Translate this page into:
Hidradenitis suppurativa unresponsive to canakinumab treatment: A case report
Correspondence Address:
Burak Tekin
Durupark A/10, Ankara Street No: 264, Gullubaglar District, 34899 Pendik, Istanbul
Turkey
| How to cite this article: Tekin B, Salman A, Ergun T. Hidradenitis suppurativa unresponsive to canakinumab treatment: A case report. Indian J Dermatol Venereol Leprol 2017;83:615-617 |
Sir,
Hidradenitis suppurativa is a chronic disorder manifesting with relapsing, deep-seated nodules and abscesses in the inverse body areas which have a tendency to form sinus tracts, fistulae, and scar tissue.[1],[2] As specified in a recent Cochrane review, none of the interventions have consistently been effective in managing this debilitating condition.[1] Similar to other dermatologic diseases, there is an increasing global trend toward pathogenesis-directed therapies in hidradenitis suppurativa, particularly tumor necrosis factor (TNF)-α inhibitors.[3] Recently, two studies revealed an increased level/expression of interleukin (IL)-1β in lesional and perilesional hidradenitis suppurativa skin, suggesting that the blockade of IL-1 pathway may be of therapeutic value.[4],[5] Accordingly, IL-1 inhibition has been studied in case reports/series and two clinical trials, mostly with encouraging results [Table - 1]. Here, we report our unfavorable experience with canakinumab in a patient with hidradenitis suppurativa and briefly review the literature.
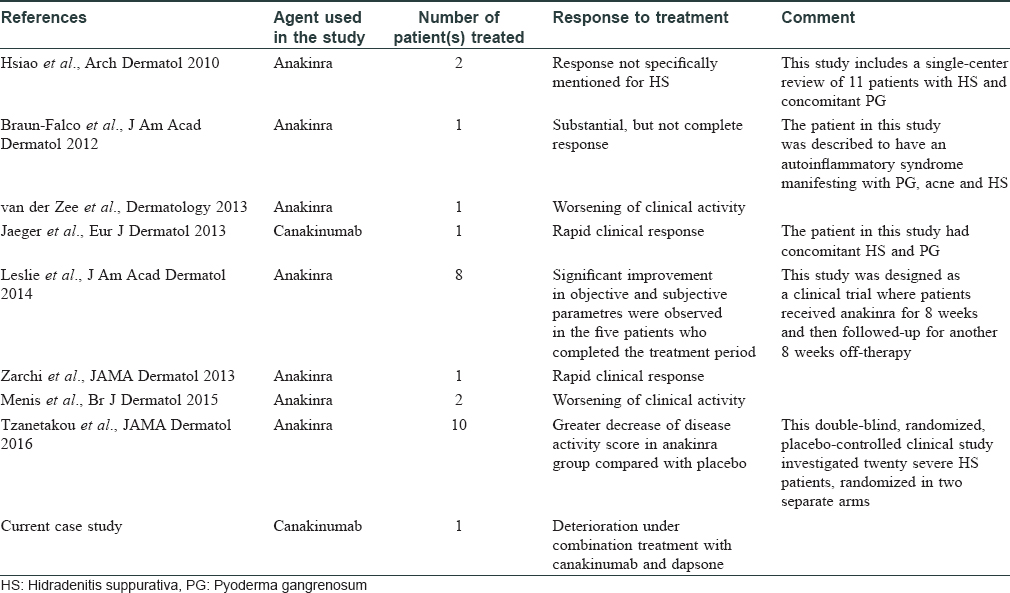
A 27-year-old nonsmoker, obese male presented with a 5-year history of hidradenitis suppurativa resistant to multiple therapies including systemic (tetracycline and clindamycin for 5 months) and topical antibiotics and oral isotretinoin. Medical history was insignificant except for hepatosteatosis and persistent elevation of transaminases contraindicating acitretin use. Dermatological examination revealed inflammatory papulopustules and nodules, sinus tracts with malodorous discharge, and hypertrophic scars in the axillary and inguinal regions. In addition, his scalp was covered with crusts roofing lakes of pus, leading to malodor. He was diagnosed with hidradenitis suppurativa (Hurley stage III) and dissecting cellulitis of the scalp. He did not have acne conglobata or pilonidal sinus and swab cultures from discharging lesions were negative. Combination treatment with dapsone (150 mg/day) and intravenous infliximab (5 mg/kg per infusion) was initiated. However, only slight improvement was observed after 8 infusions. Based on previous reports of efficacy of IL-1 blockade, off-label treatment with subcutaneous canakinumab (150 mg, every 4 weeks) was started following an informed consent while continuing dapsone. Subsequently, three canakinumab injections were administered, resulting in objective (measured by Sartorius score) and subjective worsening of the lesions [Figure 1a], [Figure 1b], [Figure 1c], [Figure 1d].
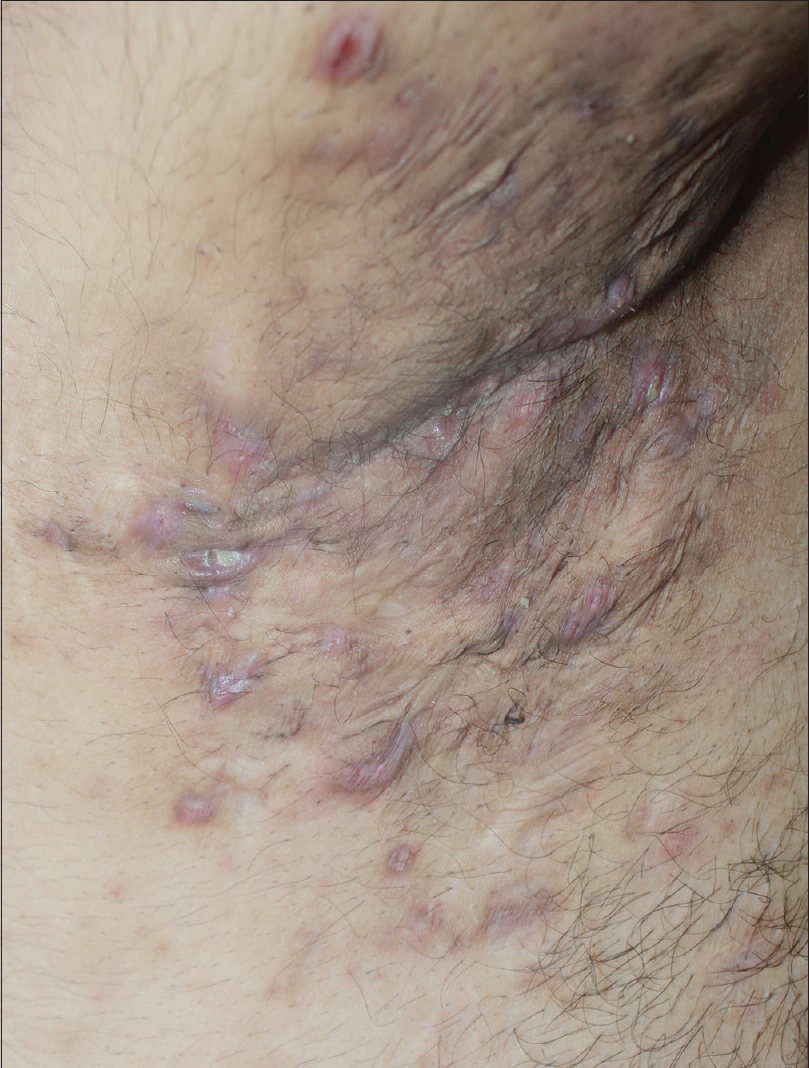 |
| Figure 1a: Right axilla of the patient at the baseline |
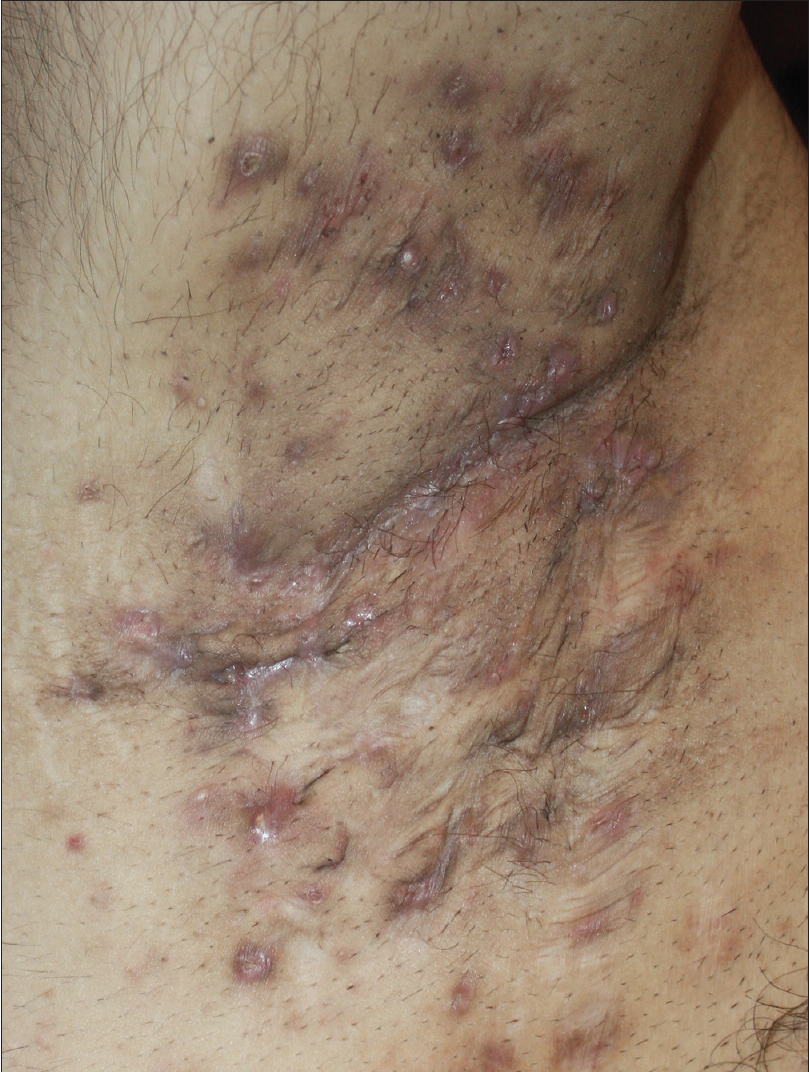 |
| Figure 1b: Right axilla of the patient before the second monthly canakinumab injection |
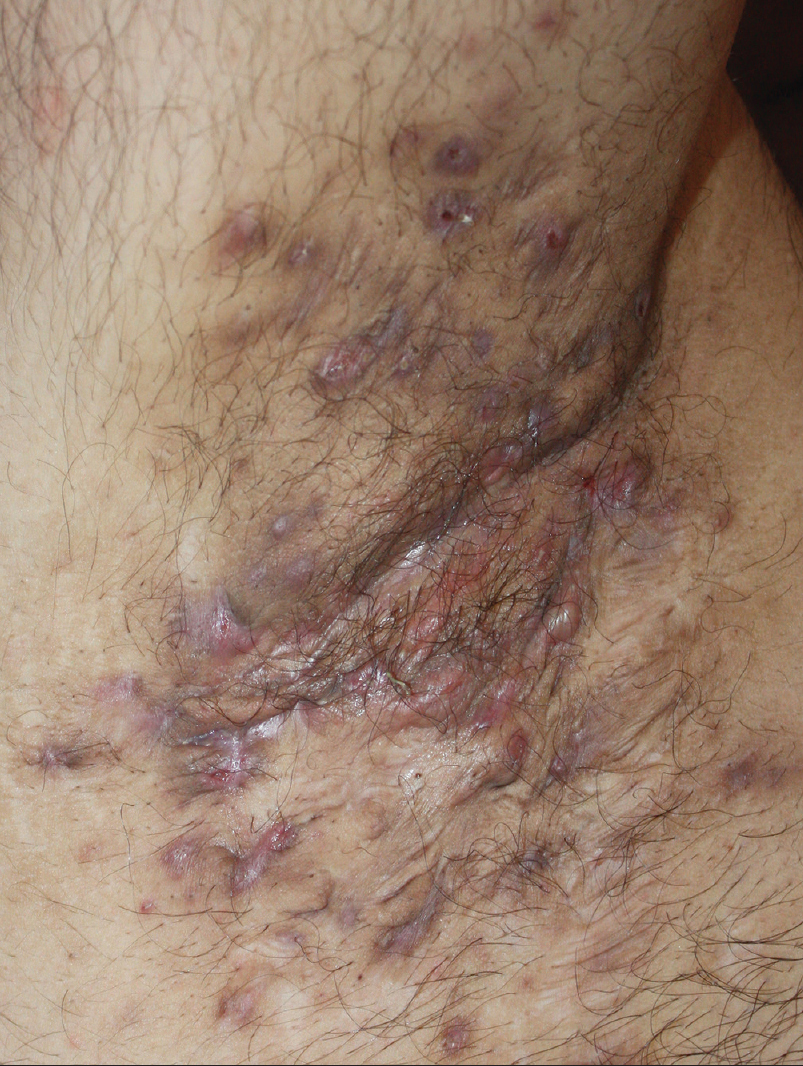 |
| Figure 1c: Right axilla of the patient three weeks after the third monthly canakinumab injection. At the final examination, the lesions were significantly more infiltrated, purulent and tender to palpation compared to baseline, and had a serious impact on the patient's quality of life, as reflected by objective and subjective parameters, respectively |
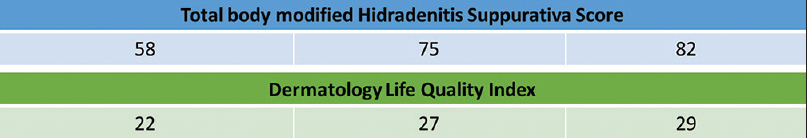 |
| Figure 1d: Total body modified hidradenitis suppurativa score was calculated as per Sartorius et al. (British Journal of Dermatology, 2009) by taking the following parameters into account: the anatomical regions involved, the numbers and scores of lesions for each region, the longest distance between two relevant lesions in each region, and whether all lesions are separated by normal skin |
Despite having a complex pathogenesis, hidradenitis suppurativa is increasingly being conceptualized as an autoinflammatory disorder associated with immune dysregulation.[4] This view is reinforced by the coexistence of hidradenitis suppurativa with other possibly autoinflammatory conditions such as Crohn's disease,[5] and demonstration of an altered cytokine profile with increased expression of IL-1β, IL-17, and TNF-α in lesional and perilesional hidradenitis suppurativa skin.[4],[5] In accordance with these findings, IL-1 blockade has been used in the treatment of hidradenitis suppurativa with variable response [Table - 1]. Because our patient was unresponsive to infliximab, we decided to use canakinumab, which is a fully humanized anti-IL-1β monoclonal antibody administered subcutaneously every 1–2 months. It was preferred to anakinra (recombinant IL-1 receptor antagonist used as a daily subcutaneous injection) mainly due to the relative ease of administration. Although data on time to response is limited, we determined efficacy at the end of a 3-month period because a dramatic response to canakinumab was reported in a patient with concomitant hidradenitis suppurativa and pyoderma gangrenosum after one canakinumab injection only.
We think that several factors help to explain the discrepancy between the reported efficacy of IL-1 blockade in the literature and the response observed in our patient. This disparity might be partly attributable to publication bias, as case studies with a more encouraging outcome are more likely to be accepted for publication. More importantly, it is speculated that there are clinical variants of hidradenitis suppurativa, possibly linked to diverse genetic backgrounds. If so, this clinicogenetic heterogeneity of disease expression could arguably contribute to the unpredictable response of hidradenitis suppurativa to different drugs in different patients.[2] Immunologically, it might be possible that activation of discrete pathways (e.g., TNF-α or IL-1 pathway) is predominantly responsible for the hidradenitis suppurativa phenotype in a particular patient. Finally, follicular hyperplasia and resultant occlusion as well as loss of follicle integrity may be the primary pathophysiological event leading to activation of the innate immune response and subsequent proinflammatory cytokine release, explaining the limited efficacy of treatments targeting immune dysregulation.[5] Overall, current data may implicate management of hidradenitis suppurativa through treatment modalities which both prevent follicular hyperkeratosis and regulate altered innate immune response, perhaps through concomitant acitretin and IL-1 antagonist or acitretin and IL-17 antagonist combinations.
Considering its limitations, our report merely suggests that IL-1 blockade is not efficacious in every patient with hidradenitis suppurativa and should not be misinterpreted as an unjustifiable challenge to the overall efficacy of this treatment approach documented in the literature. In future, clinical studies that aim to identify which patients are most likely to benefit from blockade of TNF-α, IL-1, IL-17, or other potentially culprit pathways would be highly desirable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ingram JR, Woo PN, Chua SL, Ormerod AD, Desai N, Kai AC, et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev 2015; DOI:10.1002/14651858.CD010081.pub2.
[Google Scholar]
|
| 2. |
van der Zee HH, Laman JD, Boer J, Prens EP. Hidradenitis suppurativa: Viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol 2012;21:735-9.
[Google Scholar]
|
| 3. |
Lee RA, Eisen DB. Treatment of hidradenitis suppurativa with biologic medications. J Am Acad Dermatol 2015;73 5 Suppl 1:S82-8.
[Google Scholar]
|
| 4. |
van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1ß and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-a and IL-1ß. Br J Dermatol 2011;164:1292-8.
[Google Scholar]
|
| 5. |
Kelly G, Hughes R, McGarry T, van den Born M, Adamzik K, Fitzgerald R, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol 2015;173:1431-9.
[Google Scholar]
|
Fulltext Views
3,916
PDF downloads
1,993





