Translate this page into:
Topical calcipotriol/betamethasone dipropionate for psoriasis vulgaris: A systematic review
Correspondence Address:
Yan Wu
Department of Dermatology, No. 1 Hospital of China Medical University, 155 North Nanjing Street, Shenyang 110001
China
| How to cite this article: Yan R, Jiang S, Wu Y, Gao XH, Chen HD. Topical calcipotriol/betamethasone dipropionate for psoriasis vulgaris: A systematic review. Indian J Dermatol Venereol Leprol 2016;82:135-144 |
Abstract
Topical calcipotriol/betamethasone dipropionate ointment/gel has been commonly used for the treatment of psoriasis vulgaris. However, the efficacy of this combination needs to be consolidated. We aimed to assess the effects and safety profile of calcipotriol/betamethasone dipropionate for the treatment of psoriasis vulgaris, using evidence based approach. Randomized controlled trials on the treatment of psoriasis vulgaris with calcipotriol/betamethasone dipropionate were identified by searching PubMed, China National Knowledge Infrastructure and the Cochrane Library. The primary outcome measure was the Psoriasis Area and Severity Index (PASI) score. Ten randomized controlled trials involving 6590 participants were included. The methodologies of the studies were generally of moderate to high quality. These trials used topical calcipotriol/betamethasone dipropionate for 4 or 8 weeks, and were compared with topical calcipotriol or betamethasone. The results showed that calcipotriol/betamethasone dipropionate was more effective than controls. A four-week treatment with calcipotriol/betamethasone dipropionate did not show any significant difference between the once-daily or twice-daily regimen. The adverse events of calcipotriol/betamethasone dipropionate were tolerable and acceptable. The reports included in this review are heterogenous and have limitations. Topical application of calcipotriol/betamethasone dipropionate once daily is an efficacious treatment for psoriasis vulgaris and is associated with few side effects.INTRODUCTION
Psoriasis is a common, chronic inflammatory cutaneous disease that affects 1-3% of the world population. [1] The pathogenesis is multifactorial and there are various triggering factors. It may lead to a low quality of life and reduces physical and mental functioning. [2] Psoriasis vulgaris is the most common form of psoriasis. Mild to moderate psoriasis vulgaris is usually treated topically with agents that may include vitamin D derivatives and corticosteroids. [3]
Calcipotriol and betamethasone dipropionate have been used as topical therapies in psoriasis vulgaris for many years. [4],[5],[6] Treatments are often used in combination to improve symptom control and tolerability. It was postulated that a combination of topical calcipotriol and corticosteroid may have an additive or synergistic treatment effect due to their different mechanisms of action. This is expected to be more efficacious and better tolerated than monotherapy with either drug. [7] However, calcipotriol and corticosteroids are usually not compatible because of their different pH requirements.
A compound with calcipotriol (50 ug/g) and betamethasone (0.5 mg/g) was introduced and recommended for mild-to-moderate psoriasis in 2001. [8] This formulation, available as both an ointment and a gel, combined the action of vitamin D3 analogues on keratinocyte differentiation with the anti-inflammatory effect of steroids. This reduced the side effect profile that was seen with single topical agents. [9],[10] A study in a human disease model showed that the ointment and gel formulations had comparable anti-psoriatic effects. [10],[11] Previous studies have used this compound, either alone or combined with other therapies, for the treatment of mild-to-moderate psoriasis. [9],[12],[13],[14],[15],[16],[17],[18],[19],[20] The primary aim of our systematic review was to examine the efficacy and tolerability of calcipotriol/betamethasone dipropionate in the treatment of psoriasis.
Materials and Methods
Eligibility criteria
We searched for studies regardless of the language or publication status, that met all of the following criteria: (i) randomized controlled trials involving psoriasis vulgaris patients; (ii) undergoing calcipotriol/betamethasone dipropionate ointment/gel therapy in the treatment group, without any other simultaneous treatment; (iii) undergoing calcipotriol or betamethasone ointment/gel therapy in the control group, without any other simultaneous treatment; (iv) psoriasis on the body, with or without scalp lesions.
Search strategy
PubMed, China National Knowledge Infrastructure and Cochrane library were independently searched by two investigators using the open search strings "calcipotriol," "betamethasone," "psoriasis", "random," "randomized control trial" and "randomized controlled trial." All studies that matched the search criteria were included until April 2015. The reference lists of prior reviews, systematic reviews and trials were also checked.
Study selection and data extraction
Two investigators independently screened all studies for inclusion into the review. Eligible studies were determined and retrieved. Disagreements were resolved by consensus. They independently extracted the data from included studies with the help of standardized forms. A third investigator was assigned the checking process. The study data included the country/region of participants, interventions, number of participants with age and gender distribution, the affected sites, baseline score, mean duration of disease, duration of treatment, evaluation time, efficacy and safety.
Quality assessment
The criteria recommended by the Cochrane Collaboration Handbook were used to assess the methodological quality of included trials. It focused on the description of randomization (sequence generation progress), allocation concealment, blinding, addressing of incomplete outcome data, reporting of selective outcome and other potential threats to validity. [21] The judgment for each entry was based on the answer of a question with "Yes" indicating "low risk of bias," "No" indicating "high risk of bias" and "unclear" indicating "either lack of information or uncertainty on the potential for bias." Disagreements were resolved by group consensus.
Outcome measures
The primary outcomes were the percentage reduction of the PASI score, the PASI score, and the proportion of patients who achieved PASI 75 score. The secondary outcomes included the proportion of "marked improvement or clearance" by investigator assessment and occurrence of any adverse events.
Data analysis and statistical methods
The statistical analyses were performed by both investigators using the duplicate data entry facility of Revman 5.0. In addition to 95% confidence intervals, mean differences were used for continuous outcomes. The I2 statistic was calculated to determine the proportion of between-study variation due to heterogeneity. The value ranges from 0% to 100% and high values indicate strong heterogeneity. If the heterogeneity was low (P > 0.1, I2 < 50%), a fixed-effect model was used for analysis, otherwise a random-effect model or describe analysis was used.
RESULTS
Eligible studies
Altogether, 391 papers were identified by a systematic literature search. After screening the titles and abstracts, 378 non-relevant papers were excluded. The full text of the 13 remaining papers was retrieved. After review, 10 randomized controlled trials were finally included. [9],[12],[13],[14],[15],[16],[17],[18],[19],[20] The study selection procedure is illustrated in a flowchart [Figure - 1].
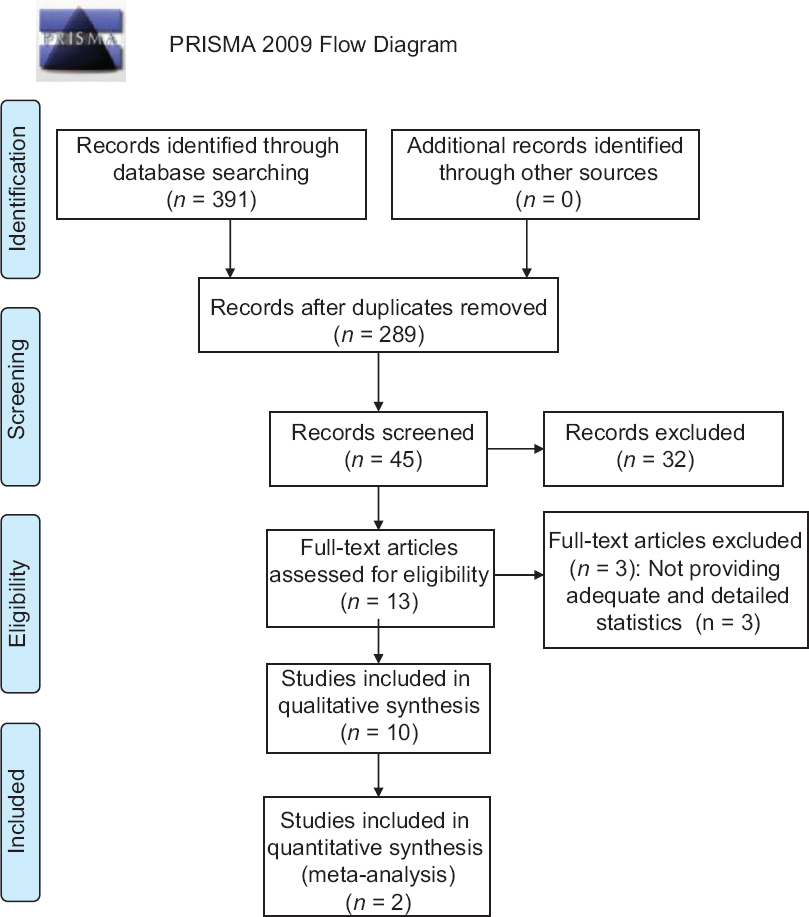 |
| Figure 1: The flow chart of the study selection process |
Risk of bias assessment and study characteristics
All studies reported random allocation of study participants, eight of them adequately described the randomization method. [9],[12],[13],[14],[16],[17],[19],[20] Six trials adequately mentioned the allocation concealment. [9],[12],[13],[14],[17],[19] Five trials performed blinding of participants and researchers and three trials mentioned blinding of outcome assessment. [12],[13],[14],[17],[19],[20] Nine trials addressed incomplete outcome data. All the ten trials addressed selective outcome reporting [Figure - 2] and [Figure - 3]. [9],[12],[13],[14],[15],[16],[17],[18],[19]
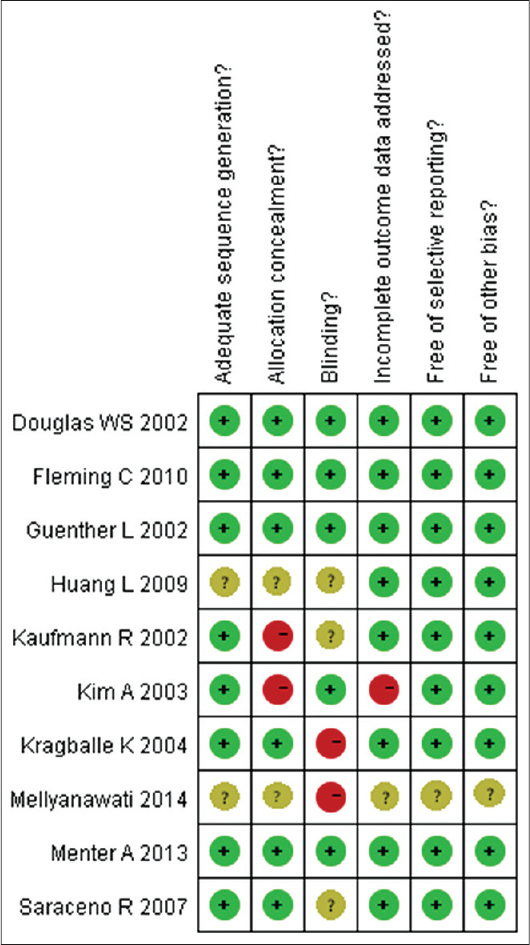 |
| Figure 2: The judgments about each risk of bias item, for each included study |
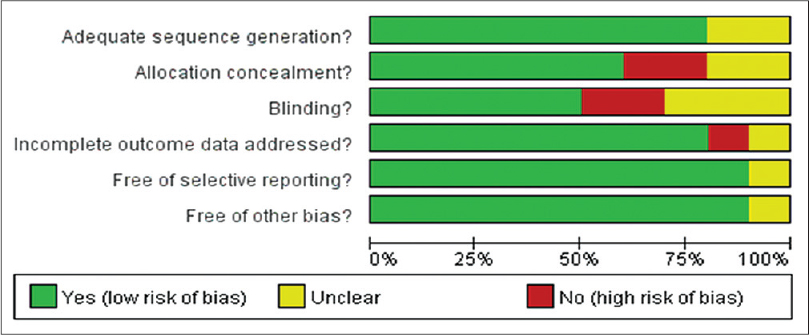 |
| Figure 3: The judgments about each risk of bias item, presented as percentages across all included studies |
Ten randomized controlled trials involving 6590 participants with psoriasis vulgaris were included in this systematic review. Five studies focused on Europeans and North Americans, two studies focused on Asians and the other three studies did not describe the origin. [9],[12],[13],[14],[15],[16],[17],[18],[19],[20] The mean age of patients was between 40-50 years. Men were relatively more affected than women. The duration of disease was more than ten years in most of the studies. The duration of treatment ranged from four to eight weeks. The characteristics of the included studies are depicted in [Table - 1].
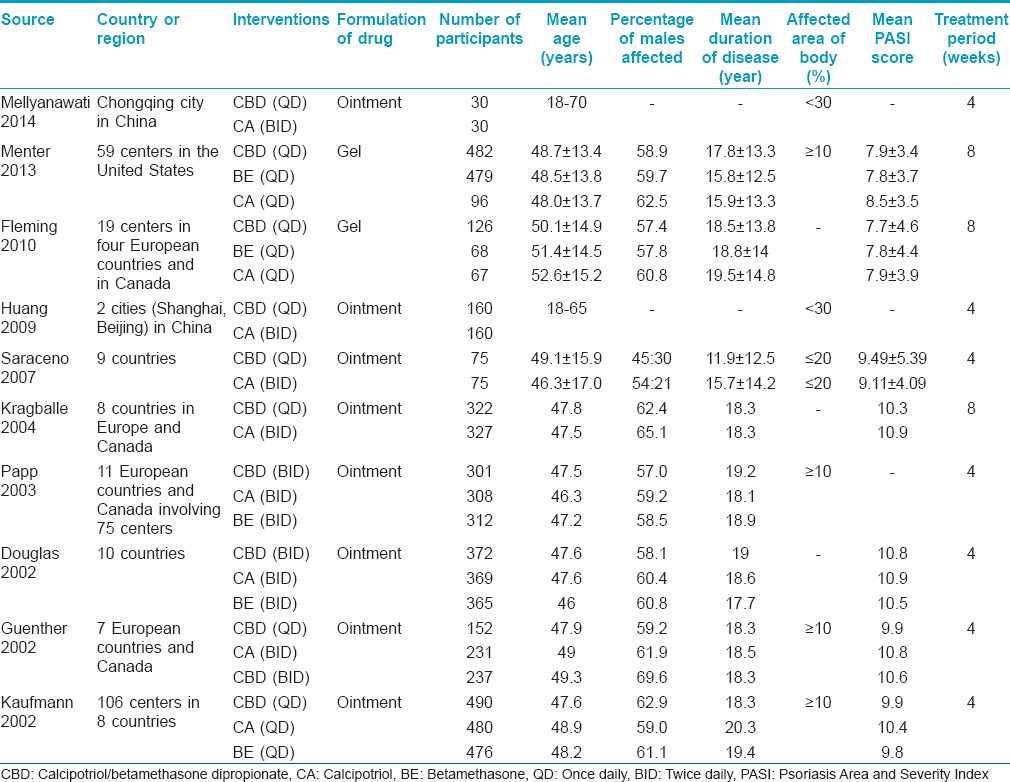
Efficacy evaluation
Comparison of calcipotriol/betamethasone dipropionate with calcipotriol [Table - 2]
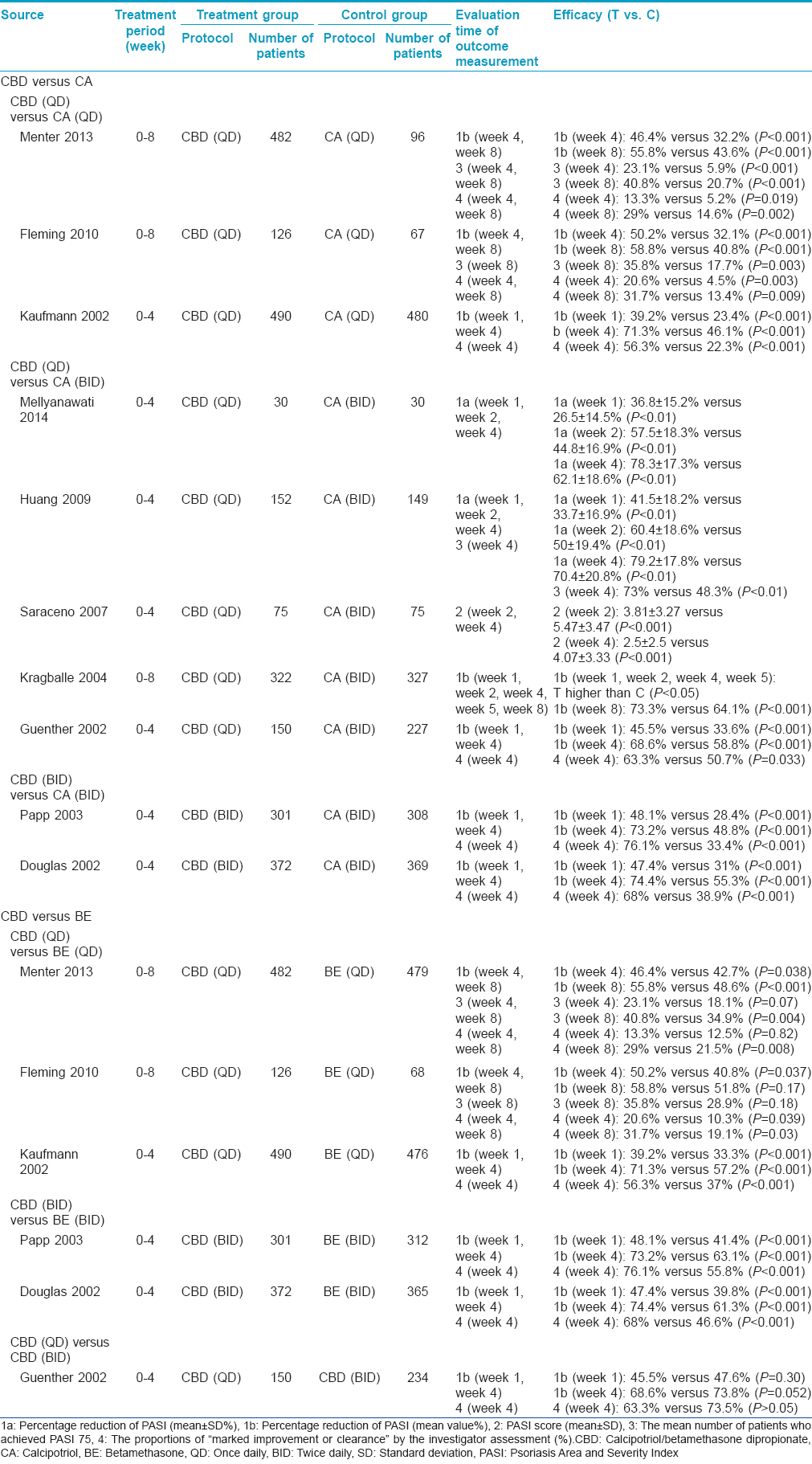
We identified ten trials for this comparison, involving a total of 4628 participants. [9],[12],[13],[14],[15],[16],[17],[18],[19],[20] The frequency of application was once daily calcipotriol/betamethasone versus once daily calcipotriol in three studies, once daily calcipotriol/betamethasone dipropionate versus twice daily calcipotriol in five studies, and twice daily calcipotriol/betamethasone dipropionate versus twice daily calcipotriol in two studies. [9],[12],[13],[14],[15],[16],[17],[18],[19],[20]
The treatment duration was 4 weeks in seven studies. [9],[12],[14],[15],[16],[18],[20] Six studies evaluated the percentage reduction of PASI score at the first week and fourth week. They showed a higher reduction in the calcipotriol/betamethasone dipropionate group (about 40% at week 1, and about 70% at week 4) compared to those in the calcipotriol group (P < 0.001). [12],[14],[15],[16],[18],[20] Two of those studies conducted a meta-analysis and showed that calcipotriol/betamethasone dipropionate was more effective than calcipotriol at all evaluation time-points (Week 1: mean difference = 8.29, 95% [4.78-11.80], P < 0.001; Week 2: mean difference = 10.85, 95% [6.98-14.73], P < 0.001; Week 4: mean difference = 10.20, 95% [6.25-14.14], P < 0.001). [15],[18] One study also demonstrated that the PASI 75 score was observed in over 70% (111/152) of patients with calcipotriol/betamethasone dipropionate therapy in contrast to only about 40% (72/149) of patients with calcipotriol treatment (P < 0.01) at week 4. [15] Another study calculated the PASI scores and showed lower values in the calcipotriol/betamethasone dipropionate group (3.81 ± 3.27 at week 2, 2.5 ± 2.5 at week 4) than those in the calcipotriol group (5.47 ± 3.47 at week 2, 4.07 ± 3.33 at week 4) (P < 0.001). [9] Four studies evaluated the proportion of "marked improvement or clearance" by the investigators assessment at week 4. [12],[14],[16],[20] All those studies showed a higher proportion of improvement in the calcipotriol/betamethasone dipropionate group (once daily being nearly 60%, and twice daily being around 70%) compared to those in the calcipotriol group (P < 0.001 or P = 0.033).
The treatment duration was eight weeks in three studies. [13],[17],[19] All the studies evaluated the percentage reduction of PASI score at week 4 and week 8 and showed a higher reduction in calcipotriol betamethasone dipropionate group (about 50% at week 4 and over 55% at week 8) than those in the calcipotriol group (P < 0.001). Two studies also demonstrated a PASI 75 score in about 20% patients at week 4 and in 40% patients at week 8 treated with calcipotriol/betamethasone dipropionate therapy. This was in contrast to a PASI 75 score seen in only about 6% patients at week 4 and 20% patients at week 8 treated with calcipotriol (Week 4, P < 0.001; Week 8, P < 0.05). These studies also evaluated the proportion of "marked improvement or clearance" by investigator assessment. They showed a higher proportion of improvement in the calcipotriol/betamethasone dipropionate group (about 15% at week 4 and 30% at week 8) compared to that seen in the calcipotriol group (Week 4, P = 0.019, P = 0.003; Week 8, P = 0.002, P = 0.009). [13],[19]
Comparison of calcipotriol/betamethasone dipropionate with betamethasone [Table - 2]
We identified five trials for this comparison, involving a total of 3471 participants. [12],[13],[16],[19],[20] The frequency of application was once daily calcipotriol/betamethasone dipropionate versus once daily betamethasone in three studies, and twice daily calcipotriol/betamethasone dipropionate versus twice daily betamethasone in two studies. [12],[13],[16],[19],[20]
Three studies treated the patients for four weeks. [12],[16],[20] The evaluation of percentage reduction of PASI score showed a higher reduction in the calcipotriol/betamethasone dipropionate group compared to that in the betamethasone group (P < 0.001). A once daily application showed nearly 40% reduction and the twice daily application showed a reduction of around 50% at week 1. There was a reduction of 70% at week 4 with both once daily and twice daily applications. The studies also showed a higher proportion of "marked improvement or clearance" by investigator assessment in the calcipotriol/betamethasone dipropionate group (once daily being 60%, and twice daily being 70%) compared to that seen in the betamethasone group (P < 0.001) at week 4.
Two studies treated the patients for eight weeks and evaluated the percentage reduction of the PASI score. [13],[19] There was a higher reduction of the PASI score in the calcipotriol/betamethasone dipropionate group (about 50% at week 4) compared to that seen in the betamethasone group (P = 0.037 and P = 0.038). However, the results of PASI score reduction was not consistent at week 8. Although one study showed better efficacy in the calcipotriol/betamethasone dipropionate group versus the betamethasone group (P < 0.001), the other study showed no significant difference (P = 0.17). These two studies compared the rate of patients who achieved a PASI 75 score. One study showed no significant difference between groups at Week 4 (P = 0.07). More patients achieved a PASI 75 score in the calcipotriol/betamethasone dipropionate group when compared to the betamethasone group at week 8 (P = 0.004). However, another trial did not show such a difference at week 8 (P = 0.18). These two studies also evaluated the proportion of "marked improvement or clearance" by investigator assessment. A higher proportion of improvement was seen in the calcipotriol/betamethasone dipropionate group compared to that seen in the betamethasone group at week 8 (P = 0.03 and P = 0.008). Improved results were seen earlier at week 4 in one study. [13]
Comparison of calcipotriol/betamethasone dipropionate once daily with calcipotriol/betamethasone dipropionate twice daily [Table - 2]
A four week trial was carried out for this comparison, with a total of 384 participants. [14] The study evaluated the percentage reduction of the PASI score at week 1 and week 4, and the proportion of "marked improvement or clearance" by investigator assessment at week 4. No statistically significant differences regarding any outcomes were seen between the two treatment protocols at any time point (P = 0.3 and P = 0.052).
Adverse effects
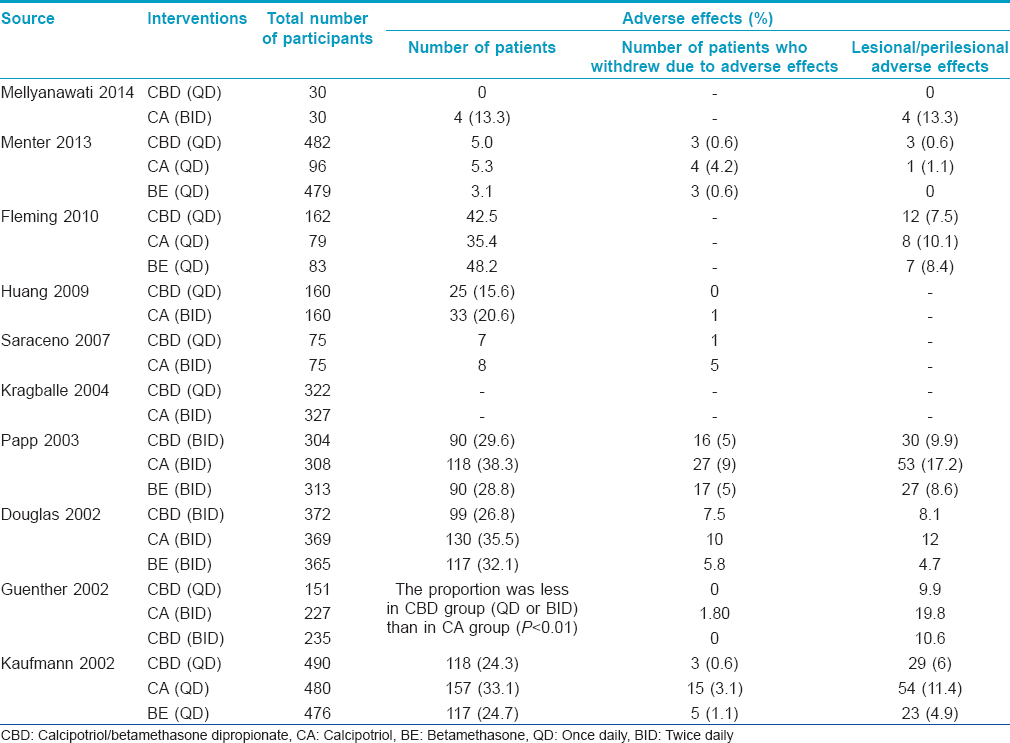
The incidence of adverse effects ranged from 0% to 42.5% with once daily calcipotriol/betamethasone dipropionate, 5.3-38.3% with calcipotriol monotherpay, 3.1-48.2% with betamethasone monotherapy and 26.8-29.6% with twice daily calcipotriol/betamethasone dipropionate. Lesional and perilesional cutaneous adverse drug reactions were recorded meticulously. The most common adverse effect noted in all groups was pruritus. Other adverse effects included pain, folliculitis, rash, pustules, dermatitis, exfoliation, erythema, aggravation of psoriasis, skin irritation, telangiectasia, etc. The proportion of patients reporting cutaneous adverse effects to calcipotriol/betamethasone dipropionate was similar across once daily (0-10%) and twice daily (8-10%) applications. The incidence of cutaneous adverse effects ranged from 11-20% with calcipotriol monotherapy, and 0-9% with betamethasone monotherapy. The proportion of patients reporting adverse effects to calcipotriol/betamethasone dipropionate was significantly less than those only on calcipotriol. The main reasons for withdrawal from the treatment protocol included unacceptable adverse effects, unacceptable treatment efficacy, poor compliance, patient being lost to follow-up or complete resolution of lesions. The percentage of patients who withdrew from treatment due to adverse effects was about 1% in the once-daily calcipotriol betamethasone dipropionate recipients, 1-10% in calcipotriol recipients, 1-6% in betamethasone recipients and 7.5% in the twice-daily calcipotriol betamethasone dipropionate recipients. The adverse effects usually resolved in a few weeks. The adverse effects are listed in [Table - 3].
DISCUSSION
We found several systematic reviews on topical calcipotriol/betamethasone dipropionate for the treatment of psoriasis. The current systematic review is conducted from various points of view. As calcipotriol/betamethasone dipropionate gel and ointment showed similar efficacies in previous studies, we included both formulations in our review. [10],[11] This is the main difference compared to previous systematic reviews. [22],[23],[24] Our study also paid close attention to the fixed compound formulation on body lesions, and not scalp lesions alone. [22],[23],[24],[25] A previous systematic review included both randomized controlled trials and non-randomized controlled trials. [24] As randomized controlled trials are considered to have a higher level of evidence, our review only included those studies. We included trials which had a duration of treatment ranging from four to eight weeks. Trials with a long term treatment duration of at least 24 weeks did not meet our inclusion criteria. [22],[24],[25],[26] Our review focused on the comparison of calcipotriol/betamethasone dipropionate with calcipotriol or betamethasone and was an update to the previous systematic reviews which included studies up to 2012. [3],[25],[26],[27],[28] According to the Cochrane library, a systematic review should be updated within 3 years. We believe that our systematic review will provide valuable and direct information about calcipotriol/betamethasone dipropionate in the treatment of psoriasis.
To assess the methodological quality, we used the criteria recommended by the Cochrane Collaboration Handbook. Of the ten studies, 80% adequately described the sequence generation and 60% described the allocation concealment so the selection bias was generally controlled. About 50% of studies performed double blinding of participants which indicates a moderate risk of performance bias. The risk of detection bias was moderately controlled as 30% of studies performed blinding of outcome assessment. Over 90% studies addressed incomplete outcome data and all studies reported the selected outcome. So, the attrition bias and reporting bias were well controlled. The overall methodological quality of included studies was moderate to high.
Our systematic review demonstrated that treatment with the two-compound formulation of calcipotriol/betamethasone dipropionate was more effective than either agent used alone. In the patients who were randomized to the calcipotriol/betamethasone dipropionate group, the percentage reduction of the PASI score was 40-48% at week 1, 50-74% at week 4 and remained at 55-73% at week 8. A higher reduction in the PASI score was observed after 1 week of treatment demonstrating the rapid onset of action of this compound. The improvement increased with continued application of the combination for four to eight weeks. The percentage of patients who showed marked improvement or clearance within 4 weeks was higher in the calcipotriol/betamethasone dipropionate group compared to the control groups, and increased further in eight weeks. All studies showed a high degree of consistency in the efficacy and tolerability results for calcipotriol/betamethasone dipropionate. [9],[12],[13],[14],[15],[16],[18],[19],[20],[29]
When once-daily calcipotriol/betamethasone dipropionate and twice-daily calcipotriol/betamethasone dipropionate were compared after four weeks of treatment, the percentage reduction of PASI score at week 1 and week 4 was not statistically or clinically significant. The proportion of "marked improvement or clearance" by investigator assessment at week 4 showed the same findings. [14] This result provides a useful reference for calcipotriol/betamethasone dipropionate application as once daily usage enables higher compliance and is economical too.
The adverse effect profile of calcipotriol/betamethasone dipropionate was similar across included studies with a comparable percentage of patients reporting cutaneous adverse reactions during treatment. The calcipotriol/betamethasone dipropionate combination was found to be safer than calcipotriol. No statistically significant differences existed between calcipotriol/betamethasone dipropionate and betamethasone, or once-daily calcipotriol/betamethasone dipropionate and twice-daily calcipotriol/betamethasone dipropionate. In a 52-week long-term study, treatment with calcipotriol/betamethasone dipropionate appeared to be safe and was well tolerated when used on its own or alternating every four weeks with calcipotriol. [30] Another six-month study showed high satisfaction and desire for repeated use of calcipotriol/betamethasone dipropionate in psoriasis vulgaris patients. [31] These results indicate a good safety profile of calcipotriol/betamethasone dipropionate.
CONCLUSION
Topical application of the two-compound product calcipotriol/betamethasone dipropionate provides good results for psoriasis vulgaris with only mild adverse effects. The optimal frequency of application is once daily. This combination is superior to either calcipotriol or betamethasone monotherapy when used for four to eight weeks, however longer durations of treatment are not recommended. We found that the studies included in this review were diverse and had limitations. Inconsistent treatment protocols, various outcome measurements, relatively short treatment duration and lack of long-term follow-up data were a few notable limitations. Randomized controlled trials that address these issues are needed in future studies.
Acknowledgement
The study was supported by skin disease research fund of China Society of Integrated Traditional Chinese and Western Medicine.
Financial support and sponsorship
Skin disease research fund of China Society of Integrated Traditional Chinese and Western Medicine.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85.
[Google Scholar]
|
| 2. |
Kragballe K, van de Kerkhof PC. Consistency of data in six phase III clinical studies of a two-compound product containing calcipotriol and betamethasone dipropionate ointment for the treatment of psoriasis. J Eur Acad Dermatol Venereol 2006;20:39-44.
[Google Scholar]
|
| 3. |
Fenton C, Plosker GL. Calcipotriol/betamethasone dipropionate: A review of its use in the treatment of psoriasis vulgaris. Am J Clin Dermatol 2004;5:463-78.
[Google Scholar]
|
| 4. |
Cunliffe WJ, Berth-Jones J, Claudy A, Fairiss G, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17-valerate ointment in patients with psoriasis vulgaris. J Am Acad Dermatol 1992;26(5 Pt 1):736-43.
[Google Scholar]
|
| 5. |
Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larkö O, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet 1991;337:193-6.
[Google Scholar]
|
| 6. |
Shupack JL, Jondreau L, Kenny C, Stiller MJ. Diflorasone diacetate ointment 0.05% versus betamethasone dipropionate ointment 0.05% in moderate-severe plaque-type psoriasis. Dermatology 1993;186:129-32.
[Google Scholar]
|
| 7. |
Kragballe K, Barnes L, Hamberg KJ, Hutchinson P, Murphy F, Møller S, et al. Calcipotriol cream with or without concurrent topical corticosteroid in psoriasis: Tolerability and efficacy. Br J Dermatol 1998;139:649-54.
[Google Scholar]
|
| 8. |
van Rossum MM, van Erp PE, van de Kerkhof PC. Treatment of psoriasis with a new combination of calcipotriol and betamethasone dipropionate: A flow cytometric study. Dermatology 2001;203:148-52.
[Google Scholar]
|
| 9. |
Saraceno R, Andreassi L, Ayala F, Bongiorno MR, Giannetti A, Lisi P, et al. Efficacy, safety and quality of life of calcipotriol/betamethasone dipropionate (Dovobet) versus calcipotriol (Daivonex) in the treatment of psoriasis vulgaris: A randomized, multicentre, clinical trial. J Dermatolog Treat 2007;18:361-5.
[Google Scholar]
|
| 10. |
Lambert J, Hol CW, Vink J. Real-life effectiveness of once-daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: 4-and 12-week interim results from the PRO-long study. J Eur Acad Dermatol Venereol 2014;28:1723-31.
[Google Scholar]
|
| 11. |
Queille-Roussel C, Hoffmann V, Ganslandt C, Hansen KK. Comparison of the antipsoriatic effect and tolerability of calcipotriol-containing products in the treatment of psoriasis vulgaris using a modified psoriasis plaque test. Clin Drug Investig 2012;32:613-9.
[Google Scholar]
|
| 12. |
Douglas WS, Poulin Y, Decroix J, Ortonne JP, Mrowietz U, Gulliver W, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol 2002;82:131-5.
[Google Scholar]
|
| 13. |
Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: A randomised, parallel group, double-blind, exploratory study. Eur J Dermatol 2010;20:465-71.
[Google Scholar]
|
| 14. |
Guenther L, Van de Kerkhof PC, Snellman E, Kragballe K, Chu AC, Tegner E, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: A randomized, double-blind, vehicle-controlled clinical trial. Br J Dermatol 2002;147:316-23.
[Google Scholar]
|
| 15. |
Huang L, Ma L, Huang Q, Yang QP, Zeng ZZ, Zhu XJ, et al. Caleipotriol betamemasone ointment in the treatment of psoriasis vulgaris: A randomized, double-blind, active-controlled, parallel group study. Chin J Dermatol 2009;42:691-4.
[Google Scholar]
|
| 16. |
Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology 2002;205:389-93.
[Google Scholar]
|
| 17. |
Kragballe K, Noerrelund KL, Lui H, Ortonne JP, Wozel G, Uurasmaa T, et al. Efficacy of once-daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. Br J Dermatol 2004;150:1167-73.
[Google Scholar]
|
| 18. |
Mellyanawati C, Huang X, Wei B, Fang S, Huuuang K, Chen AJ. Efficacy safety and quality of life of topical calcipotriol betamethasone dipropionate in treatment of psoriasis vulgaris. Chongqing Medicine 2014;43:1851-6.
[Google Scholar]
|
| 19. |
Menter A, Gold LS, Bukhalo M, Grekin S, Kempers S, Boyce BM, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: A randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol 2013;12:92-8.
[Google Scholar]
|
| 20. |
Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. J Am Acad Dermatol 2003;48:48-54.
[Google Scholar]
|
| 21. |
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration′s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
[Google Scholar]
|
| 22. |
Daudén E, Bewley A, Lambert J, Girolomoni G, Cambazard F, Reich K. Expert recommendations: The use of the fixed combination calcipotriol and betamethasone dipropionate gel for the topical treatment of psoriasis. J Eur Acad Dermatol Venereol 2014;28 Suppl 2:22-32.
[Google Scholar]
|
| 23. |
Kragballe K, van de Kerkhof P. Pooled safety analysis of calcipotriol plus betamethasone dipropionate gel for the treatment of psoriasis on the body and scalp. J Eur Acad Dermatol Venereol 2014;28 Suppl 2:10-21.
[Google Scholar]
|
| 24. |
Augustin M, Mrowietz U, Bonnekoh B, Rosenbach T, Thaçi D, Reusch M, et al. Topical long-term therapy of psoriasis with vitamin D3 analogues, corticosteroids and their two compound formulations: Position paper on evidence and use in daily practice. J Dtsch Dermatol Ges 2014;12:667-82.
[Google Scholar]
|
| 25. |
Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev 2013;3:CD005028.
[Google Scholar]
|
| 26. |
Samarasekera EJ, Sawyer L, Wonderling D, Tucker R, Smith CH. Topical therapies for the treatment of plaque psoriasis: Systematic review and network meta-analyses. Br J Dermatol 2013;168:954-67.
[Google Scholar]
|
| 27. |
Vakirlis E, Kastanis A, Ioannides D. Calcipotriol/betamethasone dipropionate in the treatment of psoriasis vulgaris. Ther Clin Risk Manag 2008;4:141-8.
[Google Scholar]
|
| 28. |
van de Kerkhof P, de Peuter R, Ryttov J, Jansen JP. Mixed treatment comparison of a two-compound formulation (TCF) product containing calcipotriol and betamethasone dipropionate with other topical treatments in psoriasis vulgaris. Curr Med Res Opin 2011;27:225-38.
[Google Scholar]
|
| 29. |
Langley RG, Gupta A, Papp K, Wexler D, Østerdal ML, Curcic D. Calcipotriol plus betamethasone dipropionate gel compared with tacalcitol ointment and the gel vehicle alone in patients with psoriasis vulgaris: A randomized, controlled clinical trial. Dermatology 2011;222:148-56.
[Google Scholar]
|
| 30. |
Kragballe K, Austad J, Barnes L, Bibby A, de la Brassinne M, Cambazard F, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris. Br J Dermatol 2006;154:1155-60.
[Google Scholar]
|
| 31. |
Claréus BW, Houwing R, Sindrup JH, Wigchert S. The DESIRE study - Psoriasis patients′ satisfaction with topical treatment using a fixed combination of calcipotriol and betamethasone dipropionate in daily clinical practice. Eur J Dermatol 2009;19:581-5.
[Google Scholar]
|
Fulltext Views
9,619
PDF downloads
3,341





