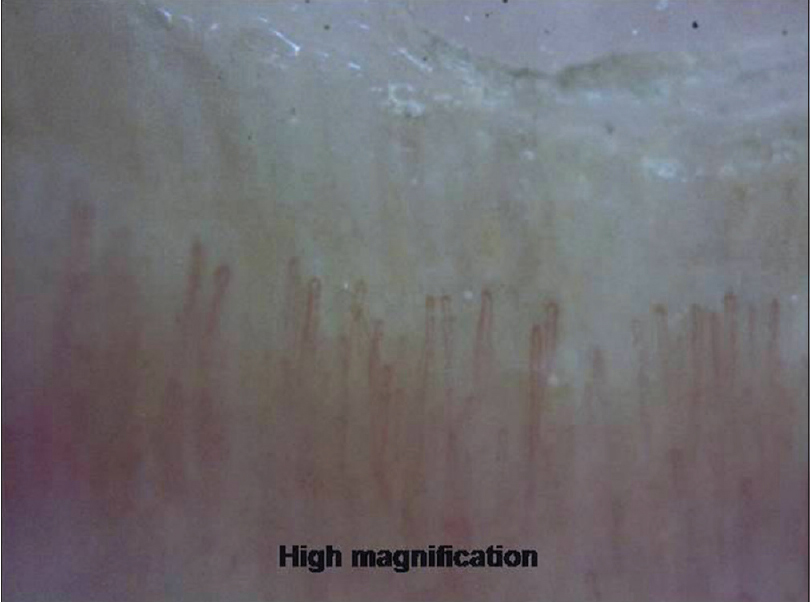 |
| Figure 5b: Examination of proximal nail fold area. At higher magnification (200X), the architecture of vessels can be appreciated |
The nail matrix is not visible in most nails. It can be partly visible in larger nails such as thumb or great toe or may be visible intraoperatively, after removal of nail plate [Figure - 6]a-c.[10]
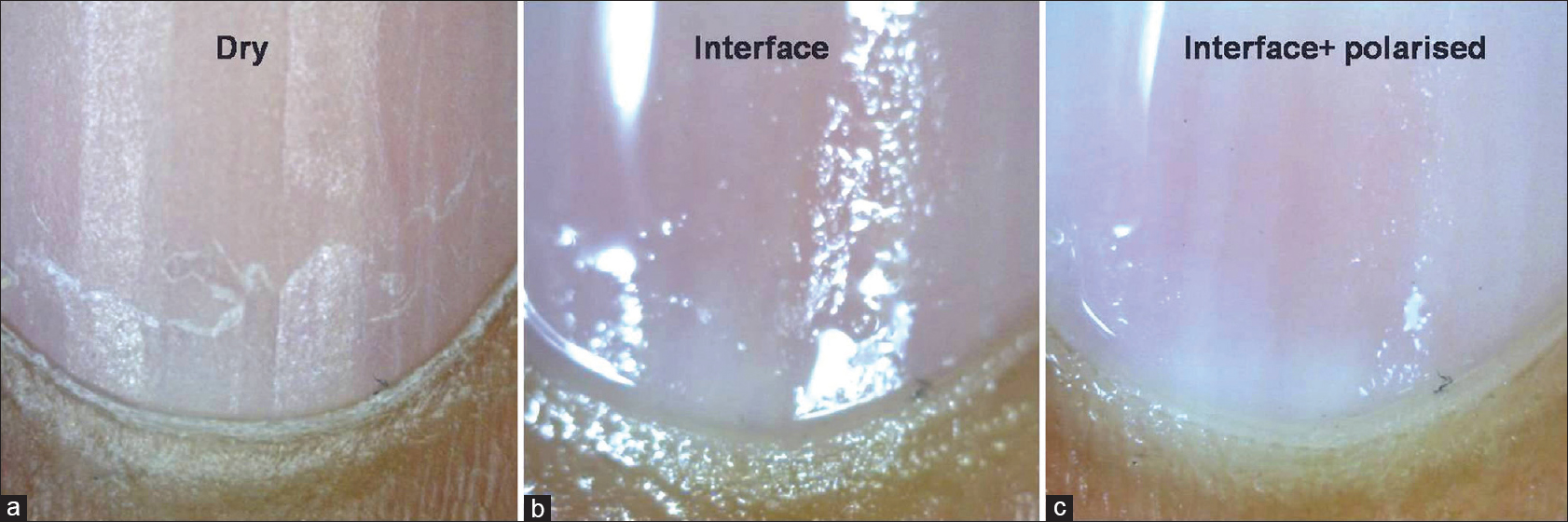 |
| Figure 6: Examination of proximal part of the nail bed. Dry examination shows detailed nail plate features (a). The use of interface medium shows underlying nail bed with the distal matrix or lunula (b). Interface medium with polarization shows underlying nail bed with lunula (c) (50X) |
Methods and Results
A PubMed search pertaining to published articles was done using the keywords “onychoscopy,” “nail fold capillaroscopy,” “dermoscopy nail” and “dermatoscopy nail.” The search yielded 6, 59, 141 and 151 indexed articles, respectively, in English. These articles were retrieved and classified into case reports (79), review articles (65) and clinical studies of various types (38). The articles were read and information pertaining to onychoscopy was collected.
It was seen that onychoscopic features of nail unit tumors were mostly in the form of isolated reports with very few studies available. Even for dermatoses affecting the nail, there were limited studies of low quality with no comparative studies available. The clinical study designs used were heterogeneous with various limitations. The final data were analyzed and presented in a narrative fashion.
Discussion
Over the years we have gained an increasing clarity about onychoscopic characteristics of various onychopathies. Furthermore, newer indications and findings are being reported. As of now, the indications for onychoscopy include nail pigmentation, especially longitudinal melanonychia;[5],[7] onychomycosis;[11] nail psoriasis;[12] nail lichen planus;[13],[14] traumatic nail abnormalities;[12],[15] subungual hemorrhage;[16] nail tumors such as onychomatricoma [17],[18] glomus tumor [19] and CTD, especially systemic sclerosis.[20],[21],[22],[23]
Disorders of nail pigmentation
One of the earliest and most common indications for onychoscopy is evaluation of nail pigmentation. Melanonychia is pigmentation of nail plate due to melanin; however, many nonmelanin pigments may also deposit in nail and are diagnostic confounders.[24]
Longitudinal melanonychia
A noninvasive diagnostic algorithm for onychoscopic evaluation of longitudinal pigmented bands has been proposed by Tosti and Argenziano.[7] The first step is to ensure that the pigment is melanin and not blood (seen as rounded globules with filamentous distal edges).[25] The next step is to assess whether melanin is a result of melanocyte activation (gray bands) or proliferation (brown-black bands) [Figure 7a] and [Figure 7b].[5],[24] Bands caused by melanocyte activation are benign and need not be evaluated pathologically.[26] Melanocyte proliferation is suggested by melanin granular inclusions (<0.1 mm) seen at higher magnification [Figure 7c].[7] The last step is to establish whether the melanocyte proliferation is benign or malignant.[27] Benign proliferations produce regular patterned, brown-black bands with uniform color and thickness, regularly spaced and parallel placed [Figure 7d].[28] Malignant proliferation is suggested by irregular patterned, varying colored, irregular spaced, bands with varying thickness and evidence of crossing over of lines.[28] However, these criteria are relative and may not be valid in children [24] and even in some adults.[27] Histopathological confirmation should always be done in doubtful cases.[6]
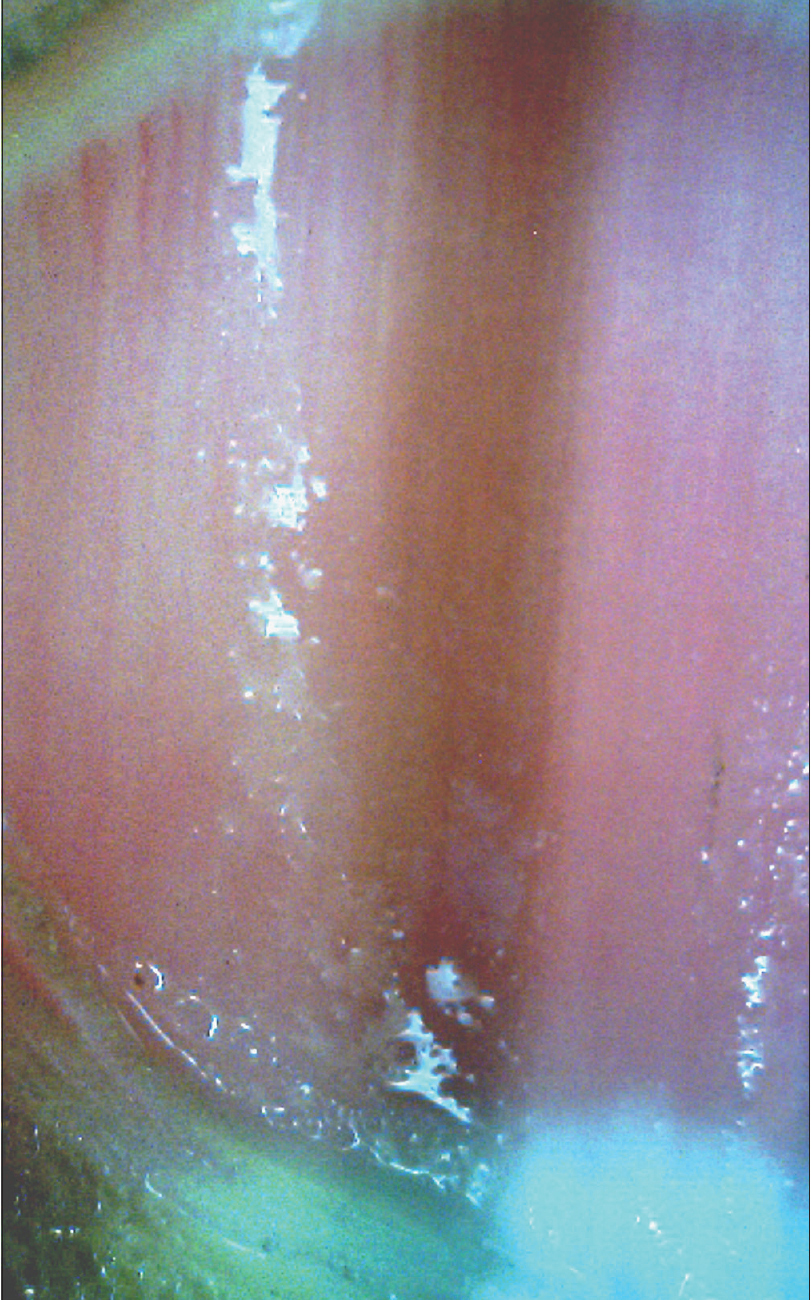 |
| Figure 7a: Onychoscopic evaluation of longitudinal pigmented bands. Longitudinal pigmented band suggestive of melanocyte activation. The bands are gray in color with not much variegation. Such bands may need no pathological evaluation (50X) |
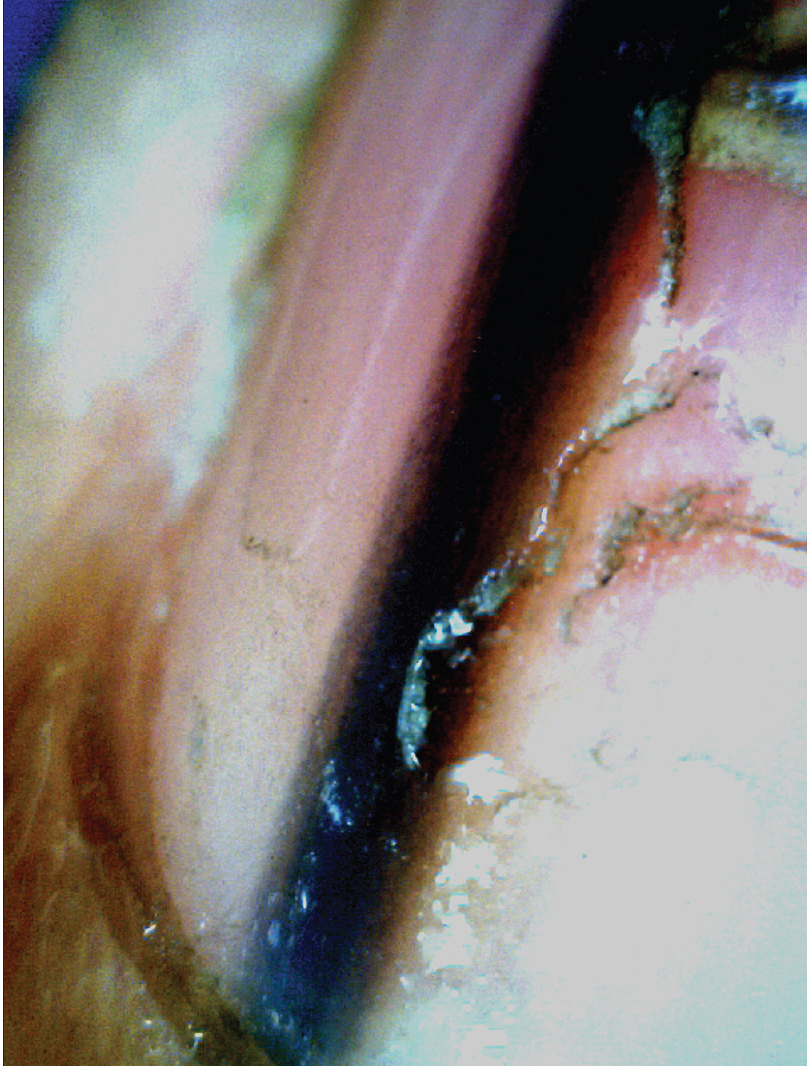 |
| Figure 7b: Onychoscopic evaluation of longitudinal pigmented bands. Longitudinal pigmented band suggestive of melanocyte proliferation. The band is regular patterned and black in color. An overall uniformity of color and thickness is seen (50X) |
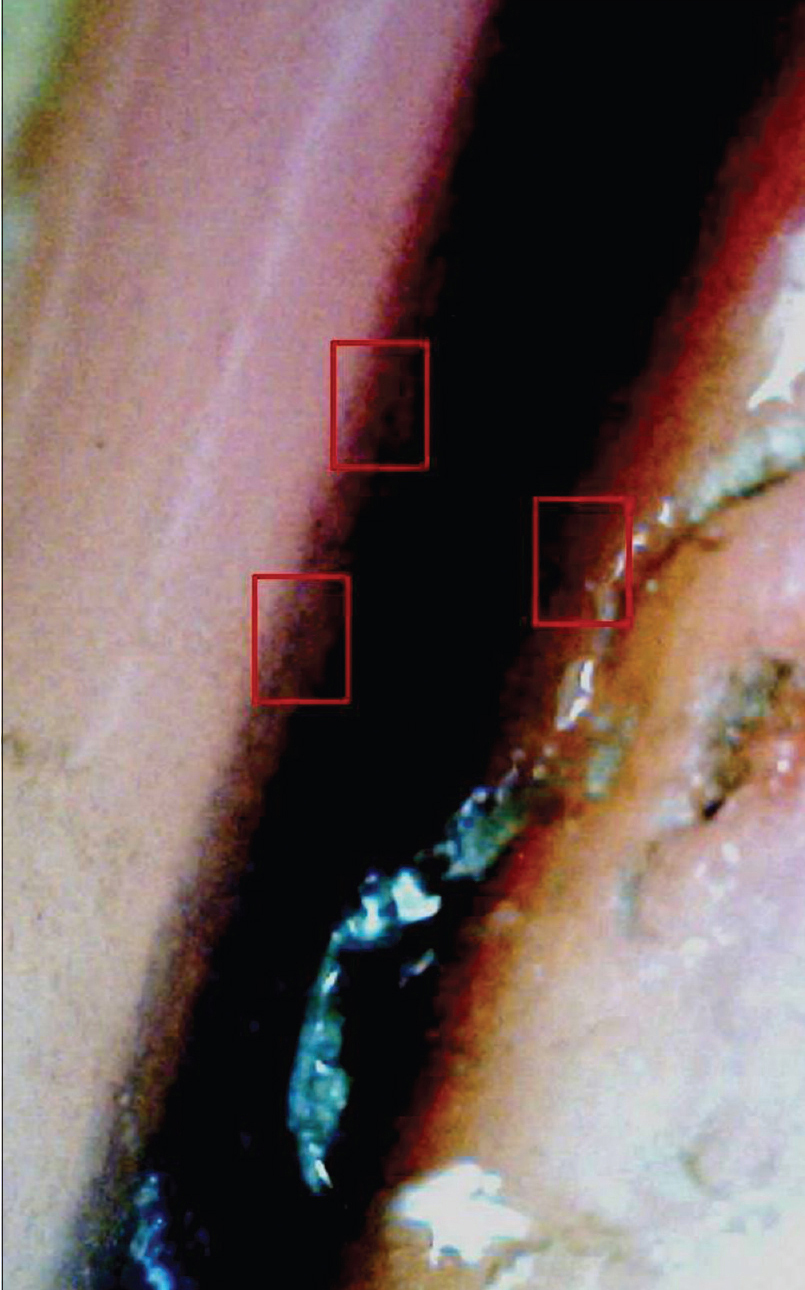 |
| Figure 7c: Onychoscopic evaluation of longitudinal pigmented bands. Higher magnification of the same band shows melanin granular inclusions at the periphery (within red boxes) (150X) |
 |
| Figure 7d: Onychoscopic evaluation of longitudinal pigmented bands. A much broader longitudinal pigmented band of brown to black is seen. Although there is significant color variation, the pigmented lines are seen to be arranged in a parallel pattern with no crisscrossing (50X) |
Onychoscopy can also help visualize the origin of the pigment. This can be through intraoperative, nail bed or matrix dermoscopy (with noncontact, polarized dermatoscope). Another way is to examine the free edge of nail plate [Figure 7e]. For pigment located in the upper portion of free edge of nail plate, the source is likely to be in the proximal matrix whereas for pigment in the lower portion, the origin is probably in the distal matrix.[10],[24],[29]
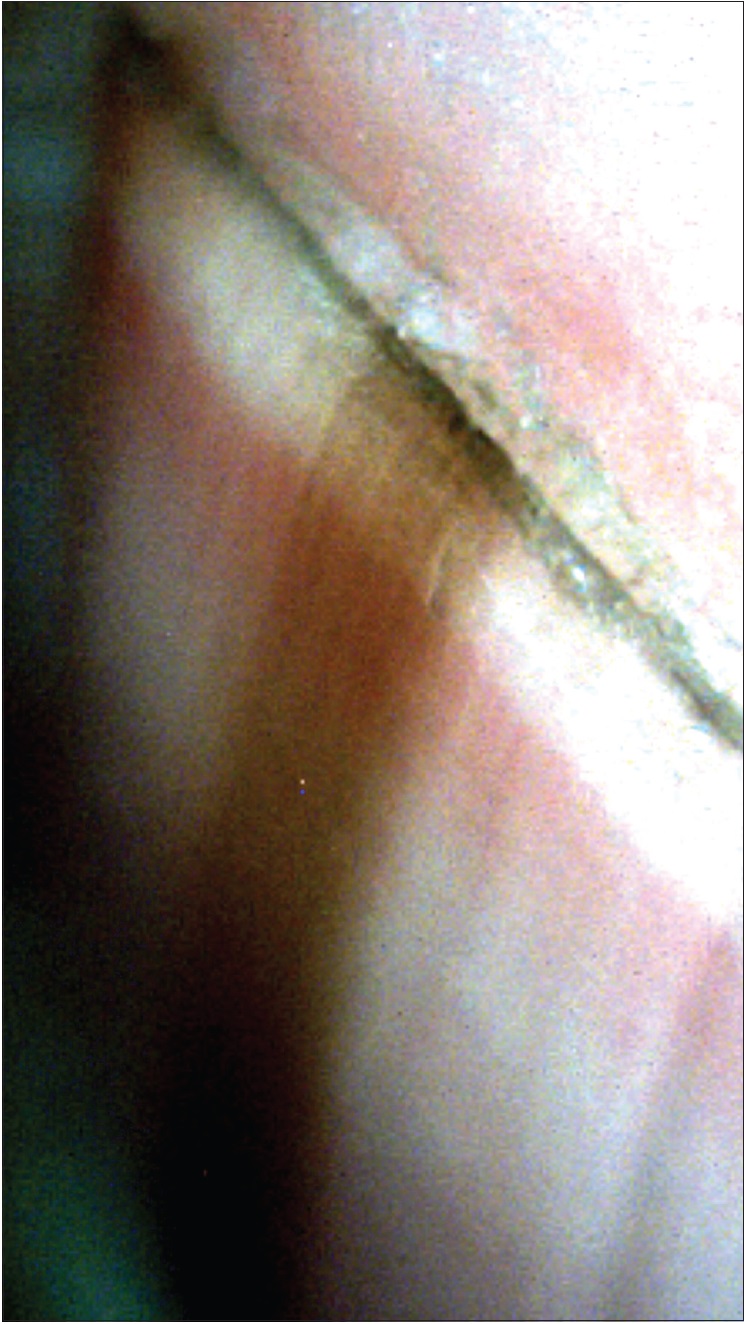 |
| Figure 7e: An evaluation of the distal free edge of the nail plate shows that the pigment is more prominent in the dorsal part of the nail plate. This suggests that the source of the pigment lies in the distal matrix (50X) |
Melanocytic nevi
Nail melanocytic nevi generally presents with longitudinal pigmented band. The suggestive onychoscopic features include brownish discoloration of background with pigmented lines that are parallel, regular in color, width and spacing [Figure - 8].[25],[30],[31] Pigmentation may be quite dark, making it difficult to observe the nature of pigmented lines.[28] A subungual blue nevus may also be visualized as bluish discoloration of the nail plate.[32]
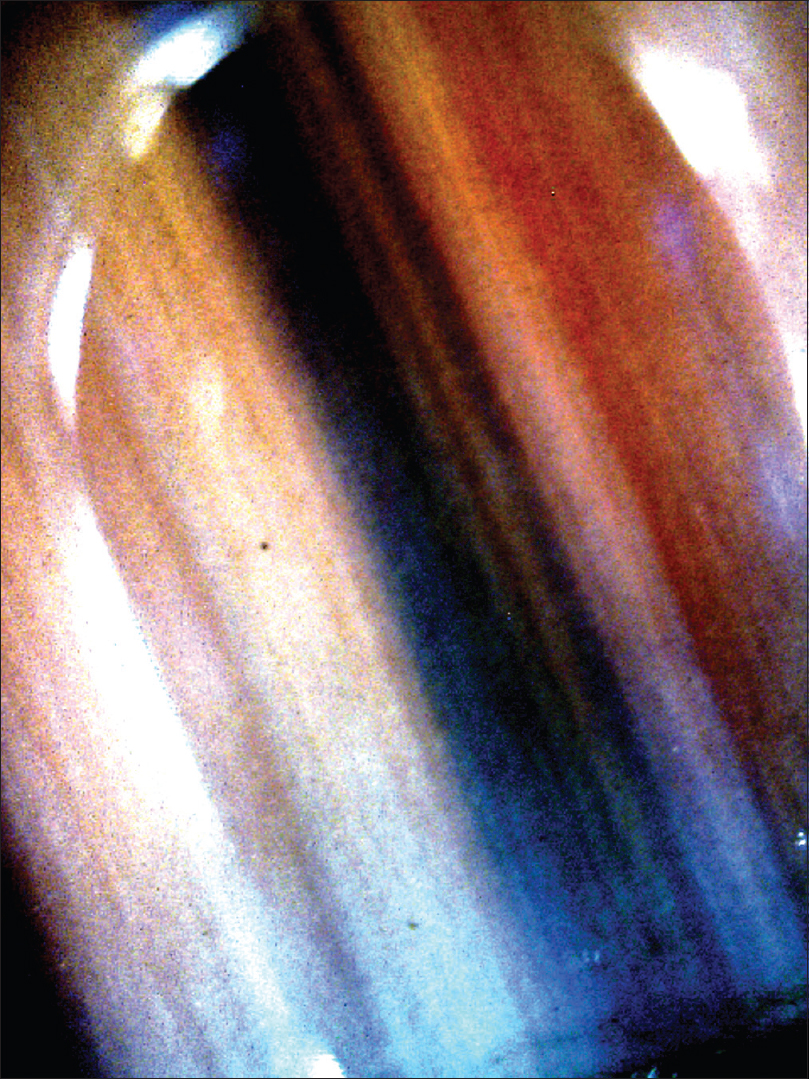 |
| Figure 8: Longitudinal pigmented band with brownish background discoloration and pigmented lines which are parallel, regular in color, width and spacing. This picture is suggestive of a nail matrix nevus |
Nail apparatus melanoma
The onychoscopic features favoring melanoma include micro-Hutchinson sign and irregular lines, crisscrossing or stopping abruptly.[6] Some cases may have a background brownish color.[5],[6] Onychoscopy make the differentiation between micro-Hutchinson sign (pigmentation of the cuticle visible only on dermoscopy and not clinically) from pseudo-Hutchinson sign (nail plate pigmentation visible under a translucent cuticle) easier.[24] The pigmented lines are irregular in parameters such as color, spacing, thickness and distribution (loss of parallel arrangement). Other suggestive features include a band which is thicker proximally and thinning out distally (suggesting a rapidly growing melanoma).[33] The presence of blood does not rule out melanoma as it may be entirely coincidental. A subtle feature is the presence of erosion or microscopic grooving of nail plate surface best appreciated on onychoscopy.[5],[28]
Diagnosis of amelanotic melanoma can be very challenging. In suspected cases, onychoscopy may aid identification of pigment remnants in nail fragments, helping differentiation from other nonmelanocytic tumors or pyogenic granuloma. Polymorphic vascular pattern with milky red areas and red spots can also be seen.[34]
Inflammatory nail disorders
Nail psoriasis
The clinical as well as onychoscopic findings vary depending on the part of the nail unit affected.[35] The nail plate characteristics include pitting in the form of irregularly sized and shaped depressions which may be surrounded by whitish halo [Figure 9a], [Figure 9b], [Figure 9c], [Figure 9d]. Longitudinal or transverse ridges and nail plate crumbling may also be seen. Characteristic nail bed features include onycholysis surrounded by an erythematous border [Figure 10a] and [Figure 10b]; salmon patch [Figure 11a] and [Figure 11b]; pustular lesions and splinter hemorrhages [Figure - 12]. The erythematous border of onycholysis shows globose, dilated blood vessels surrounded by a prominent halo [Figure 10a] and [Figure 10b].[36] Salmon patches are irregular in size and shape and variable in color (red to orange) [Figure 11a] and [Figure 11b]. Splinter hemorrhages are longitudinal brown, black or purple linear hemorrhages oriented along the direction of nail growth [Figure - 12]. The hyponychium and proximal nail fold show characteristic vascular pattern of psoriasis (irregularly distributed, dilated and tortuous capillaries) [Figure - 13].[37] In pustular psoriasis, nail bed may show small pustules, scaling, hemorrhages and dilated vessels.
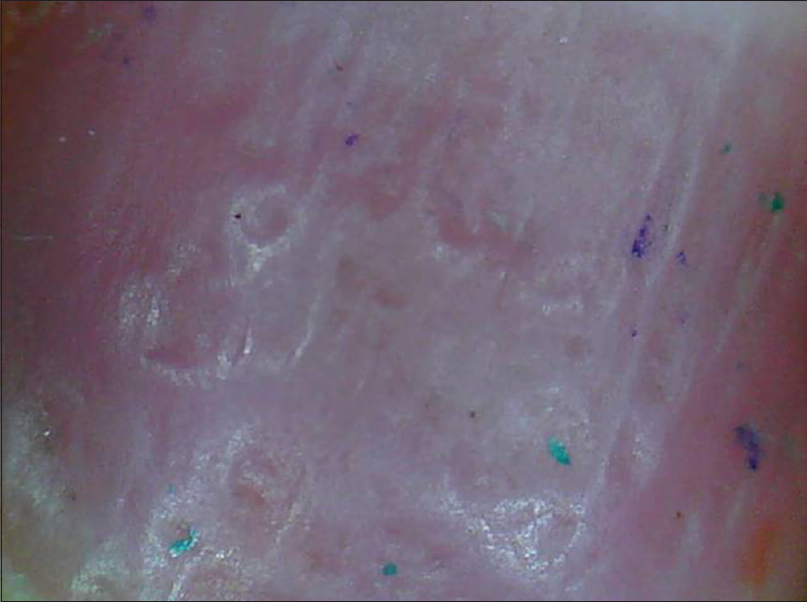 |
| Figure 9a: Nail plate pits. As seen on 'dry examination', the pits are prominent, irregular in size, depth and distribution (50X) |
 |
| Figure 9b: Nail plate pits. On 'wet examination', the glare may make the pits less visible (50X) |
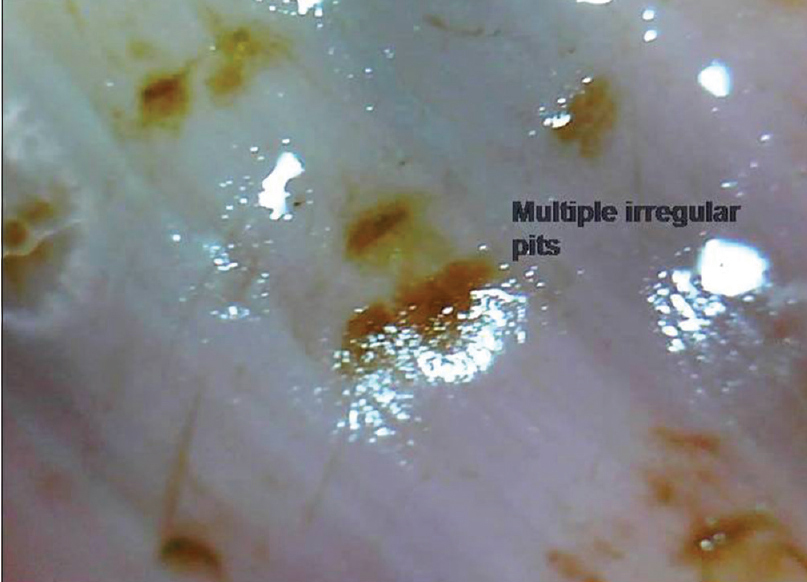 |
| Figure 9c: Nail plate pits. Another representative case where pits are seen prominently due to entrapped dye (henna) (50X) |
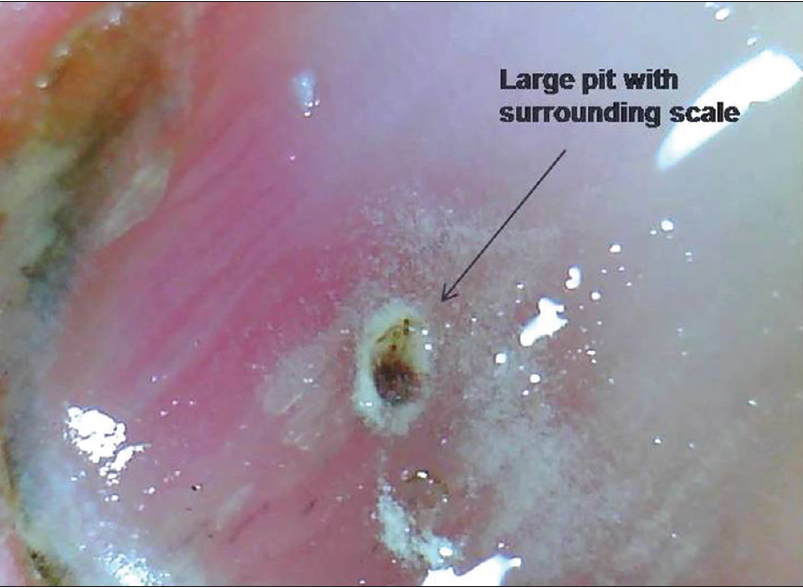 |
| Figure 9d: Nail plate pits. The surrounding scale is prominently apparent around this large pit (50X) |
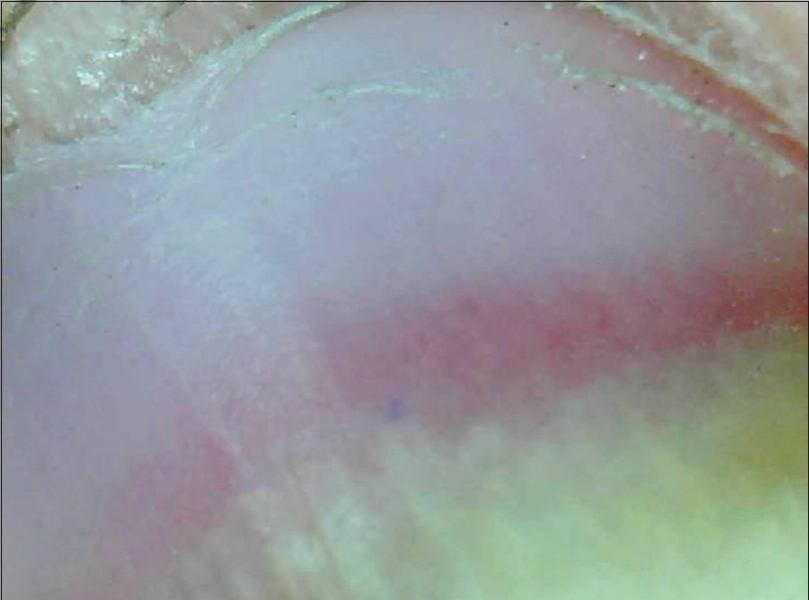 |
| Figure 10a: Distal onycholysis with a prominent erythematous border (50X) |
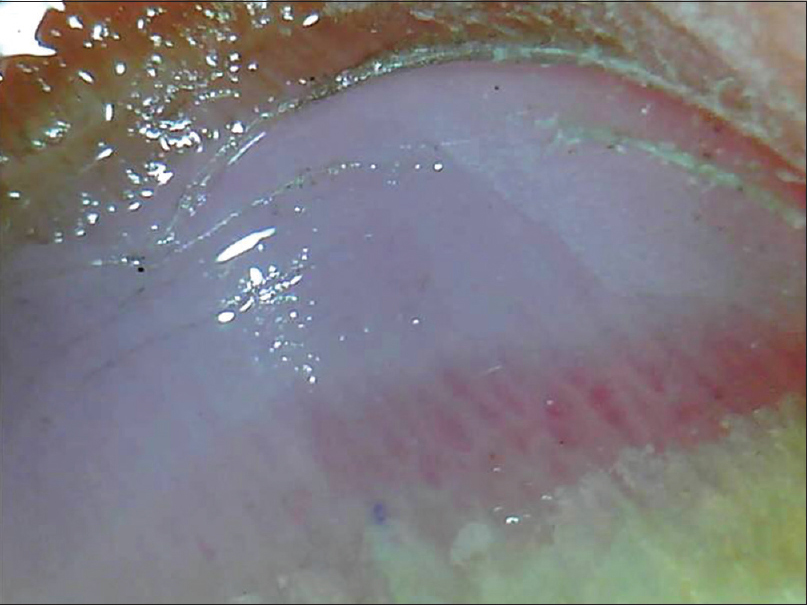 |
| Figure 10b: The erythema is better delineated with the use of interface medium to show globose, dilated blood vessels surrounded by whitish halo (50X) |
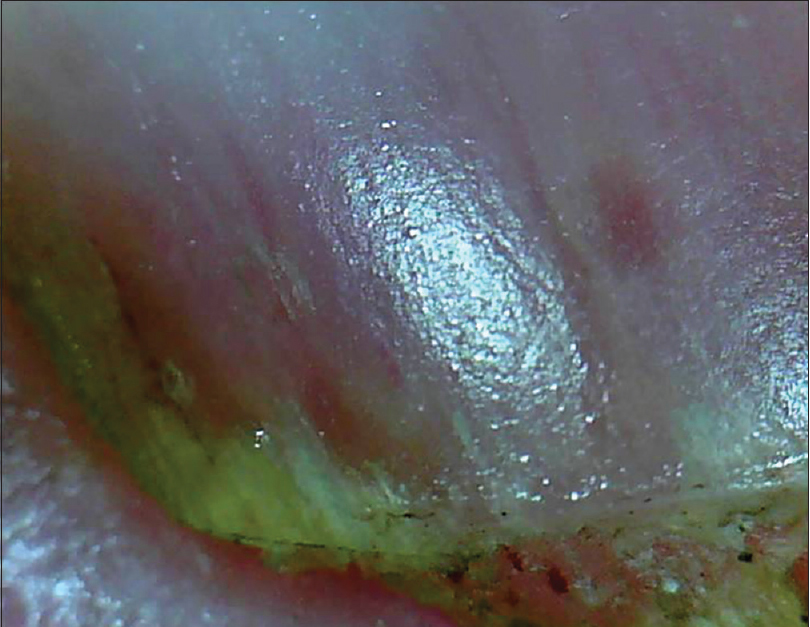 |
| Figure 11a: Salmon patch as seen on onychoscopy. These are irregular in size and shape and may vary in color (50X) |
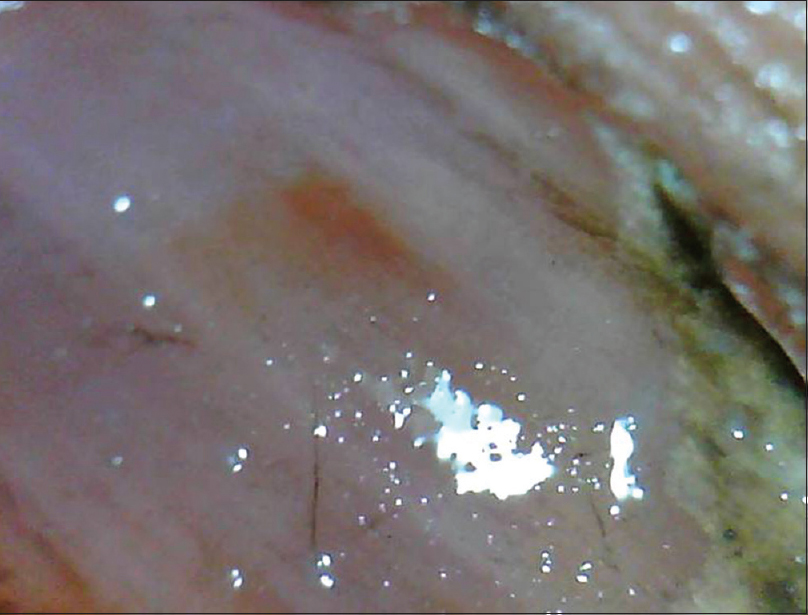 |
| Figure 11b: Salmon patch. Another representative case with a larger patch (50X) |
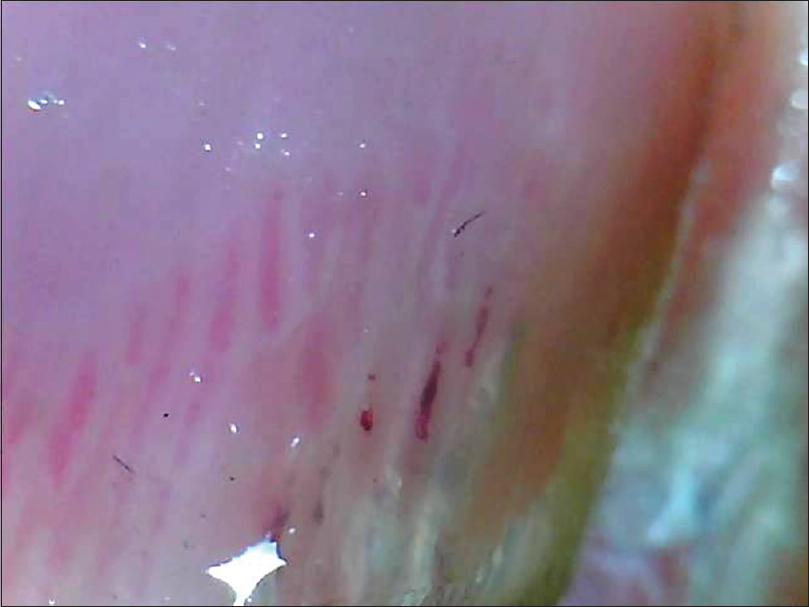 |
| Figure 12: Splinter hemorrhages seen as longitudinal red to brown hemorrhages, oriented linearly (50X). The darker linear bands are the splinter hemorrhages. The fainter erythema is the erythema in the nail bed and not a hemorrhage |
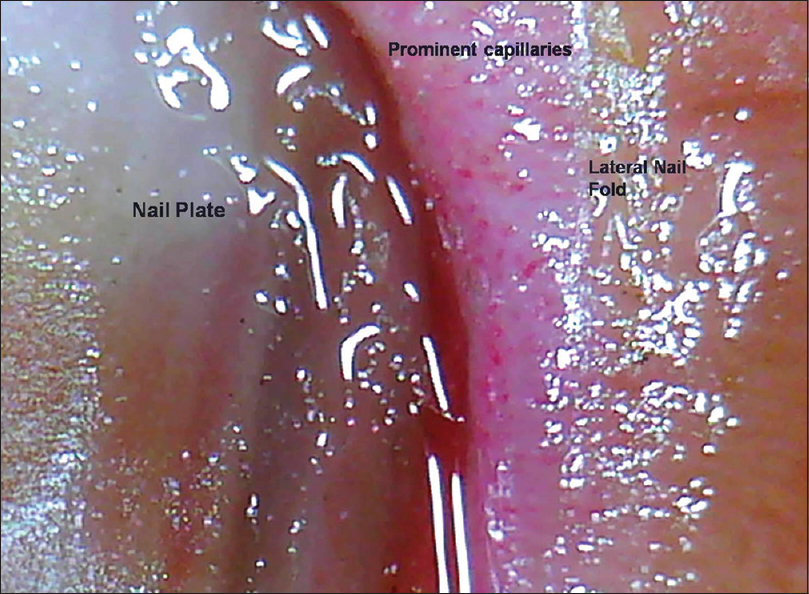 |
| Figure 13: The characteristic vascular pattern of psoriasis seen as irregularly distributed, dilated and tortuous capillaries in the lateral and proximal nail fold (50X) |
Nail Lichen planus
In a study of 79 affected nails, the onychoscopic abnormalities demonstrated were of nail matrix, bed and paronychial origin.[13] Nail matrix features seen were trachyonychia (in 40.6% cases), pitting [Figure 14a] and [Figure 14b] (34.2%), pterygium (21.5%) and red lunula (3.8%). Nail bed features highlighted were chromonychia (55.7% cases) [Figure 15a] and [Figure 15b], nail fragmentation (50.6%) [Figure 15a] and [Figure 15b], splinter hemorrhages (35.4%), onycholysis (27.8%) and subungual keratosis (7.5%). Paronychia was seen in 31.6% cases although no capillaroscopic changes were observed. Others features were longitudinal streaks and anonychia.[13] It was concluded that nail bed features and converging longitudinal streaks are suggestive of aggressive characteristics, indicating a poor prognosis and possibly a poor response to therapy.[13] These could help decide whether or not to initiate aggressive therapy.
 |
| Figure 14a: Pitting can be appreciated in a case of nail lichen planus. Very superficial numerous pits are seen more commonly (20X) |
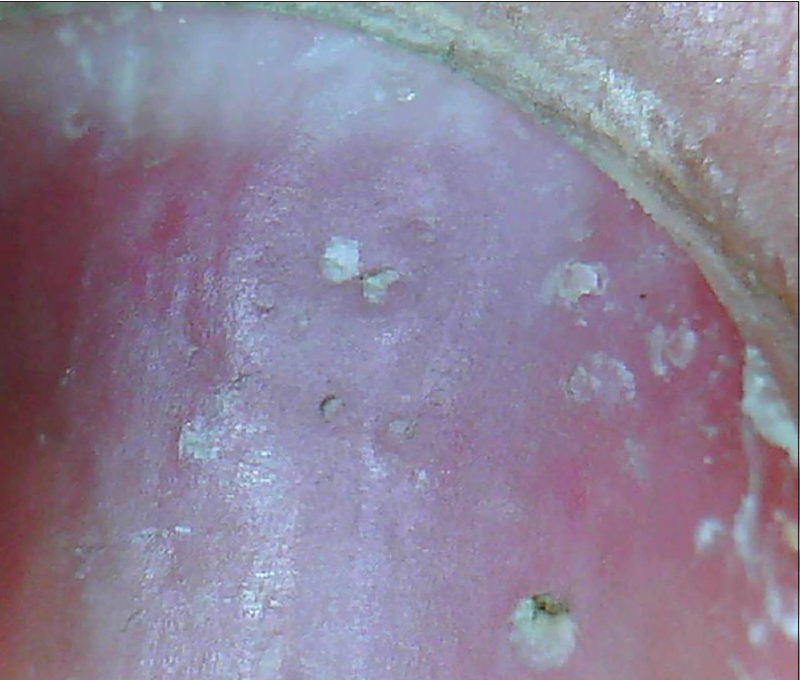 |
| Figure 14b: Pitting in Nail lichen planus. Occasional cases may have larger, deeper pits (50X) |
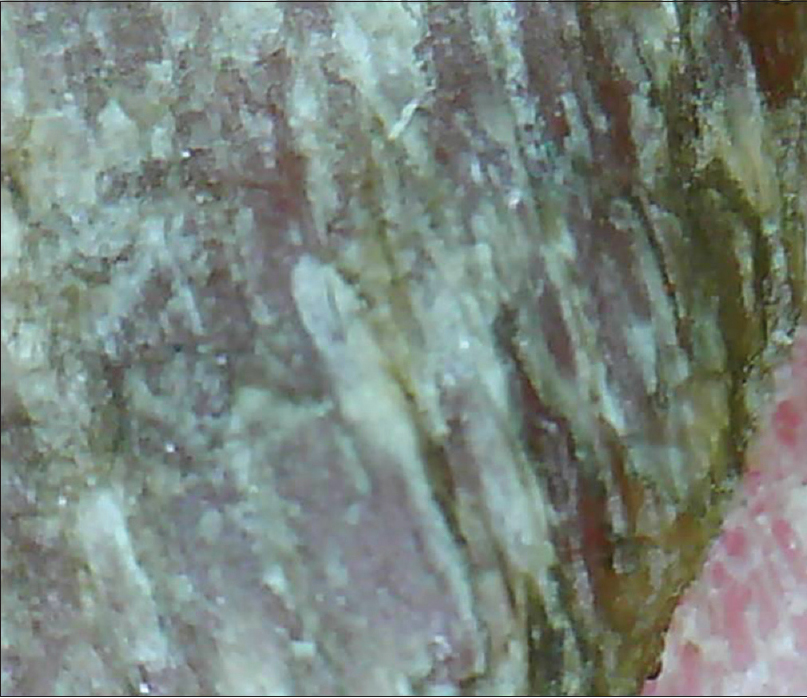 |
| Figure 15a: Chromonychia in a case of severe nail lichen planus. Examination without interface medium shows varying colors of the nail plate (150X) |
 |
| Figure 15b: Chromonychia in a case of severe nail lichen planus. Accentuated with the use of mineral oil. The fragmentation of the nail plate can also be appreciated (150X) |
Infective nail disorders
Onychomycosis
The salient onychoscopic features include jagged proximal edge of onycholytic area; “spikes” in onycholytic areas [Figure 16a] and [Figure 16b]; white-yellow longitudinal striae within the onycholysed nail plate and parallel bands of varying colors (“Aurora Borealis” pattern).[38] The other not so common features include blackish dots and globles, matte homogenous color of detached nail plate and dryness and scaling of adjacent skin. Piraccini et al. suggested that onychomycosis can be differentiated from traumatic onycholysis as the latter has a linear edge instead of a jagged edge.[11],[38] However, Jesús-Silva et al. reported a linear edge in 13.5% of patients with onychomycosis concluding that onychoscopy may not be the sole diagnostic criterion.[39] They also described a distinctive pattern of distal irregular thickening in patients with total dystrophic and distal subungual onychomycosis.[39] De Crignis et al. described “ruin appearance” subungual keratosis along with “distal to proximal subungual longitudinal spikes” as characteristic feature in ten cases with distal subungual onychomycosis.[40]
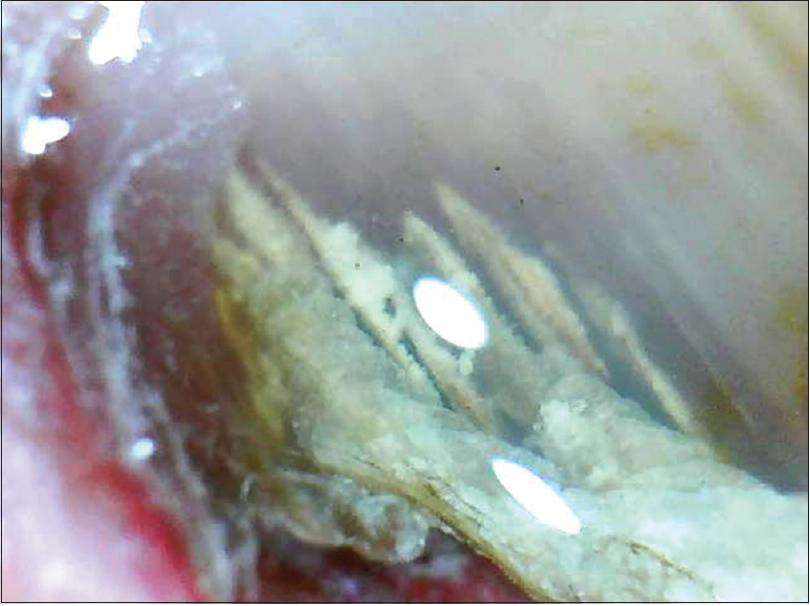 |
| Figure 16a: Onychomycosis (150X). The distal onycholysis with a typical "jagged proximal edge" can be appreciated |
 |
| Figure 16b: Onychomycosis (150X). The distal onycholysis with a typical "spike pattern" can be appreciated |
Pseudomonas infection
Features suggestive of a pseudomonas infection include greenish discoloration of nail plate with proximal chronic paronychia and onycholysis [Figure - 17]. Peripheral fading of the green colour may also be appreciated.[15]
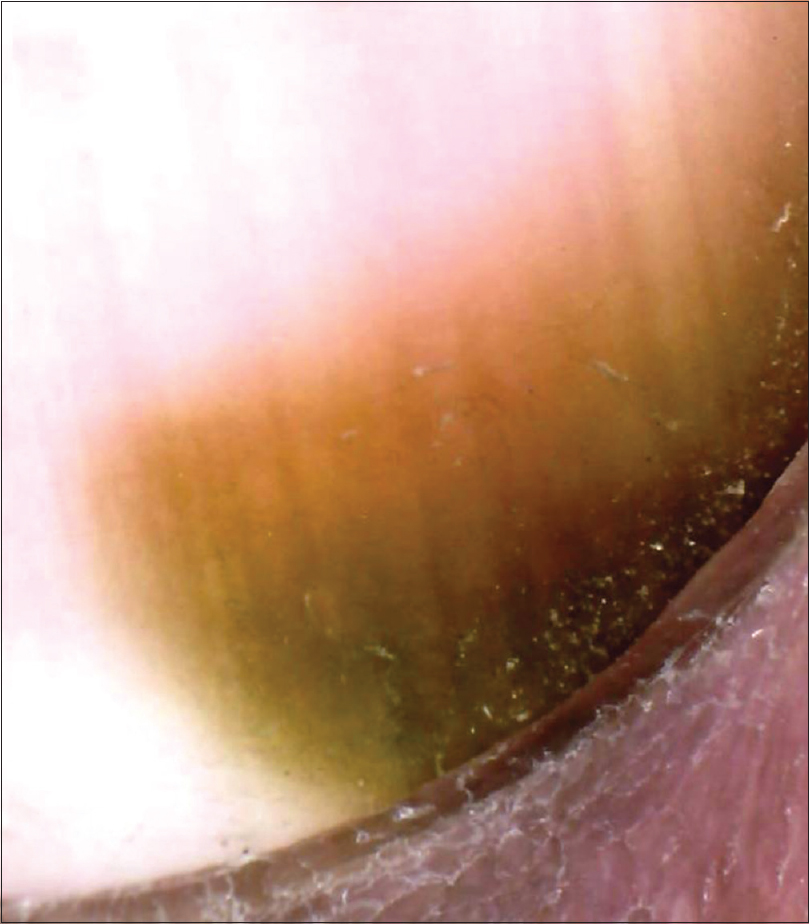 |
| Figure 17: Pseudomonas infection. Greenish discoloration seen arising near the proximal nail fold. Disrupted cuticle can also be appreciated (150X) |
Warts
Recognition of warts, especially the early stage lesion, is a distinct advantage offered by onychoscopy.[41] Dermoscopic and vascular patterns typical for warts have been described in various locations.[41] Small periungual lesions which clinically appear as scaly papules can be diagnostically identified on dermoscopy with the help of dilated and thrombosed vessels [Figure - 18].[15]
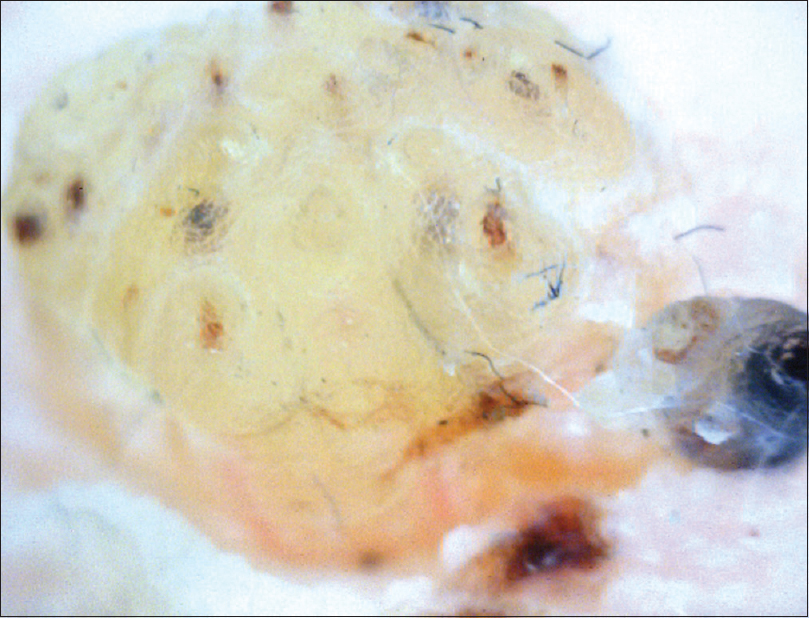 |
| Figure 18: Periungual wart involving the distal nail fold (200X). The hyperkeratotic structure with numerous punctate capillaries and small hemorrhages can be appreciated |
Traumatic nail disorders
Subungual hematoma
These are visible as well-delineated globular structures with color variation ranging from black, purple, red to brown [Figure - 19]. The color generally fades toward periphery. The globules are rounded proximally and streaked or filamentous distally.[16] Periungual hemorrhages may also be present. Recent hematoma tends to be deeply located, reddish purple with irregular margins; whereas older hematomas are more superficial, roundish and deep red.[16] Mostly, the hemorrhage grows out progressively with the growth of nail plate; hence, a follow-up of 3–4 months clarifies the issue. However, in some cases, it may not migrate, raising suspicion of subungual melanoma. In a study comparing onychoscopic feature of subungual melanoma and subungual hematoma, it was found that Hutchinson's sign (seen in 100% cases with melanoma); longitudinal irregular bands (81%); triangular structure of bands (25%) and vascular pattern (6%) were signs specifically associated with melanoma. These were absent in ninety cases of subungual hematomas examined. In addition, subungual hematomas exhibit variety of colors, even combination of more than one color (80% cases). Thus, onychoscopy is a useful investigation in this scenario.[16]
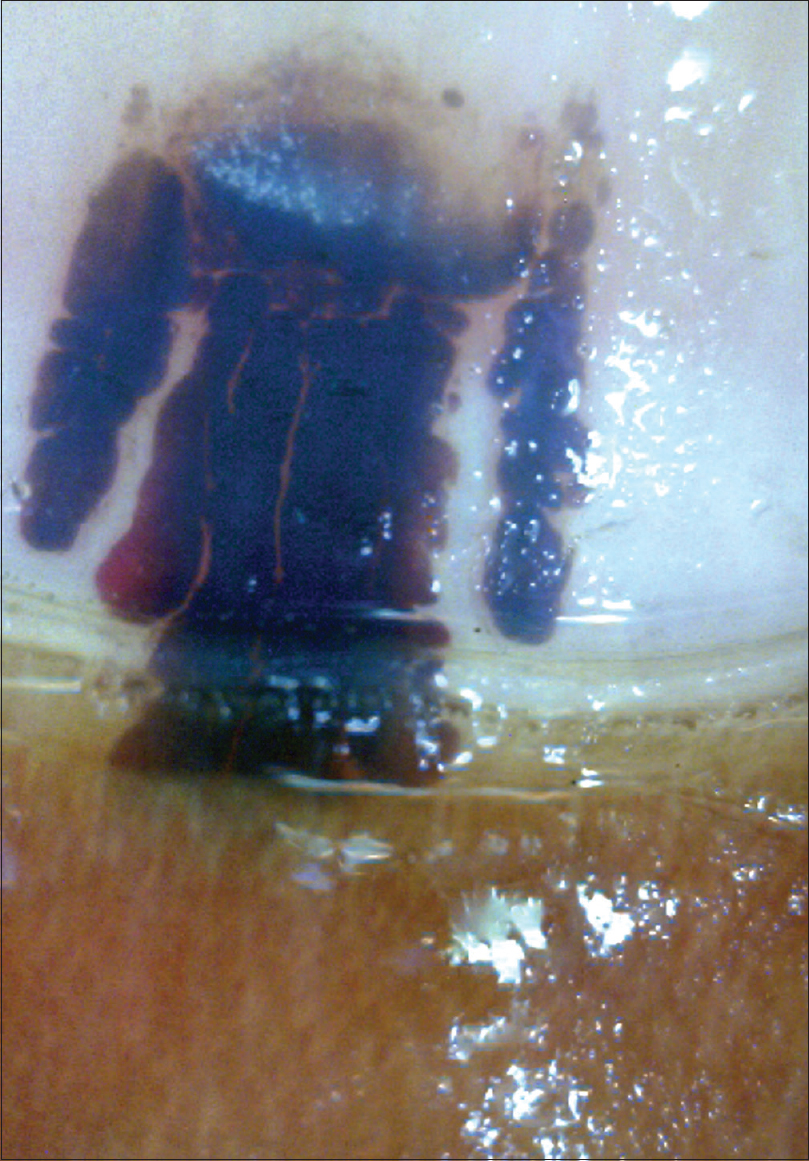 |
| Figure 19: Subungual hematoma at low magnification (50X). The globose erythema with peripheral fading of color can be appreciated |
Nail biting
Onychoscopic features of nail biting include scaling and crusting of nail folds, shortening of nail plate, exposure of nail bed epithelium and presence of hemorrhages.[15]
Nail tumors
Onychomatricoma
It is a rare benign tumor of matrix, presenting with a classical tetrad of xanthonychia, ungual hyperkeratosis, splinter hemorrhages and transverse plus longitudinal over-curvature of nail plate. Onychoscopic examination of 34 cases revealed the commonly present features in the form of longitudinal parallel white lines, splinter hemorrhages, dark dots, free-edge nail pitting and thickening. It was suggested that as dermoscopic features for this tumor are more frequently present and less subject to divergent interpretation, they could be a reliable diagnostic criteria for this condition.[18]
Glomus tumors
Glomus tumor represents 1%–5% of soft tissue tumors of hands. Diagnosis can be accomplished by clinical examination and imaging. Onychoscopy can be helpful in some cases by revealing discrete linear vascular structures suggestive of glomus;[19] however a more useful role is intraoperative (after nail plate removal). It can help localize the lesion, delineate surgical margins, thus ensuring complete removal and minimizing recurrence.[19]
Onychopapilloma
Clinically, this tumor presents as longitudinal erythronychia, leukonychia or melanonychia, associated with splinter hemorrhages. There is a characteristic subungual keratotic mass with distal fissuring. Onychoscopy can show a longitudinal homogenous red band extending from proximal nail fold to distal free edge. As the distal end is onycholysed, the band is seen white distally. Onychoscopy helps clearly delineate the presence of splinter hemorrhages. The distal nail plate may show longitudinal splitting and wedge-shaped notches. Onychoscopy of free edge of nail plate shows small subungual keratotic mass, providing a definitive clue for diagnosis.[42]
Connective tissue diseases
Changes in nail fold capillaries can be visualized in most CTD's, especially well in the fourth finger. It has been shown that even with some training; identifying structural capillary abnormalities is possible on dermatoscopy.[20] This is in contrast to the use of dermoscopy for skin lesions where the importance of training or previous exposure is well recognized.[20] The typical nail fold capillaroscopy pattern described in systemic sclerosis is also known as “scleroderma pattern.” Similar changes may be appreciated in dermatomyositis, mixed connective tissue disease, Raynaud's phenomenon and other systemic diseases as well. Nail fold capillaroscopy is particularly useful in evaluating cases with Raynaud's phenomenon. The capillary architecture is essentially normal in primary Raynaud's phenomenon, unlike the secondary variant.
The nail fold capillaroscopy changes described in systemic sclerosis are best discerned on videocapillaroscopy. Based on morphology, they could be classified as early changes (some enlarged capillaries [Figure 20a] and some hemorrhages); active disease (frequently enlarged capillaries and frequent hemorrhages [Figure 20b] and late changes (irregular enlargement, severe loss of capillaries and avascular areas [43] [Figure 20c]. Capillaries can be called dilated if they are more than three times wider than surrounding hairpin-shaped capillaries.[21] Dogan et al. classified scleroderma capillaries as Group I (capillary dilation); Group II (giant capillaries); Group III (disrupted vascular configuration) and Group 1V (both giant and dilated capillaries [44]). Moore et al. developed a practical, less time-consuming, four-point grading scale in cases with suspected systemic sclerosis.[20] Beltrán et al. also concluded that dermoscopy (even at lower magnification) had 77% sensitivity and 71% specificity for diagnosing systemic sclerosis.[45]
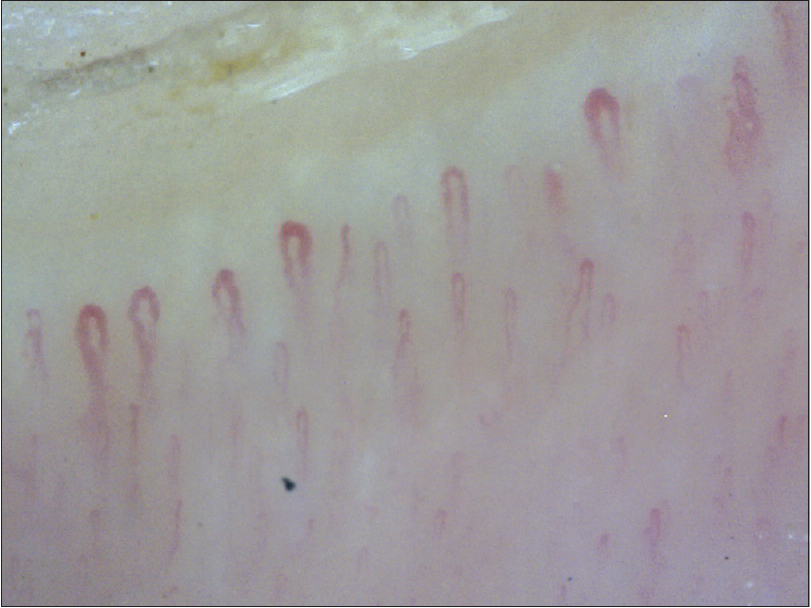 |
| Figure 20a: Nail fold capillaroscopy in patients with systemic sclerosis (200X). Early changes seen in the form of capillary dilations. There is no/ minimal change in the normal capillary arrangement |
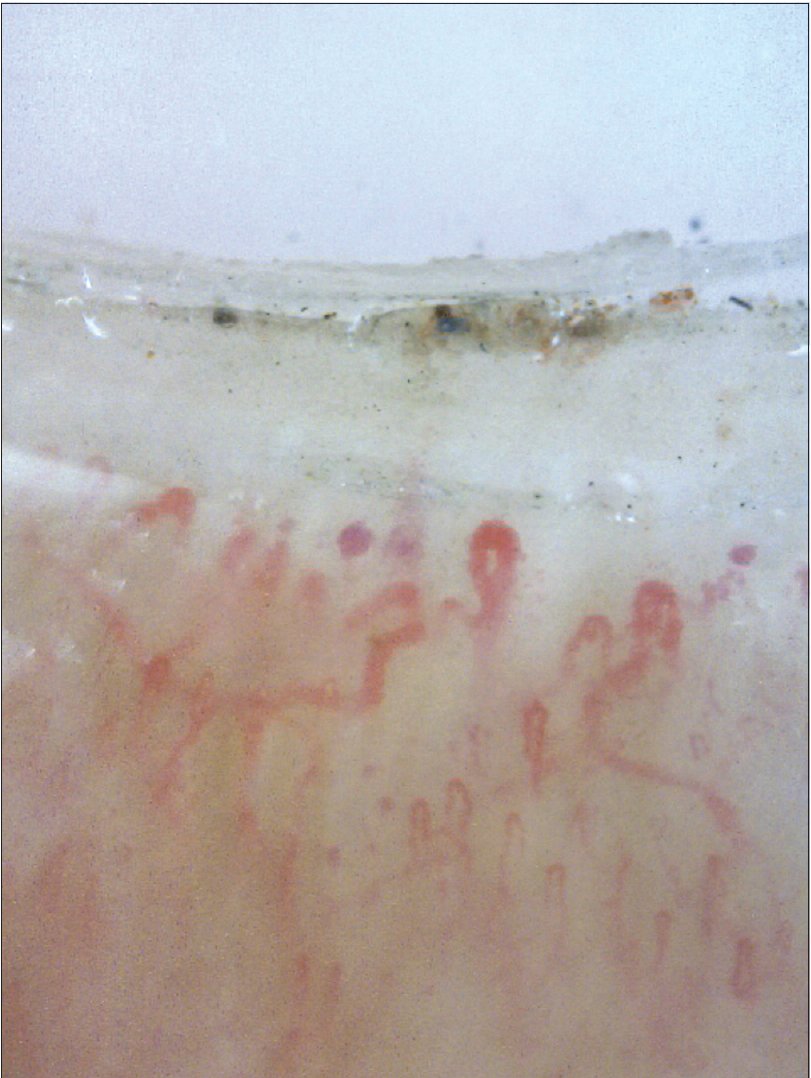 |
| Figure 20b: Nail fold capillaroscopy in patients with systemic sclerosis (200X). Features of active disease can be seen in the form of disruption of normal capillary arrangement with frequent dilated vessels and frequent hemorrhages. Some capillary drop outs also seen |
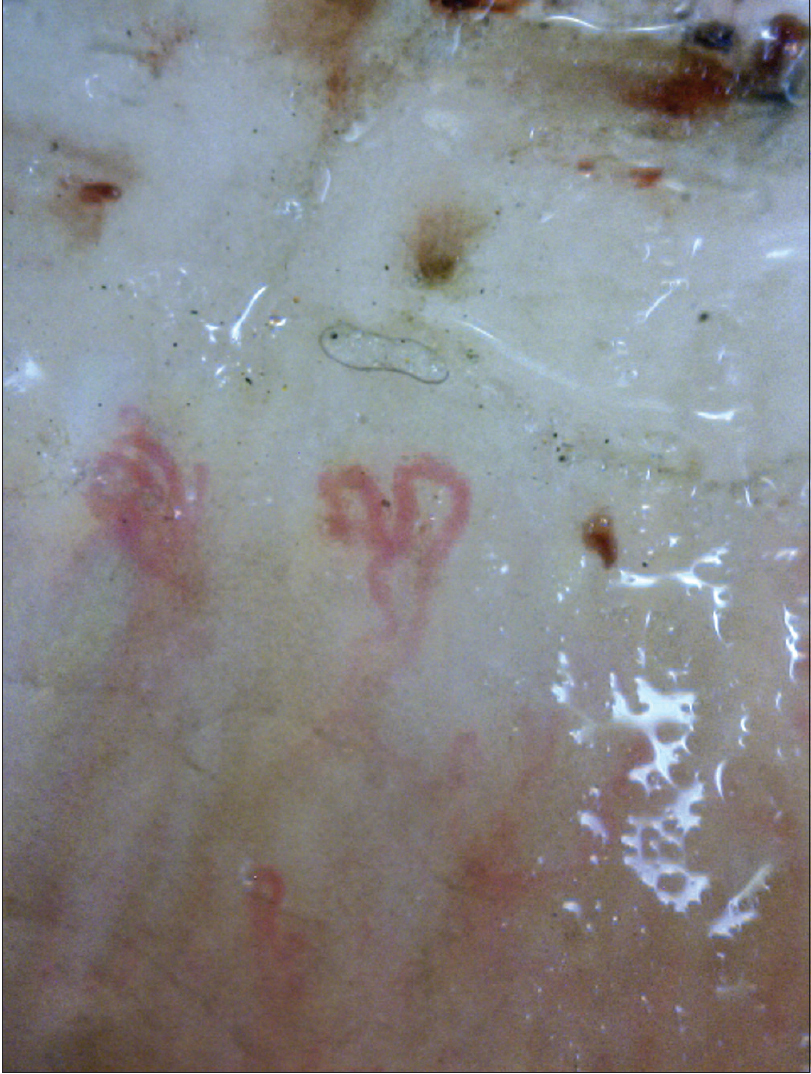 |
| Figure 20c: Nail fold capillaroscopy in patients with systemic sclerosis (200X). Features of late disease showing irregularly enlarged giant capillaries, microvascular hemorrhages and avascular areas. Arborizing vessels can also be seen |
Although not diagnostic, nail fold capillaroscopy can still be useful in identifying other CTD as well. A systemic sclerosis-dermatomyositis pattern is defined as presence of two or more findings, in two or more nail folds. The findings to be evaluated include enlarged capillary loops, loss of capillaries (moderate or extensive), disorganization of normal distribution, budding (bushy) capillaries, twisted (tortuous, crossing, ramified) capillaries and capillary hemorrhages (>2 punctate hemorrhages in a finger).[21] Ohtsuka reported this pattern in 76.4% patients with systemic sclerosis; 63.6% patients with dermatomyositis and 50% patients with mixed connective tissue disease. These criteria show a sensitivity of 76.9% and specificity of 96.9% in cases with systemic sclerosis.[21]
Conclusion
Onychoscopy has come a long way from being an investigational fad to a reliable diagnostic tool. It definitely permits better visualization of nail signs. For some diseases, it adds a lot to clinical examination and reveals suggestive features, not otherwise visible to the naked eye. Both “dry” and “wet” examinations are needed to form an opinion. The technique, however, may not be easy to perform and requires a good knowledge of anatomy and nail diseases. It also has critical limitations and cannot replace a thorough clinical examination or diagnostic histopathology of the nails as of now. Increasingly, onychoscopy-based diagnostic criteria for various nail diseases are being refined and evolved. Larger number of studies are needed to increase the diagnostic sensitivity and specificity of this valuable, noninvasive investigational tool.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Saphier J. Dermatoscopy. I. Communication. Arch Dermatol Syph 1920;128:1-19.
[Google Scholar]
|
| 2. |
Goldman L. Some investigative studies of pigmented nevi with cutaneous microscopy. J Invest Dermatol 1951;16:407-27.
[Google Scholar]
|
| 3. |
Goldman L. A simple portable skin microscope for surface microscopy. AMA Arch Derm 1958;78:246-7.
[Google Scholar]
|
| 4. |
MacKie RM. An aid to the preoperative assessment of pigmented lesions of the skin. Br J Dermatol 1971;85:232-8.
[Google Scholar]
|
| 5. |
Ronger S, Touzet S, Ligeron C, Balme B, Viallard AM, Barrut D, et al. Dermoscopic examination of nail pigmentation. Arch Dermatol 2002;138:1327-33.
[Google Scholar]
|
| 6. |
Lencastre A, Lamas A, Sá D, Tosti A. Onychoscopy. Clin Dermatol 2013;31:587-93.
[Google Scholar]
|
| 7. |
Tosti A, Argenziano G. Dermoscopy allows better management of nail pigmentation. Arch Dermatol 2002;138:1369-70.
[Google Scholar]
|
| 8. |
Tasli L, Oguz O. The role of various immersion liquids at digital dermoscopy in structural analysis. Indian J Dermatol Venereol Leprol 2011;77:110.
[Google Scholar]
|
| 9. |
Gewirtzman AJ, Saurat JH, Braun RP. An evaluation of dermoscopy fluids and application techniques. Br J Dermatol 2003;149:59-63.
[Google Scholar]
|
| 10. |
Hirata SH, Yamada S, Almeida FA, Enokihara MY, Rosa IP, Enokihara MM, et al. Dermoscopic examination of the nail bed and matrix. Int J Dermatol 2006;45:28-30.
[Google Scholar]
|
| 11. |
Piraccini BM, Balestri R, Starace M, Rech G. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol 2013;27:509-13.
[Google Scholar]
|
| 12. |
Farias DC, Tosti A, Chiacchio ND, Hirata SH. Dermoscopy in nail psoriasis. An Bras Dermatol 2010;85:101-3.
[Google Scholar]
|
| 13. |
Nakamura R, Broce AA, Palencia DP, Ortiz NI, Leverone A. Dermatoscopy of nail lichen planus. Int J Dermatol 2013;52:684-7.
[Google Scholar]
|
| 14. |
Nakamura RC, Costa MC. Dermatoscopic findings in the most frequent onychopathies: Descriptive analysis of 500 cases. Int J Dermatol 2012;51:483-5.
[Google Scholar]
|
| 15. |
Piraccini BM, Bruni F, Starace M. Dermoscopy of non-skin cancer nail disorders. Dermatol Ther 2012;25:594-602.
[Google Scholar]
|
| 16. |
Mun JH, Kim GW, Jwa SW, Song M, Kim HS, Ko HC, et al. Dermoscopy of subungual haemorrhage: Its usefulness in differential diagnosis from nail-unit melanoma. Br J Dermatol 2013;168:1224-9.
[Google Scholar]
|
| 17. |
Lee DY, Lee JH. Use of dermoscopy to identify nail plate cavities as a clinical diagnostic clue for onychomatricoma. Int J Dermatol 2016;55:e108-10.
[Google Scholar]
|
| 18. |
Lesort C, Debarbieux S, Duru G, Dalle S, Poulhalon N, Thomas L. Dermoscopic features of onychomatricoma: A study of 34 cases. Dermatology 2015;231:177-83.
[Google Scholar]
|
| 19. |
Maehara Lde S, Ohe EM, Enokihara MY, Michalany NS, Yamada S, Hirata SH. Diagnosis of glomus tumor by nail bed and matrix dermoscopy. An Bras Dermatol 2010;85:236-8.
[Google Scholar]
|
| 20. |
Moore TL, Roberts C, Murray AK, Helbling I, Herrick AL. Reliability of dermoscopy in the assessment of patients with Raynaud's phenomenon. Rheumatology (Oxford) 2010;49:542-7.
[Google Scholar]
|
| 21. |
Ohtsuka T. Dermoscopic detection of nail fold capillary abnormality in patients with systemic sclerosis. J Dermatol 2012;39:331-5.
[Google Scholar]
|
| 22. |
Hughes M, Moore T, O'Leary N, Tracey A, Ennis H, Dinsdale G, et al. Astudy comparing videocapillaroscopy and dermoscopy in the assessment of nailfold capillaries in patients with systemic sclerosis-spectrum disorders. Rheumatology (Oxford) 2015;54:1435-42.
[Google Scholar]
|
| 23. |
Hasegawa M. Dermoscopy findings of nail fold capillaries in connective tissue diseases. J Dermatol 2011;38:66-70.
[Google Scholar]
|
| 24. |
Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg 2009;28:49-54.
[Google Scholar]
|
| 25. |
Braun RP, Oliviero M, Kolm I, French LE, Marghoob AA, Rabinovitz H. Dermoscopy: What's new? Clin Dermatol 2009;27:26-34.
[Google Scholar]
|
| 26. |
Husain S, Scher RK, Silvers DN, Ackerman AB. Melanotic macule of nail unit and its clinicopathologic spectrum. J Am Acad Dermatol 2006;54:664-7.
[Google Scholar]
|
| 27. |
Di Chiacchio N, Hirata SH, Enokihara MY, Michalany NS, Fabbrocini G, Tosti A. Dermatologists' accuracy in early diagnosis of melanoma of the nail matrix. Arch Dermatol 2010;146:382-7.
[Google Scholar]
|
| 28. |
Thomas L, Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol Ther 2007;20:3-10.
[Google Scholar]
|
| 29. |
Braun RP, Baran R, Saurat JH, Thomas L. Surgical Pearl: Dermoscopy of the free edge of the nail to determine the level of nail plate pigmentation and the location of its probable origin in the proximal or distal nail matrix. J Am Acad Dermatol 2006;55:512-3.
[Google Scholar]
|
| 30. |
John RH, Izakovic J. Dermatoscopy/ELM for the evaluation of nail-apparatus pigmentation. Dermatol Surg 2001;27:315-22.
[Google Scholar]
|
| 31. |
Kawabata Y, Ohara K, Hino H, Tamaki K. Two kinds of Hutchinson's sign, benign and malignant. J Am Acad Dermatol 2001;44:305-7.
[Google Scholar]
|
| 32. |
Causeret AS, Skowron F, Viallard AM, Balme B, Thomas L. Subungual blue nevus. J Am Acad Dermatol 2003;49:310-2.
[Google Scholar]
|
| 33. |
Braun RP, Baran R, Le Gal FA, Dalle S, Ronger S, Pandolfi R, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol 2007;56:835-47.
[Google Scholar]
|
| 34. |
Phan A, Dalle S, Touzet S, Ronger-Savlé S, Balme B, Thomas L. Dermoscopic features of acral lentiginous melanoma in a large series of 110 cases in a white population. Br J Dermatol 2010;162:765-71.
[Google Scholar]
|
| 35. |
Tosti A, Pirraccini BM, Farias DC. Nail dermoscopy. In: Micali G, editor. Videodermatoscopy in Clinical Practice. London: Informa Healthcare; 2009.
[Google Scholar]
|
| 36. |
Yadav TA, Khopkar US. Dermoscopy to detect signs of subclinical nail involvement in chronic plaque psoriasis: A study of 68 patients. Indian J Dermatol 2015;60:272-5.
[Google Scholar]
|
| 37. |
Iorizzo M, Dahdah M, Vincenzi C, Tosti A. Videodermoscopy of the hyponychium in nail bed psoriasis. J Am Acad Dermatol 2008;58:714-5.
[Google Scholar]
|
| 38. |
Piraccini BM, Alessandrini A. Onychomycosis: A review. J Fungi 2015;1:30-43.
[Google Scholar]
|
| 39. |
Jesús-Silva MA, Fernández-Martínez R, Roldán-Marín R, Arenas R. Dermoscopic patterns in patients with a clinical diagnosis of onychomycosis-results of a prospective study including data of potassium hydroxide (KOH) and culture examination. Dermatol Pract Concept 2015;5:39-44.
[Google Scholar]
|
| 40. |
De Crignis G, Valgas N, Rezende P, Leverone A, Nakamura R. Dermatoscopy of onychomycosis. Int J Dermatol 2014;53:e97-9.
[Google Scholar]
|
| 41. |
Dong H, Shu D, Campbell TM, Frühauf J, Soyer HP, Hofmann-Wellenhof R. Dermatoscopy of genital warts. J Am Acad Dermatol 2011;64:859-64.
[Google Scholar]
|
| 42. |
Tosti A, Schneider SL, Ramirez-Quizon MN, Zaiac M, Miteva M. Clinical, dermoscopic, and pathologic features of onychopapilloma: A review of 47 cases. J Am Acad Dermatol 2016;74:521-6.
[Google Scholar]
|
| 43. |
Cutolo M, Sulli A, Pizzorni C, Accardo S. Nail fold videocapillaroscopy assessment of microvascular damge in systemic sclerosis. J Rheumatol 2000;27:155-160
[Google Scholar]
|
| 44. |
Dogan S, Akdogan A, Atakan N. Nailfold capillaroscopy in systemic sclerosis: Is there any difference between videocapillaroscopy and dermatoscopy? Skin Res Technol 2013;19 (4):446-9.
[Google Scholar]
|
| 45. |
Beltrán E, Toll A, Pros A, Carbonell J, Pujol RM. Assessment of nailfold capillaroscopy by ×30 digital epiluminescence (dermoscopy) in patients with Raynaud phenomenon. Br J Dermatol 2007;156:892-8.
[Google Scholar]
|
Fulltext Views
14,105
PDF downloads
3,869





