Translate this page into:
Viva questions from the IJDVL
2 Department of Dermatology, K J Somaiya Medical College and Research Centre, Mumbai, Maharashtra, India
Correspondence Address:
Vishalakshi Viswanath
Department of Dermatology, Rajiv Gandhi Medical College, Thane, Maharashtra
India
| How to cite this article: Viswanath V, Vasani R. Viva questions from the IJDVL. Indian J Dermatol Venereol Leprol 2016;82:589-594 |
Focus: Demodex in Dermatology
Identify the ectoparasite depicted in [Figure - 1] and describe its characteristic features
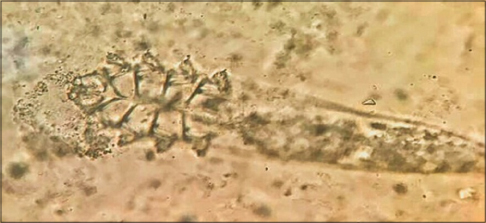 |
| Figure 1: Identify the ectoparasite |
The ectoparasite belongs to Demodex species, an obligate human ectoparasite which is commonly found at or near the pilosebaceous units.
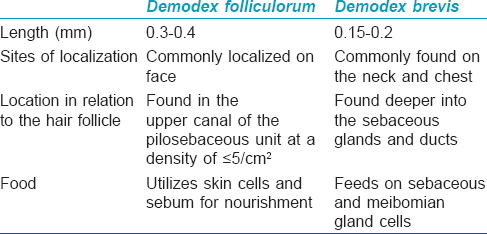
The two species of dermatological importance include Demodex folliculorum and Demodex brevis. The characteristic differentiating features of these species are outlined in [Table - 1].
What are the sites of predilection for Demodex infestation?
The sites of predilection for Demodex infestation are as follows:
- Face – forehead, cheeks, nose, chin, temples, scalp, external ears, eyelash follicles, eyebrows and neck
- Seborrheic areas – nasolabial fold, periorbital areas, upper and medial regions of chest and back
- Other areas where it can be found are penis, mons veneris, buttocks and ectopic sebaceous glands of buccal mucosa.
What are the methods to detect Demodex?
- Microscopic examination of mites by potassium hydroxide examination of skin scrapings. The test is considered positive when there are more than five mites either in one follicle or in one low power field by scraping.
Adhesive bands, skin scrapings, skin impressions, expressed follicular contents, comedone extraction, hair epilation and punch biopsies can be used to demonstrate Demodex microscopically.
- Skin surface biopsy technique with cyanoacrylate adhesion. The test is considered positive when there are more than five mites in 1 cm 2 area by standardized skin surface biopsy.
- Newer diagnostic modalities include dermatoscopy, confocal laser scanning microscopy and high-definition optical coherence tomography. On dermatoscopy, the characteristic findings are presence of Demodex tails (creamy gelatinous tails representing the mite) and follicular openings (round, coarse openings with light brown/grayish plugs surrounded by an erythematous halo).
When is infestation with Demodex mites of significance?
Mere presence of Demodex does not indicate pathogenicity. Penetration of Demodex into the dermis or, more commonly, an increase in the number of mites in the pilosebaceous unit of >5/cm 2 is believed to cause inflammation. Some authors consider the density of >5 mites per follicle as a pathogenic criterion.
What are the predisposing factors for demodicosis?
- Primary immunosuppression based on hereditary defect of T-cells
- Secondary immunosuppression following corticosteroid or immunosuppressant therapy, or due to diseases of an immunocompromised nature such as malignant neoplasia, hepatopathies, lymphosarcoma and human immunodeficiency virus infection
- Genetic predisposition.
What are the dermatological conditions in which Demodex has been implicated?
There are various dermatological conditions in which there is an increase in Demodex mite density.[1]
- Rosacea: that cause immunosuppression oily skin, absent follicular scaling and more deeply seated lesions
- Rosacea-like demodicosis: dryness, follicular scaling, superficial vesicles and pustules
- Steroid rosacea
- Demodicosis gravis (granulomatous rosacea-like demodicosis)
- Lupus miliaris disseminatus faciei
- Non-specific facial dermatitis
- Androgenetic alopecia
- Madarosis
- Dissecting folliculitis
- Miscellaneous: perioral dermatitis, blepharoconjunctivitis, Grover's disease, eosinophilic folliculitis, Demodex abscess.
Recently, a new classification of demodicosis has been proposed as primary and secondary forms based on clinical manifestations.[2]
Primary demodicosis
There is an abnormal increase in mite colonization in the absence of preexisting or concurrent inflammatory dermatoses such as acne, rosacea or perioral dermatitis. Remission of the disease occurs only after topical or systemic acaricides but not with antibiotics possessing anti-inflammatory effects such as doxycycline or macrolides. This form is characterized by late-onset disease and is usually asymptomatic or may present with mildly pruritic lesions commonly seen on the face and especially the periorificial areas. Variants include spinulate demodicosis, Demodex folliculitis, ocular and auricular demodicosis.
Secondary demodicosis
It can occur as a part of known skin or systemic diseases such as human immunodeficiency virus infection, leukemia or chronic renal failure. Associations include perioral dermatitis, papulopustular rosacea, seborrheic dermatitis and tumors such as eyelid basal cell carcinoma or mycosis fungoides. It may be seen in patients on topical or systemic steroids, topical calcineurin inhibitors, phototherapy and epidermal growth factor inhibitors. This form is characterized by an early onset with more diffuse facial distribution or truncal involvement with more extensive inflammation.
Outline the preventive and therapeutic modalities for demodicosis
Preventive measures include regular facial cleansing with avoidance of oil-based cleansers and greasy cosmetics. Therapeutic modalities for demodicidosis include:
- Systemic: metronidazole, ivermectin, tetracyclines
- Topical: metronidazole 0.75–2%, salicylic acid, gamma benzene hexachloride, sublimed sulfur, permethrin, crotamiton, benzyl benzoate 10%, azelaic acid 15–20%.
Ichthyosis Follicularis, Alopecia and Photophobia Syndrome
What is ichthyosis follicularis, alopecia and photophobia (IFAP) syndrome?
It is a syndrome of ichthyosis follicularis, atrichia and photophobia. It is an X-linked recessive condition caused by missense mutations in membrane-bound transcription factor protease site 2 gene causing impaired cholesterol homeostasis and ability to cope with endoplasmic reticulum stress.
What are the clinical features of ichthyosis follicularis, alopecia and photophobia syndrome?
The clinical manifestations of ichthyosis follicularis, alopecia and photophobia syndrome includes:
- Ichthyosis follicularis: spiny follicular projections typically described as “ prickly surface of a rose leaf”
- Congenital alopecia
- Photophobia can occur early in life or later in childhood. Superficial corneal ulceration and vascularization can lead to progressive corneal scarring and photophobia
- The most frequent neurological features are intellectual disability and seizures
- Other clinical features are short stature, dysmorphic features such as frontal bossing, choanal atresia and large ears
- Various intestinal anomalies, renal, cardiac, vertebral anomalies and cleft hands have been reported.
Urethritis
A 25-year-old unmarried sexually active man presents with symptoms of dysuria with mild whitish urethral discharge for the past 1 week. There is a positive history of unprotected exposure with a commercial sex worker 15 days before the onset of symptoms.
What is the likely diagnosis?
The prior history of unprotected sexual exposure accompanied by dysuria and urethral discharge in a man points to a diagnosis of urethritis. The presence of scanty mucoid urethral discharge would go more in favor of non-gonococcal urethritis though the final diagnosis could be confirmed after the demonstration of the organism on smear/culture.
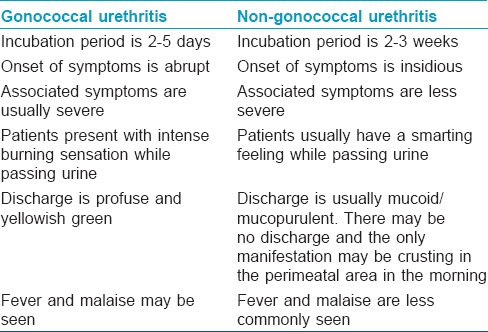
Enumerate the differences between gonococcal and non-gonococcal urethritis
There are certain differences between gonococcal and non-gonococcal urethritis [Table - 2].
Which is the bedside investigation for diagnosing the origin of urethral discharge?
A simple bedside investigation that can be done to know the origin of the urethral discharge (from anterior/posterior urethra or bladder) is a two-glass and three-glass urine test. (Though this is not done as a routine and is an outdated test, this can be posed as a viva question).
In this test, the patient is asked to pass urine in two separate glasses.
- In cases of anterior urethritis, the first specimen is cloudy/hazy while the second glass is clear
- If the second glass is hazy, it should be considered as a case of posterior urethritis
- If both glasses are hazy, one should think of bacterial cystitis.
For better accuracy, one can perform the three-glass urine test. Here, the anterior urethra is irrigated with a colorless antiseptic (1:8000 oxycyanide of mercury) until washings in the first glass are clear. Then, patient is asked to pass urine in two glasses. If the first sample contains pus, the infection lies in the posterior urethra and if the second glass contains pus, the infection lies in the bladder.
How can a diagnosis be confirmed in the above case scenario?
To confirm the diagnosis, urethral material needs to be obtained using meatal or intraurethral swabs by milking the penis from base to the glans (since in this case, the discharge is very scanty). Patient should be asked not to pass urine for 2 hours prior to the test. The swab is introduced for 2–3 cm inside the urethra and then withdrawn gradually. This smear is then stained with Gram stain.
- Presence of Gram-negative diplococci inside the polymorphonuclear leukocytes is diagnostic of gonococcal urethritis. If Gram-negative diplococci are seen only extracellularly, it has to be confirmed by culture
- Non-gonococcal urethritis is diagnosed when there are ≥5 white blood cells per oil immersion field in the absence of Gram-negative diplococci.
Chlamydia trachomatis is the most common cause of non-gonococcal urethritis. Other pathogens are Trichomonas vaginalis, Mycoplasma hominis, Ureaplasma urealyticum. For a more specific diagnosis of C. trachomatis, the sample can be sent for culture (HeLa 229 and McCoy cell lines). Other non-culture, non-invasive diagnostic tests are included under a broad classification as antigen detection tests/nucleic acid hybridization/nucleic acid amplification/urethral lymphocyte isolation.
How will you manage a case of non-gonococcal urethritis?
The patient should be screened for other treatable sexually transmitted infections and should be offered counseling and serologic testing for human immunodeficiency virus. An effort should be made to identify and treat the infected partner.
The treatment of non-gonococcal urethritis would include azithromycin 1 g as a single dose, orally or doxycycline 100 mg twice daily for 7 days or alternatively, erythromycin stearate 500 mg 4 times a day can be prescribed. The patient should be instructed to abstain from sex for 7 days after therapy, even after treatment with a single dose of azithromycin.
What is post-gonococcal urethritis?
Non-gonococcal urethritis occurring soon after curative therapy of gonorrhea is called post-gonococcal urethritis. These patients probably acquire gonorrhea and chlamydial infection simultaneously but because of the longer incubation period of the latter, develop a biphasic illness if the gonorrhea is treated with an agent that does not eradicate chlamydia.
Porokeratotic Eccrine Ostial and Dermal Duct Nevus
Describe the clinical features and associations of porokeratotic eccrine ostial and dermal duct nevus
Clinically, porokeratotic eccrine ostial and dermal duct nevus has punctate pits with comedo-like plugs typically localized to palms and soles. However, lesions at other sites as well as blaschkoid and systemized patterns have been reported. Lesions may become verrucous, especially on hair-bearing skin beyond the palms and soles.
Associations include breast hypoplasia, palmoplantar keratoderma, psoriasis, hemiparesis, seizure disorder, scoliosis, polyneuropathy, hyperthyroidism, developmental delay, onychodystrophy or squamous cell carcinoma.
Describe the characteristic histologic findings of porokeratotic eccrine ostial and dermal duct nevus
Histopathological examination shows the characteristic cornoid lamella (epidermal acanthosis and a deep epidermal invagination of a parakeratotic column with underlying absence of granular layer) and a dilated acrosyringium beneath the cornoid lamella.
Enumerate the differential diagnoses of porokeratotic eccrine ostial and dermal duct nevus
Differential diagnoses include linear porokeratosis, nevus comedonicus, inflammatory linear verrucous epidermal nevus, verrucous epidermal nevus, linear psoriasis and punctate keratoderma.
Porokeratotic eccrine ostial and dermal duct nevus and linear porokeratosis are difficult to distinguish both clinically and histopathologically, especially on non-acral sites. Clinically, non-acral porokeratotic eccrine ostial and dermal duct nevus lacks typical keratotic comedo-like lesions, is less scaly and has an appearance similar to conventional porokeratosis. Histopathological features may overlap and a diagnosis of linear porokeratosis may be made if adnexa are not included in the section showing a cornoid lamella. Conversely, a misdiagnosis of porokeratotic eccrine ostial and dermal duct nevus may be made if the cornoid lamella in a conventional case of porokeratosis is associated with underlying adnexa. Therefore, multiple biopsies and deeper sections are recommended.
What are the pathogenic mechanisms for development of porokeratotic eccrine ostial and dermal duct nevus?
A keratinization defect with abnormally dilated parakeratotically plugged acrosyringium and increased proliferation of basal keratinocytes may be the pathogenic mechanism. Lack of carcinoembryonic antigen expression, genetic mosaicism and somatic gene mutation are other proposed theories.
What are the therapeutic modalities for porokeratotic eccrine ostial and dermal duct nevus?
Various therapies include topical keratolytics, corticosteroids, calcipotriol, anthralin, tar, urea, phototherapy, cryotherapy, electrocautery, surgical excision and ultrapulsed carbon dioxide laser.
Metabolic Syndrome and Psoriasis
Enumerate the components of metabolic syndrome?
The diagnosis of metabolic syndrome is based on the presence of ≥3 criteria of the modified National Cholesterol Education Program's Adult Treatment Panel:
- Waist circumference >102 cm in men or >88 cm in women
- Hypertriglyceridemia ≥150 mg/dL
- High-density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women
- Blood pressure ≥130/85 mmHg
- Fasting plasma glucose ≥100 mg/dL.
Which dermatological conditions can be associated with metabolic syndrome?
The dermatological conditions associated with metabolic syndrome include psoriasis, polycystic ovarian syndrome (cutaneous signs of hyperandrogenism such as acne, seborrhea, hirsutism, alopecia, or frank virilization), lipodystrophies, androgenetic alopecia, acne inversa, lichen planus, acrochordons, acanthosis nigricans, systemic lupus erythematosus and cutaneous malignancies.
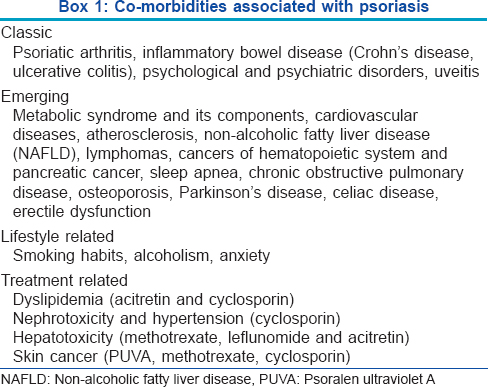
What are the co-morbidities associated with psoriasis?
The comorbidities associated with psoriasis are outlined in [Box 1].[3]
Angioma Serpinginosum
Describe the clinical features of angioma serpiginosum
Angioma serpiginosum is a rare, benign, acquired, vascular nevoid, mosaic condition of unknown etiology. It is a progressive anomaly that starts in early childhood, stabilizes in adults and may partially involute with time. It has a female preponderance and chiefly involves the lower extremities and buttocks. The lesions are copper to bright red, punctate, non-blanchable or partially blanchable, grouped macules that may develop into papules with a background of erythema. Lesions enlarge by developing new lesions at the periphery with clearing of lesions in the center and this leads to a serpiginous or gyrate or ring-like morphology.
What are the dermatoscopic and histological characteristics of angioma serpiginosum?
Dermatoscopy reveals the characteristic presence of round to oval red lagoons.
Histopathology shows proliferating and dilated capillaries in the superficial papillary dermis. Periodic acid–Schiff stain shows deposits around involved vessels and absence of erythrocyte extravasation or hemosiderin deposits or inflammatory cells; this helps to differentiate angioma serpiginosum from pigmented purpuric dermatoses and unilateral nevoid telangiectasia.
Giant Cell Arteritis
What is giant cell arteritis?
Giant cell arteritis is a rare systemic immune-mediated vasculitis that affects the elderly, characterized by a pan-arteritis of medium and large arteries, especially the extracranial branches of the carotid artery. The synonyms are temporal arteritis or cranial arteritis. It may be associated with the HLA-DR4 haplotype with a genetic predisposition. Other studies suggest a possible association with chlamydia pneumonia, parvovirus B19 or herpes zoster infection.
Describe the clinical manifestations of giant cell arteritis
The clinical features occur due to the inflammation and occlusion of affected arteries. Fever, weight loss, headache, visual loss, facial pain, ataxia, vertigo or deafness may be the presenting features. Uncommon manifestations include cerebrovascular accidents, neuropsychiatric manifestations, angina or myocardial infarction, bowel angina or infarction, leg claudication, unilateral or bilateral scalp ulceration and tongue or corneal ulceration.
Enumerate the treatment modalities for giant cell arteritis
High-dose corticosteroids are the treatment of choice. Additional immunosuppressive therapy, such as azathioprine, mycophenolate mofetil or methotrexate may be needed. A newer modality, humanized monoclonal anti-interleukin-6-receptor antibody, tocilizumab has shown good response in some cases. Aspirin may be recommended if there are no contraindications.
Livedoid Vasculopathy
What are the synonyms of livedoid vasculopathy?
Livedoid vasculopathy is a hyalinizing vascular disease characterized by thrombosis and ulceration of the lower extremities. Various synonyms include livedoid vasculitis, segmental hyalinizing vasculitis, atrophie blanche, livedo reticularis with ulcerations and painful purpuric ulcers with reticular pattern on the lower extremities.
Enumerate the conditions associated with livedoid vasculopathy?
The various conditions which can be associated with livedoid vasculopathy include:
- Inherited or acquired thrombophilias: protein C and S deficiency, homocysteinemia, cryoglobulinemia, cryofibrinogenemia, antiphospholipid syndrome
- Autoimmune connective tissue diseases: rheumatoid arthritis, scleroderma, systemic lupus erythematosus, mixed and undifferentiated connective tissue diseases
- Malignancies: hematological (multiple myeloma), solid organ tumors.
Describe the cutaneous features of livedoid vasculopathy
Livedoid vasculopathy has a chronic, recurrent course with episodic exacerbations. The classical triad of manifestations includes livedo reticularis, leg ulcerations and atrophie blanche.
Livedo reticularis or livedo racemosa
Lesions are distributed symmetrically on dorsa of the feet, ankles and lower legs. The initial lesions are purpuric macules, papules or ecchymotic net-like streaks which develop due to abnormal perfusion of the cutaneous microcirculation.
Leg ulceration
Burning pain precedes the ulceration; painful, necrotic ulcers are classically located asymmetrically on the ankle extending to the dorsum of the foot and up to distal leg. The ulcers are characteristically small, irregular, painful and recurrent and rarely may coalesce to form big ulcers. Ulcers develop due to thrombotic ischemia.
Atrophie blanche
These are porcelain-white stellate scars usually surrounded by telangiectasias and hyperpigmentation and represent irreversible infarction.
Enumerate the causes of atrophie blanche
The causes of atrophie blanche include:
- Vascular causes: peripheral vascular disease, vasculitis, especially polyarteritis nodosa, livedoid vasculopathy, stasis dermatitis, cryoglobulinemia, Sneddon's syndrome, Degos' disease
- Hematological causes: polycythemia vera, thalassemia, essential thrombocytosis, chronic myeloid leukemia
- Connective tissue disorders: systemic lupus erythematosus, scleroderma
- Miscellaneous: pyoderma gangrenosum, factitial, post-pulsed dye laser, drug induced: hydroxyurea.
Describe the histopathological findings in livedoid vasculopathy
Histopathological findings depend on the stage of the disease. Examination of the ulcers is diagnostic. It is important to include the borders of the ulcer while doing a biopsy rather than taking only the base.
Characteristic fibrin occlusion and thrombus formation involving the upper and mid-dermal capillaries are seen in the early stage. Perivascular infiltrate is minimal and predominantly lymphocytic. Extravasation of red blood cells results from vessel wall damage and there is endothelial proliferation. Leukocytoclasia is usually absent and if neutrophils are found, it is usually secondary to the necrotizing ulcerative process. In the stage of atrophie blanche, there is hyalinization of dermis and capillary walls.
Enumerate treatment modalities in livedoid vasculitis
Treatment modalities depend on the associated condition and stage of presentation. Anticoagulants, antiplatelet agents, fibrinolytics, vasodilators, anti-inflammatory drugs and immunosuppressants form the mainstay. Intravenous immunoglobulin, hyperbaric oxygen, psoralen ultraviolet A therapy (PUVA) and supplements can be used. Supportive measures such as rest, leg elevation and compression stockings are important.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rather PA, Hassan I. Human Demodex mite: The versatile mite of dermatological importance. Indian J Dermatol 2014;59:60-6.
[Google Scholar]
|
| 2. |
Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol 2014;170:1219-25.
[Google Scholar]
|
| 3. |
Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: Classical and emerging comorbidities. An Bras Dermatol 2015;90:9-20.
[Google Scholar]
|
Fulltext Views
8,181
PDF downloads
2,106





