Translate this page into:
Nonsegmental vitiligo follows Blaschko's lines and embryonic pigmentary segments
Correspondence Address:
Nilendu Sarma
P N Colony, Sapui Para, Bally, Howrah - 711 227, West Bengal
India
| How to cite this article: Sarma N. Nonsegmental vitiligo follows Blaschko's lines and embryonic pigmentary segments. Indian J Dermatol Venereol Leprol 2020;86:350-358 |
Abstract
Background: Pathogenic mechanism that determines the localization of vitiligo patches and thus a patterned distribution in patients with nonsegmental vitiligo has remained poorly elucidated. A distributional similarity of the vitiligo patches with Blaschko's lines has been documented in patients with segmental vitiligo, both isolated segmental vitiligo and mixed vitiligo but never in cases of nonsegmental vitiligo.
Methods: Distribution of nonsegmental vitiligo patches on face and neck regions was assessed and compared with Blaschko's lines and also with embryonic pigmentary segments on the face.
Results: This study has documented distributional similarity of the nonsegmental vitiligo patches on face and neck with Blaschko's lines and the “embryonic pigmentary segments” among 154 (58.6%) cases. Patches around the palpebral and other fissures like periorbital, perinasal, perioral, and periaural were more common. In addition to the vitiligo patches, the spared areas were also found to respect the embryonic segmental outlines and follow the Blaschko's lines.
Conclusion: Distributional pattern of the individual nonsegmental vitiligo patches along the Blaschko's lines and embryonic pigmentary segments suggests that mosaicism might control the susceptibility to the disease process in a patterned manner.
Limitation: No genetic testing could be performed to confirm the hypothesis. Evaluation of nonsegmental vitiligo was done only on the face and neck areas.
Introduction
Vitiligo has been classified into two clinical subtypes namely, segmental vitiligo and nonsegmental vitiligo.[1] Presence of both forms in a single individual is termed as mixed vitiligo, and a single patch of vitiligo that has not evolved into either subtypes for 1–2 years is called focal vitiligo.[1] The simplicity in this phenotype-based classification, that suggests distinct pathogenic mechanisms, is considered better than most other existing systems of classification and is thus widely accepted.
Mosaicism is a genetic mechanism and is defined as the presence of two or more different cell lines in an individual (mosaic). Mosaicism limits the extent of genetic aberration in the individual. Nevi are common cutaneous conditions resulting from mosaicism. Cutaneous conditions developing due to mosaicism follow some definite patterns. Blaschko's lines are the most common clinical patterns that indicate underlying mosaicism.[2] Striking similarity in the distribution pattern between the segmental vitiligo and Blaschko's lines and occasionally some other patterns of mosaicism has been reported.[3],[4],[5],[6],[7],[8] Based on these clinical observations, somatic mosaicism has been suggested to exist in segmental vitiligo.[3],[4],[5],[6],[7],[8]
A role of mosaicism in nonsegmental vitiligo was hypothetically suggested.[9] However, the authors did not compare the patterns in those cases with Blaschko's lines or any of the known patterns of mosaicism.[9]
This study has documented the distribution patterns of nonsegmental vitiligo patches along the Blaschko's lines,[10],[11] and embryonal pigmentary units in a large number of cases suggesting a role of mosaicism in these cases.
Methods
This was a descriptive type of observational study, cross-sectional in design, conducted in the dermatology outpatient department of a tertiary care hospital and medical college in the eastern part of India. Patient population attending the hospital was from all over the state (West Bengal) and also from the nearby states and consisted of a mixed profile with regard to the socioeconomic profile.
All consecutive patients with bilateral nonsegmental vitiligo having a distribution over the face and neck regions were included. All mixed vitiligo (segmental vitiligo along with nonsegmental vitiligo), nonconsenting and moribund patients were excluded. Written consent from the patients or from the parents (in case of minor patients) was obtained in all cases. Face and neck area was selected as the Blaschko's lines are most distinctive on this region.
All cases were divided into two categories, cases with limited involvement (Category A) and cases with near-complete involvement with very limited areas of sparing (Category B). Pattern analysis was done with vitiligo patches in the first group and with the spared areas in the second group.
Specific history was asked about the location of initial patches and on subsequent progression of the patches. When the spared patches were evaluated for the pattern (Category B), it was enquired whether such patches were truly spared since the beginning or were actually repigmented. Photographs of the lateral and frontal view of the face and neck of the patients were taken.
Spatial patterns like shape, size, and orientation of the vitiligo patches on head and neck regions were graphically drawn on a diagram of a human face and compared with “Blaschko's lines” and also “embryonic pigmentary segments and units on face”[10],[11] [Figure - 1] and [Figure - 2].
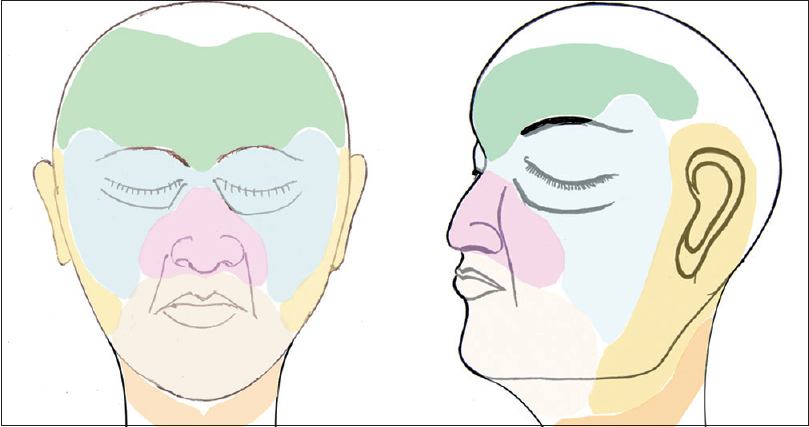 |
| Figure 1: Segmental distribution of nonsegmental vitiligo patches on face and neck. Area around palpebral fissure and on lips have been additionally marked to show the common occurrence of patches within the respective segments |
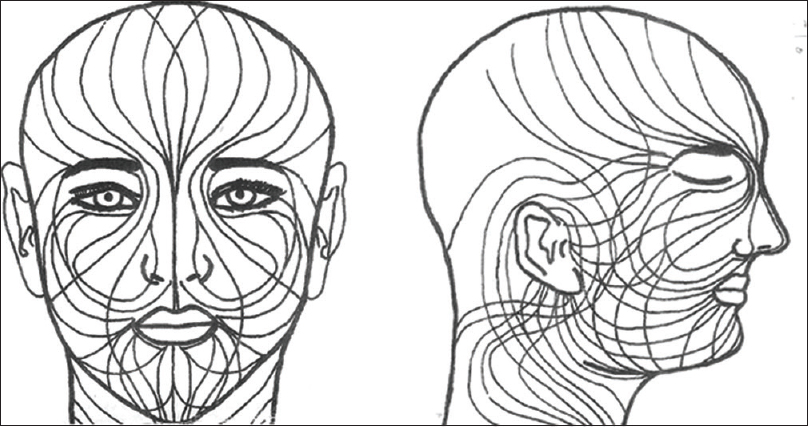 |
| Figure 2: Blaschko's lines on face and neck[10] |
The study was approved by the institutional ethics committee before the study was started.
Results
A total of 263 cases were selected for pattern analysis, 224 (85.20%) cases in Category A and 39 (14.80%) cases in Category B. The mean age at presentation and at onset was much higher (51.5 and 23.9 years) in Category B than Category A (24.3 and 15.2 years). Pattern analysis was found to be difficult on the hairy scalp in most of the cases and thus avoided.
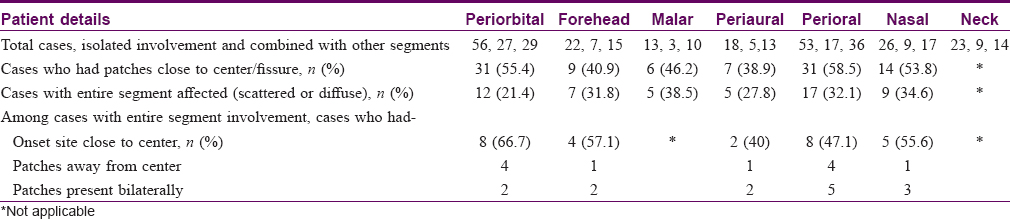
Out of 263 cases, some definite patterns were observed in 154 (58.6%) cases [Table - 1]. It was noticed that vitiligo patches were distributed into six areas or segments on face and into one segment on the neck (each side). The facial segments were forehead, periorbital (eyelids and surrounding skin), malar, nasal (nares and surrounding skin), perioral (lips and surrounding skin), and periaural (around ear) [Table - 2] and [Figure - 1]. These segments matched the pattern of Blaschko's lines and embryonic pigmentary segments and units [Figure - 2] and [Figure - 3].
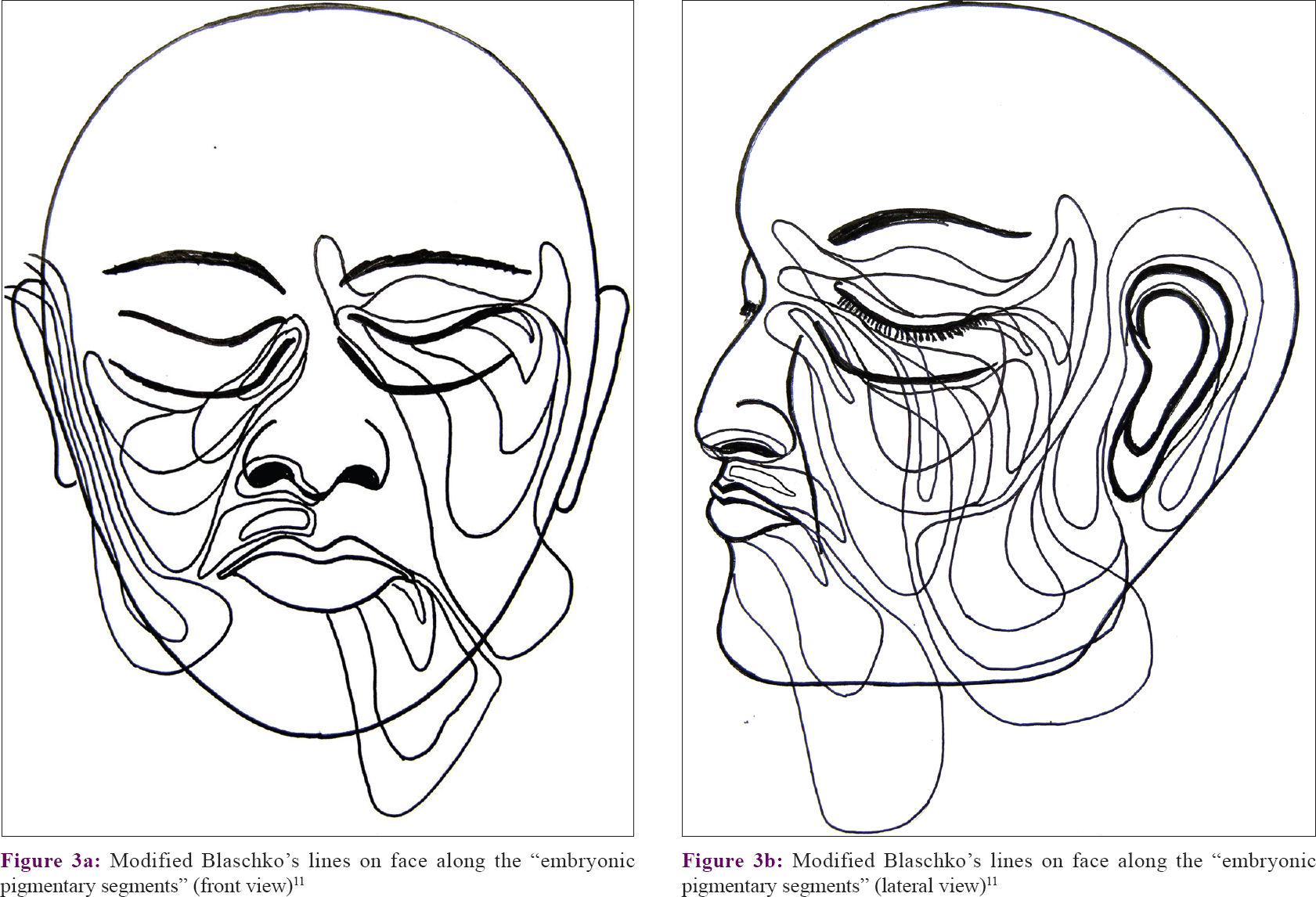 |
| Figure 3: |
Vitiligo patches that involved only a single segment, partially or completely, were noted among 77 cases and patches involving more than one segment were affected among 55 cases.
In the upper part of the face, three different patterns and thus three segments were noted. A majority of these patches (>42%) developed around the orbital fissure with a mediolateral orientation pattern. More laterally, they followed a curved distribution and turned upward on the temporal area or downward on the zygomatic areas [Figure - 4].
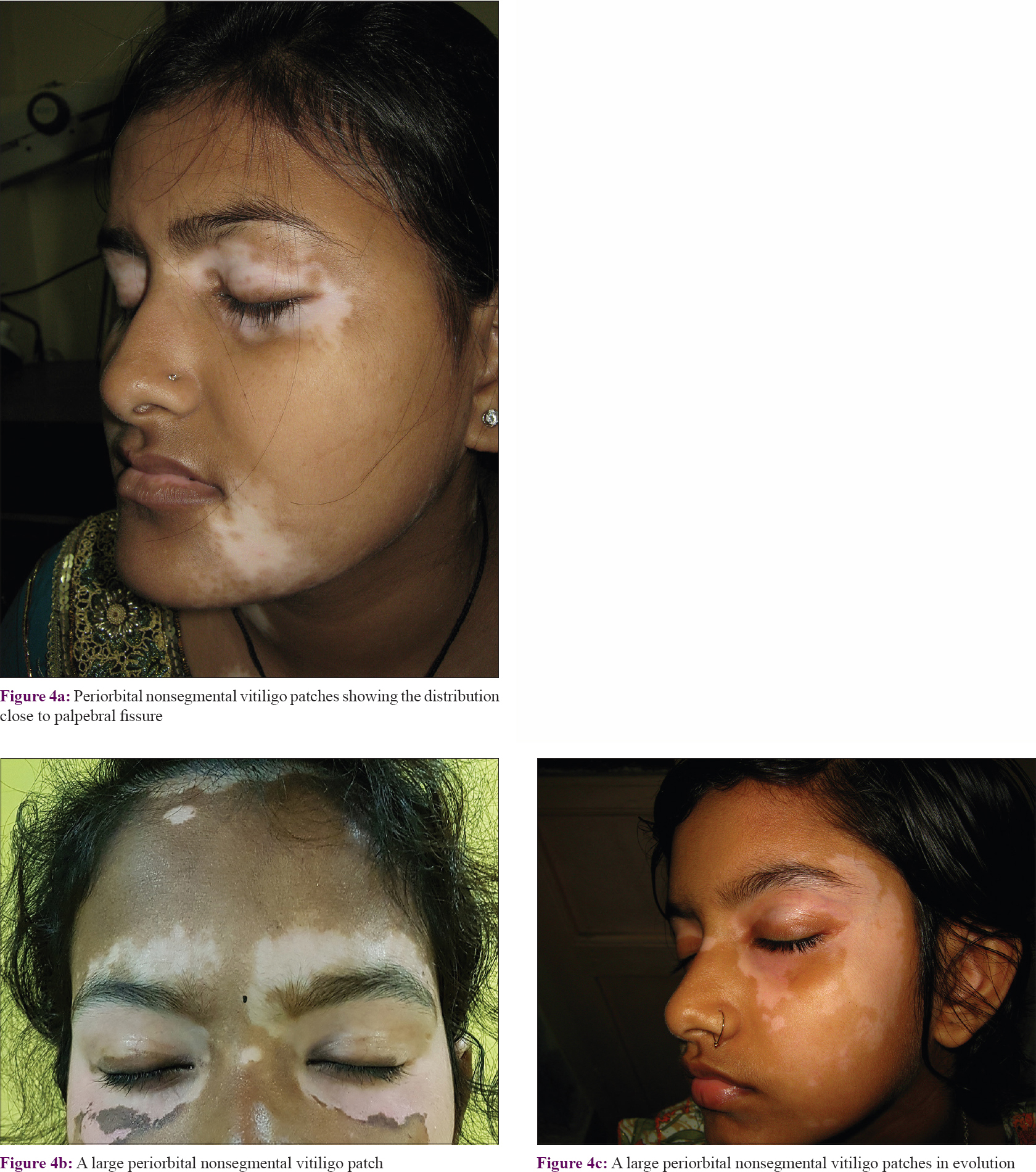 |
| Figure 4: |
Patches on forehead had an upward curve to cover frontal scalp and temporal region [Figure - 5]. Malar area was the least commonly affected segment (>9%). More than 61% of all malar patches merged with periorbital patches.
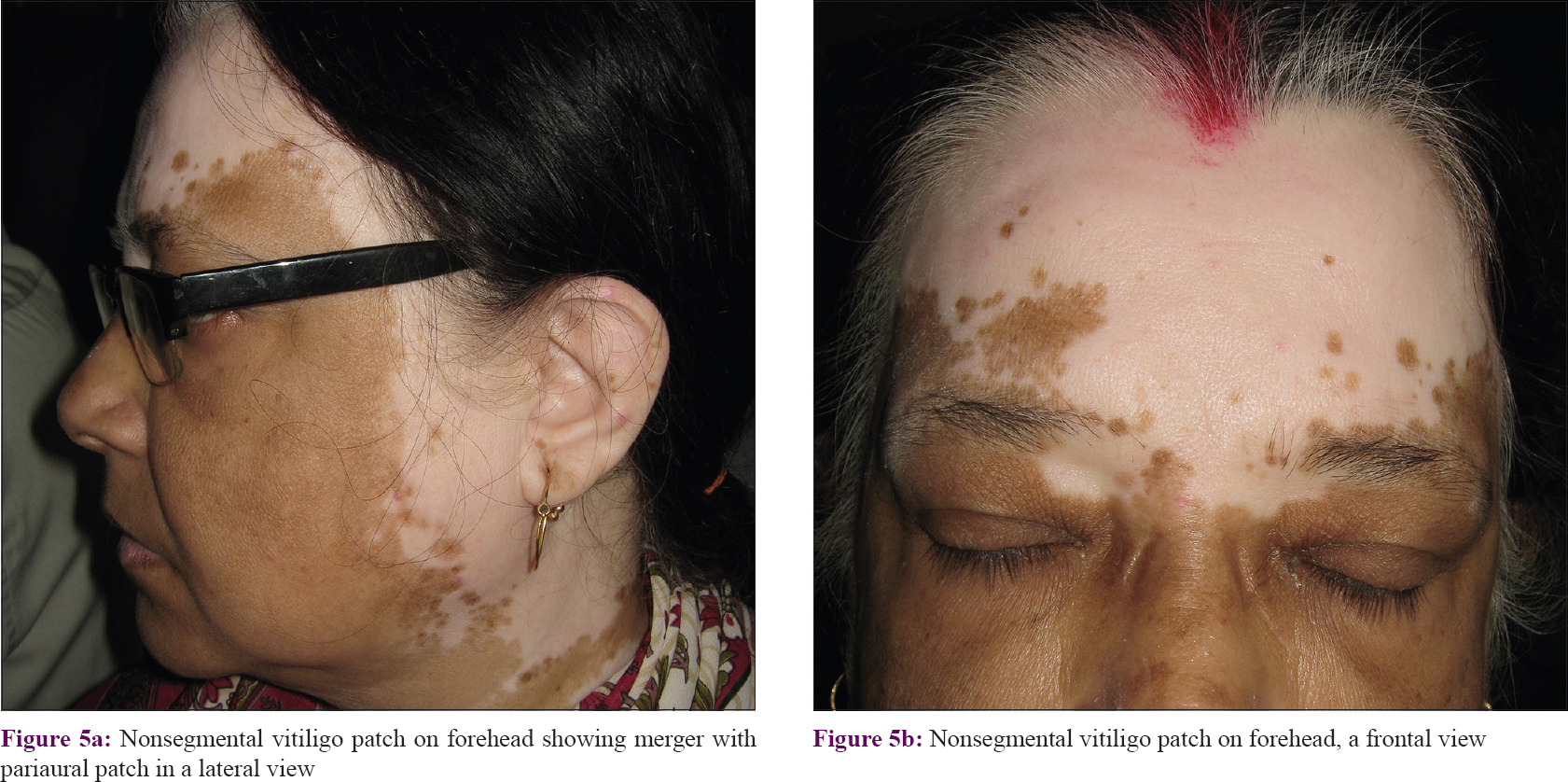 |
| Figure 5: |
Patches in the lower part of the face developed around nares (perinasal), oral fissure (perioral), and on and around the external ear (periaural). Perinasal and perioral patches frequently merged to form an apparently single larger patch in more than 50% cases [Figure - 6]. Perinasal and perioral patches also had a similar orientation pattern, that is, downward and laterally. Perioral patches progressed laterally to reach over the mandible to merge with periaural patches or progress over to the neck.
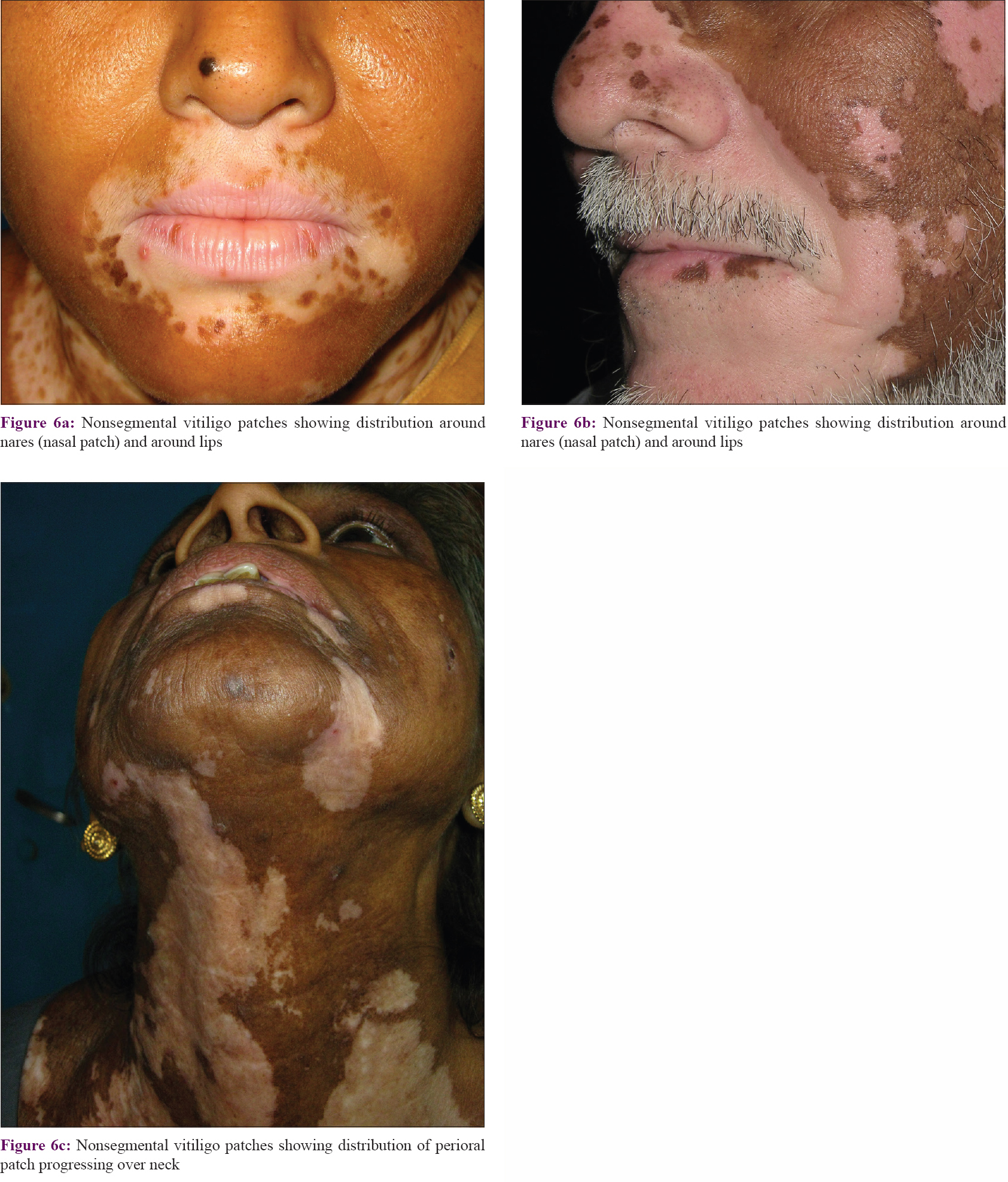 |
| Figure 6: |
Patches on the periaural area, unlike other patches on face, advanced downward and anteriorly (medially) on the mandible (ramus and body) or on the neck [Figure - 7]. Thus, large patches on mandible were often found to be linked to both the periaural areas and perioral areas. Patches on the neck were oriented downward and anteriorly. Some of the upper patches on neck originated around the ear and lower patches from the back of the scalp.
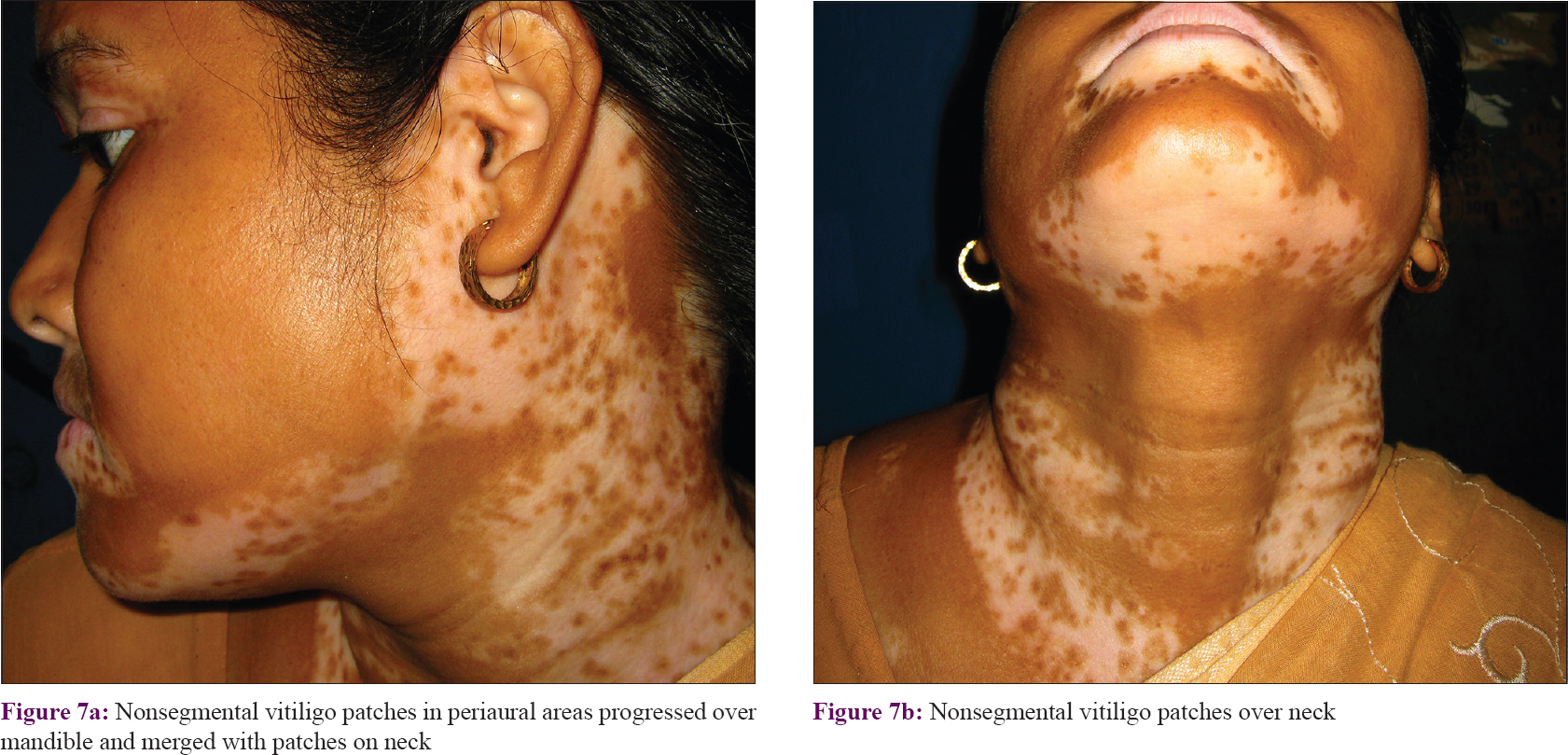 |
| Figure 7: |
Segmental involvement was also discernible in cases of extensive and multisegmental involvement (Category B). In these cases, evaluation of the distribution pattern was simpler with spared areas and these were found to follow the segmental pattern [Figure - 8] and [Figure - 9]. A total of 56.4% (n = 22) of these cases followed Blaschko's lines and embryonic pigmentary segments/units [Table - 1].
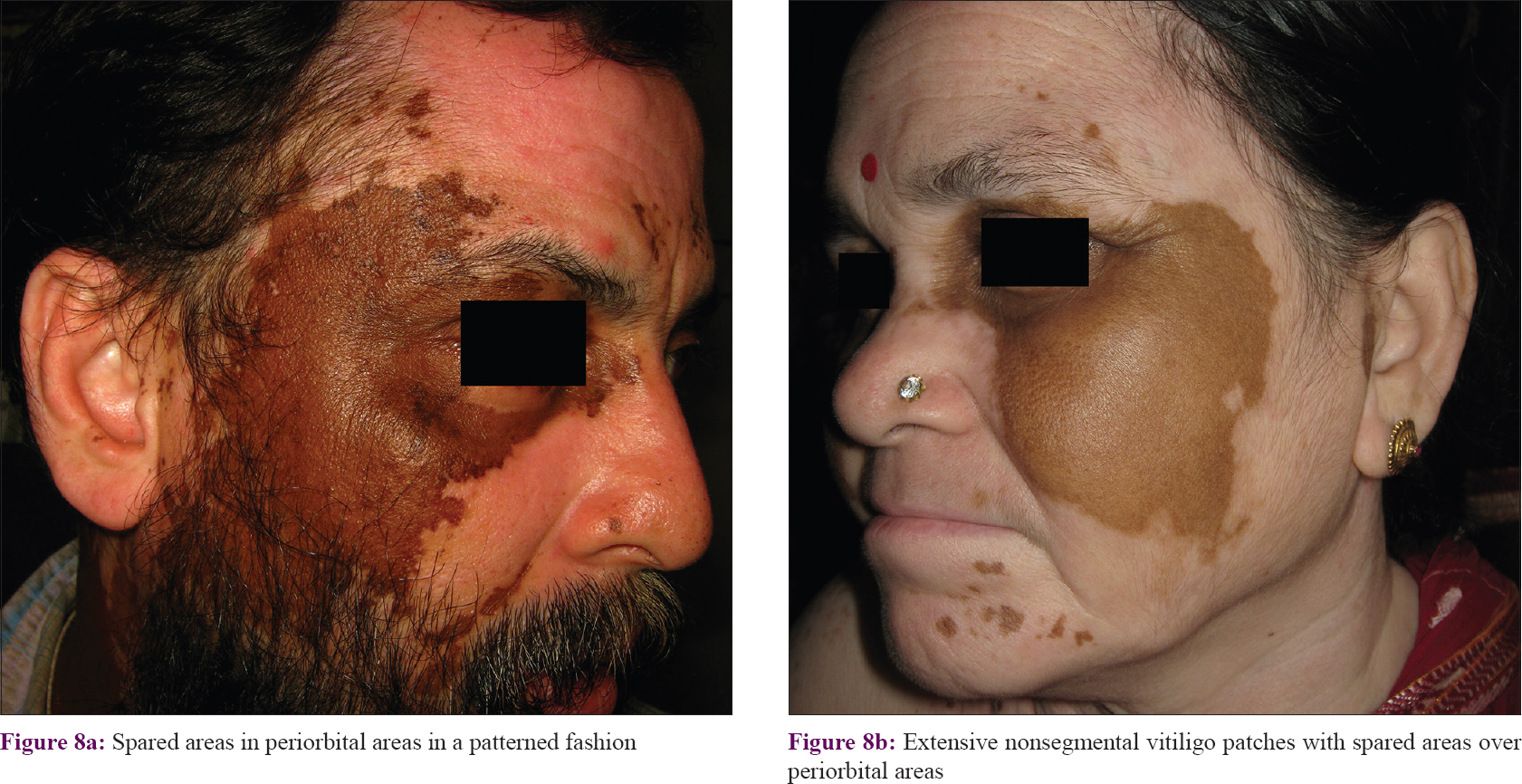 |
| Figure 8: |
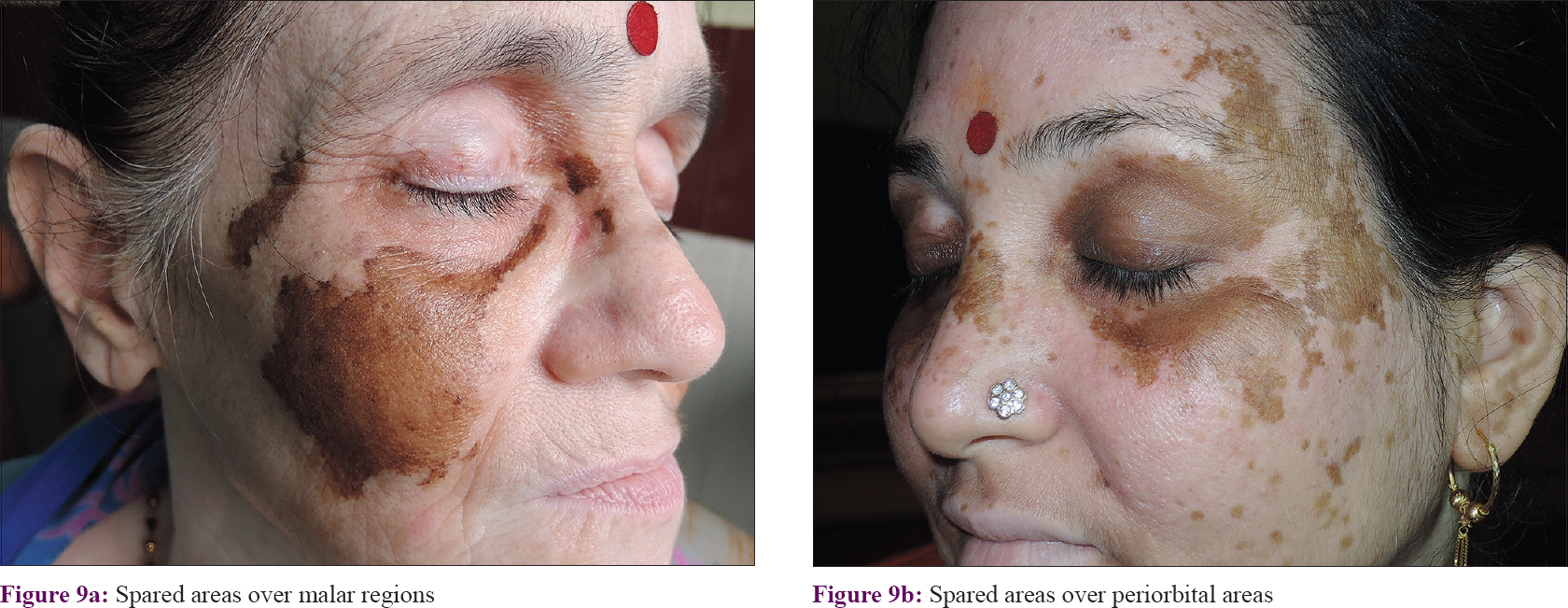 |
| Figure 9: |
Overall, it was noted that upper facial patches (forehead, periorbital, and malar) were centered on a region around the root of nose. Many patches had a tendency to develop around the fissures like palpebral fissure, nares, oral fissure, and external ear [Figure - 10]. With further progression, patches involved areas away from these areas.
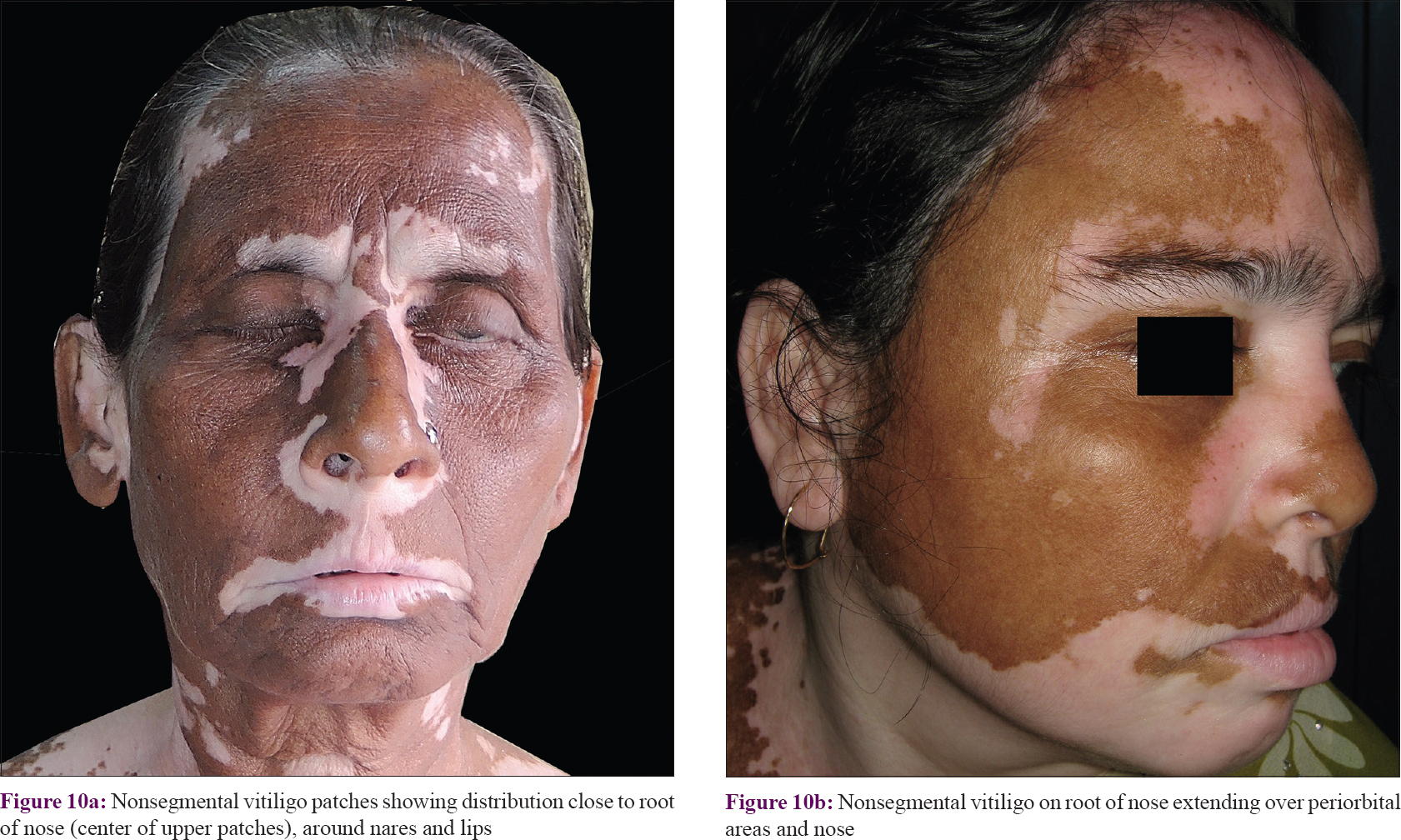 |
| Figure 10: |
Periorbital and perioral areas were the two most commonly affected segments. Malar area (segment) was found to be a less common site to develop depigmentation and a common site to develop early repigmentation.
The size of the segments varied considerably but the patterns were maintained. The segments were complementary to each other to cover the entire area of the face and neck. Patches merged with adjoining segments during progression and such merger also had some preferences. Thus, periorbital segment merged commonly with malar; nasal with perioral; and forehead, periaural and neck segment merged to each other [Figure - 4], [Figure - 5], [Figure - 6], [Figure - 7], [Figure - 8].
Discussion
Nonsegmental vitiligo is currently understood to be a polygenic disease and susceptible loci have been detected onchromosomes 1, 4, 6, 7, 8, 17e, and 22 in various linkage analysis studies.[12] Studies have identified the genes that are known to increase the autoimmune susceptibility like microRNA-211, NLRP1 (17p13), XBP1 (22q12), PTPN22, and CTLA4.[13],[14],[15],[16]
Presence of dendritic cells, IL17 cells,[17] and CD8+ cytotoxic T lymphocytes[18] in the lesional margins and along with reduced number of lesional regulatory T cells (TREGs)[19],[20] indicate on-going dysregulated immune process.
Intracellular oxidative stress that results from external exposure to chemicals and harsh environmental factors along with failure of the intracellular systems to overcome the stress induces targeted melanocytic destruction. This leads to expression of mediators like inducible Heat Shock protein 70 (HSP70i), IL-1β, IL-18, IL17, NLRP-1 inflammasome,[21],[22] and non-histone high-mobility group box1 (HMGB1) DNA-binding protein.[23] CD8+ T cells (CXCL16) are reported to play the critical role in the final targeted destruction of the melanocyte.[24]
Thus, unlike segmental vitiligo, melanocyte destruction in nonsegmental vitiligo is mediated through diverse, intricate, and interconnected mechanisms.
Blaschko's lines reflect an underlying mosaicism. Some other rare clinical patterns of mosaicism are checkerboard, phylloid, and garment-type patterns.[2] Segmental vitiligo was reported to show distributional similarity with Blaschko's lines[3],[4],[5] and rarely other patterns of mosaicism like checkerboard pattern[6] implying an underlying mosaicism in them.
Although less common, a generalized disease may be preceded by or subsequently develop an additional segmental component of the same disease in the same individual. The primary disease can be either monogenic (e.g. neurofibromatosis type 1, Hailey–Hailey disease, and porokeratosis) or polygenic in nature (e.g. atopic dermatitis, psoriasis and vitiligo). The development of the segmental component in a generalized disease is caused by mosaicism. In monogenic disorders, this is termed as “type 2 segmental manifestations” and in polygenic diseases, this mechanism has been termed as “superimposed segmental manifestation.” These have been explained with a mechanism called “loss of heterozygosity” of the affected genes.[25],[26]
In contrast to classical segmental vitiligo or superimposed segmental vitiligo in mixed vitiligo, mosaicism was never known to exist in nonsegmental vitiligo.
Recently, the role of mosaicism in nonsegmental vitiligo has been hypothesized on the basis of some form of distributional patterns.[9] However, evidence provided by the authors appeared to be weak as the patterns of distribution of the vitiligo patches were not compared to any of the established patterns of mosaicism. Rather, to support the role of mosaicism, authors highlighted some common distribution patterns like “bilateral symmetry” which are already well-known to be a typical phenotypic expression in nonsegmental vitiligo and do not indicate mosaicism.[9]
This study evaluated individual patches of nonsegmental vitiligo on face and neck areas and it has, possibly for the first time documented scientific evidence in favor of an intimate relation with the Blaschko's lines, embryonic pigmentary segments/units, and thus mosaicism.
As per our present knowledge, none of the known pathogenic mechanisms like autoimmune or oxidative stress–induced destruction of melanocytes could explain any blaschkoid distribution of patches in case of nonsegmental vitiligo. This present finding has been explained with the help of a recently proposed mechanism for the development of Blaschko's lines and “embryonic pigmentary segments.”[11]
Blaschko's lines have so far been known as hypothetical lines that represent the migration lines of the embryonic cells like primitive melanoblasts from the neural crest to skin. The present author previously proposed that in addition to the line of migration, proliferation pattern of the embryonic cells at some later stage might have contributed significantly to the final orientation patterns of Blaschko's lines. It was suggested that the Blaschko's lines, at least in the context of melanocyte, were not just “lines” but actually represented “closed outlines” that encircled the circumscribed areas developed from the proliferation of the embryonic clone of melanocytes.
The area developed from a primitive melanoblast was named as “embryonic pigmentary unit.”[11] A more primitive melanoblast develops into a larger patch and vice versa. At an early developmental stage during the development, the exact timing of which is yet to be understood, primitive embryonic melanoblasts are dedicated to form a larger area with distinctive patterns, mentioned as 'embryonic pigmentary segment'.[11] All the smaller units and the larger segments mutually cover the entire face. Similar phenomena should also exist in other body areas. Each unit and segment were proposed to be margined by Blaschko's lines.[11]
In addition to nevi, some non-nevoid and apparently physiological conditions like “acquired idiopathic patterned facial pigmentations” that includes periorbital and perioral pigmentation were also shown to follow Blaschko's lines and match the distribution pattern of “embryonic pigmentary segments.”[27] These patterned pigmented patches developed after birth and showed progressive intensity under the influence of physiological stimuli like sun exposure, aging, or other unknown physiological factors. It appeared that the capacity for pigmentation under identical stimulation varied among these embryonic units/segments leading to the disparity in pigmentation among these segments. Thus, existence of a form of “functional mosaicism” was proposed to exist within these embryonic units/segments and such functional variation possibly evolved during development due to the fact that each unit/segment is developed from a different primitive embryonic melanoblast.[27]
Possibly, the same functional differences among the units and segments influence the degree of susceptibility to the various mechanisms that operate against the melanocytes in vitiligo (biochemical, immunologic, stress, environmental, or other unknown factors). Thus, segments with higher susceptibility develop vitiligo patches. Moreover, depending on the degree of susceptibility some segments are affected earlier or in greater extent while some segments either remain unaffected or resist the disease process for a longer period of time. Even in an advanced disease, distribution pattern is found to respect the segmental outlines and the Blaschko's lines. Due to the same reason, repigmentation follows the similar pattern.
Thus, the theory of embryonic units/segments having different cell lines of origin and harboring qualitative differences among them seems to effectively explain the observed patterns in non-segmental vitiligo.[27] From the above observation, it is hypothesized that the functional mosaicism that might exist in this units/ segments might imply a loss or a gain of function leading to higher susceptibility or endurance, respectively. This creates a functional barrier to the disease process, clinically manifesting in well-defined patterns limited by the Blaschko's lines.
Preferential clustering of nonsegmental vitiligo around some areas like root of nose, palpebral fissure, nares, lips, and external ear was found to be common. The reason for this remains unexplained.
It must be mentioned here that no genetic or other laboratory testing could be done to confirm the proposed hypothesis. This is the major limitation of this study. Since Blaschko's lines are known to be highly distinctive over the head and neck region,[10] evaluation of nonsegmental vitiligo was done only on head and neck region. Lack of evaluation over other body regions may be considered another limitation of this study.
In summary, based on the distributional similarity with the Blaschko's lines, mosaicism is considered a strong possibility even in nonsegmental vitiligo. Despite various proposed mechanisms of vitiligo pathogenesis, the process that determines the localization of patches in nonsegmental vitiligo has so far remained largely unknown. This study has proposed a embryonic developmental mechanism for the mosaic patterns observed in nonsegmental vitiligo. It is expected that suitable investigational studies will be available in near future to support or negate this hypothesis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients/guardian have given their consent for their images and other clinical information to be reported in the journal. The patients/guardian understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: The vitiligo global issues consensus conference. Pigment Cell Melanoma Res 2012;25:E1-13.
[Google Scholar]
|
| 2. |
Happle R. Mosaicism in human skin. Understanding the patterns and mechanisms. Arch Dermatol 1993;129:1460-70.
[Google Scholar]
|
| 3. |
Taïeb A. Intrinsic and extrinsic pathomechanisms in vitiligo. Pigment Cell Res 2000;13 Suppl 8:41-7.
[Google Scholar]
|
| 4. |
van Geel N, Speeckaert R, Melsens E, Toelle SP, Speeckaert M, De Schepper S, et al. The distribution pattern of segmental vitiligo: Clues for somatic mosaicism. Br J Dermatol 2013;168:56-64.
[Google Scholar]
|
| 5. |
Kim DY, Oh SH, Hann SK. Classification of segmental vitiligo on the face: Clues for prognosis. Br J Dermatol 2011;164:1004-9.
[Google Scholar]
|
| 6. |
van Geel N, Bosma S, Boone B, Speeckaert R. Classification of segmental vitiligo on the trunk. Br J Dermatol 2014;170:322-7.
[Google Scholar]
|
| 7. |
Taïeb A, Morice-Picard F, Jouary T, Ezzedine K, Cario-André M, Gauthier Y. Segmental vitiligo as the possible expression of cutaneous somatic mosaicism: Implications for common non-segmental vitiligo. Pigment Cell Melanoma Res 2008;21:646-52.
[Google Scholar]
|
| 8. |
van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin 2017;35:145-50.
[Google Scholar]
|
| 9. |
Attili VR, Attili SK. Anatomical segmentations in all forms of vitiligo: A new dimension to the etiopathogenesis. Indian J Dermatol Venereol Leprol 2016;82:379-88.
[Google Scholar]
|
| 10. |
Happle R, Assim A. The lines of Blaschko on the head and neck. J Am Acad Dermatol 2001;44:612-5.
[Google Scholar]
|
| 11. |
Sarma N. Pigmentary nevi on face have unique patterns and implications: The concept of Blaschko's lines for pigmentary nevi. Indian J Dermatol 2012;57:30-4.
[Google Scholar]
|
| 12. |
Tarlé RG, Nascimento LM, Mira MT, Castro CC. Vitiligo – part 1. An Bras Dermatol 2014;89:461-70.
[Google Scholar]
|
| 13. |
Sahoo A, Lee B, Boniface K, Seneschal J, Sahoo SK, Seki T, et al. MicroRNA-211 regulates oxidative phosphorylation and energy metabolism in human vitiligo. J Invest Dermatol 2017;137:1965-74.
[Google Scholar]
|
| 14. |
Chen JX, Shi Q, Wang XW, Guo S, Dai W, Li K, et al. Genetic polymorphisms in the methylenetetrahydrofolate reductase gene (MTHFR) and risk of vitiligo in Han Chinese populations: A genotype-phenotype correlation study. Br J Dermatol 2014;170:1092-9.
[Google Scholar]
|
| 15. |
Spritz RA. Recent progress in the genetics of generalized vitiligo. J Genet Genomics 2011;38:271-8.
[Google Scholar]
|
| 16. |
Vrijman C, Kroon MW, Limpens J, Leeflang MM, Luiten RM, van der Veen JP, et al. The prevalence of thyroid disease in patients with vitiligo: A systematic review. Br J Dermatol 2012;167:1224-35.
[Google Scholar]
|
| 17. |
Sanchez-Sosa S, Aguirre-Lombardo M, Jimenez-Brito G, Ruiz-Argüelles A. Immunophenotypic characterization of lymphoid cell infiltrates in vitiligo. Clin Exp Immunol 2013;173:179-83.
[Google Scholar]
|
| 18. |
Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res 2012;25:88-98.
[Google Scholar]
|
| 19. |
van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol 2011;32:110-6.
[Google Scholar]
|
| 20. |
Le Poole IC, Mehrotra S. Replenishing regulatory T cells to halt Depigmentation in vitiligo. J Investig Dermatol Symp Proc 2017;18:S38-45.
[Google Scholar]
|
| 21. |
Xie H, Zhou F, Liu L, Zhu G, Li Q, Li C, et al. Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci 2016;81:3-9.
[Google Scholar]
|
| 22. |
Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, et al. Vitiligo: Interplay between oxidative stress and immune system. Exp Dermatol 2013;22:245-50.
[Google Scholar]
|
| 23. |
Kim JY, Lee EJ, Seo J, Oh SH. Impact of high-mobility group box 1 on melanocytic survival and its involvement in the pathogenesis of vitiligo. Br J Dermatol 2017;176:1558-68.
[Google Scholar]
|
| 24. |
Li S, Zhu G, Yang Y, Jian Z, Guo S, Dai W, et al. Oxidative stress drives CD8+ T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J Allergy Clin Immunol 2017;140:177-89.e9.
[Google Scholar]
|
| 25. |
Happle R. A rule concerning the segmental manifestation of autosomal dominant skin disorders. Review of clinical examples providing evidence for dichotomous types of severity. Arch Dermatol 1997;133:1505-9.
[Google Scholar]
|
| 26. |
Happle R. Superimposed segmental manifestation of polygenic skin disorders. J Am Acad Dermatol 2007;57:690-9.
[Google Scholar]
|
| 27. |
Sarma N, Chakraborty S, Bhattacharya SR. Acquired, idiopathic, patterned facial pigmentation (AIPFP) including periorbital pigmentation and pigmentary demarcation lines on face follows the lines of Blaschko on face. Indian J Dermatol 2014;59:41-8.
[Google Scholar]
|
Fulltext Views
9,468
PDF downloads
2,573





