Translate this page into:
Afatinib-induced hypertrichosis of the eyelashes and eyebrows
2 Department of Oncology, Hospital Ramon y Cajal, E-28034 Madrid, Spain
Correspondence Address:
Laura Miguel-Gomez
Department of Dermatology, Hospital Ramon y Cajal, E-28034 Madrid,
Spain
| How to cite this article: Miguel-Gomez L, Vano-Galvan S, Garrido-Lopez P, Jaen-Olasolo P. Afatinib-induced hypertrichosis of the eyelashes and eyebrows. Indian J Dermatol Venereol Leprol 2016;82:192-193 |
Sir,
Afatinib is an irreversible tyrosine kinase inhibitor that blocks the epidermal growth factor receptor. It has been recently approved as a first-line treatment for patients with advanced non-small cell lung cancer with epidermal growth factor receptor mutations. It has been demonstrated in randomized trials that progression-free survival is significantly higher with afatinib than with other existing treatments. Several adverse reactions have been reported in patients treated with epidermal growth factor receptor inhibitors, most affecting the gastrointestinal and respiratory tracts and the skin. Although cutaneous side effects are usually mild, they may be cosmetically unacceptable to patients. [1]
A 68-year-old woman was diagnosed with stage IV lung adenocarcinoma; epidermal growth factor receptor mutation status of the tumor was positive. She was a non-smoker and her past medical history was significant for hypertension, hypercholesterolemia and hypothyroidism. She was treated with pemetrexed (500 mg/m 2 /intravenous) and cisplatin (75 mg/m 2 /intravenous) as palliative therapy and there was no tumor progression noted after 4 months. Despite four cycles of chemotherapy, she developed a left pleural effusion and millimetric nodules in both lung fields. The treatment was switched to afatinib (40 mg orally once daily) and was tolerated well except for a moderate oral mucositis. After 4 months of afatinib therapy, she complained of excessive growth of both eyelashes and eyebrows [Figure - 1]. She was on no other treatment and denied the application of any topical product. Physical examination revealed trichomegaly and excessive growth of the eyebrow. The patient was reassured and tolerated this adverse effect well, requiring only regular trimming of the eyelashes and eyebrows over the next few months.
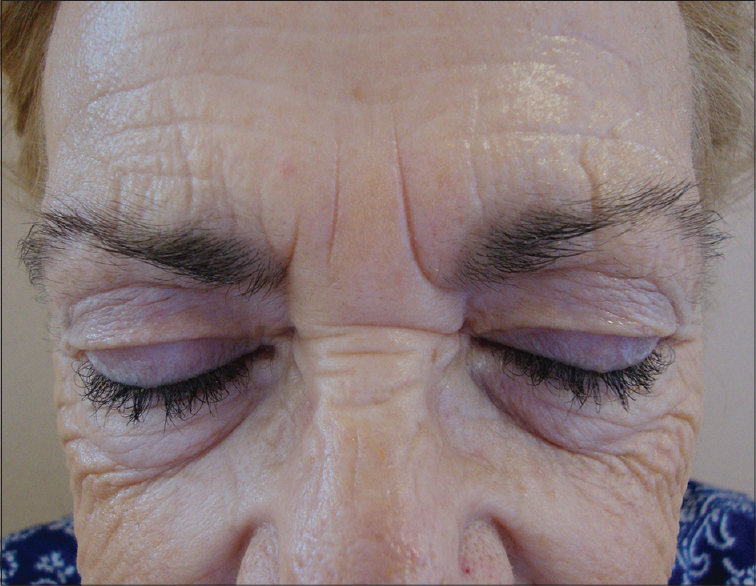 |
| Figure 1: Trichomegaly and an abnormal growth of eyelashes are shown |
Epidermal growth factor receptor inhibitors are associated with multiple cutaneous side effects such as acneiform eruptions, paronychia, mucositis, pruritus, xerosis, telangiectasia and hyperpigmentation. Hair changes such as alopecia, facial hypertrichosis, eyebrow growth and trichomegaly have also been reported. [2],[3] Trichomegaly is an abnormal growth of eyelashes causing discomfort. This side effect has been described in association with cetuximab, erlotinib, gefitinib, and panitumumab. [4]
The epidermal growth factor receptor is involved in the development and differentiation of the hair follicle. This receptor is expressed in keratinocytes of the hair follicle and regulates the transformation from anagen to catagen. Its inhibition can stimulate the formation of a disorganized hair follicle with abnormal hair growth. Trichomegaly usually appears 10-14 weeks after the beginning of treatment with these medications. [5] It is usually a mild side effect that does not require discontinuation of the drug. Nevertheless, in some patients it may be a cosmetic and ophthalmological problem because keratitis, conjunctivitis or trichiasis may occur. [4] Trichomegaly is usually reversible with discontinuation of treatment. It is not yet known if epidermal growth factor receptor inhibitor-associated trichomegaly occurs only in patients whose tumor is responding to treatment. We were unable to find any previous reports of this adverse effect.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Munoz J, Hanbali AS. Epidermal growth factor receptor-induced hirsutism and trichomegaly. Mayo Clin Proc 2011;86:e50.
[Google Scholar]
|
| 2. |
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006;55:657-70.
[Google Scholar]
|
| 3. |
Vergou T, Stratigos AJ, Karapanagiotou EM, Matekovits AE, Dilana KD, Tsimboukis S, et al. Facial hypertrichosis and trichomegaly developing in patients treated with the epidermal growth factor receptor inhibitor erlotinib. J Am Acad Dermatol 2010;63:e56-8.
[Google Scholar]
|
| 4. |
Jeon SH, Ryu JS, Choi GS, Kim JS, Kwon HY, Kim MS, et al. Erlotinib induced trichomegaly of the eyelashes. Tuberc Respir Dis (Seoul) 2013;74:37-40.
[Google Scholar]
|
| 5. |
Cohen PR, Escudier SM, Kurzrock R. Cetuximab-associated elongation of the eyelashes: Case report and review of eyelash trichomegaly secondary to epidermal growth factor receptor inhibitors. Am J Clin Dermatol 2011;12:63-7.
[Google Scholar]
|
Fulltext Views
3,760
PDF downloads
1,867





