Translate this page into:
Primary cutaneous marginal zone B-cell lymphoma with unusual manifestation and spontaneous regression
Correspondence Address:
Lin Wang
No. 37, Guo Xue Xiang, Chengdu, Sichuan 610041
China
| How to cite this article: Tang X, Tang J, Liu W, Wang L. Primary cutaneous marginal zone B-cell lymphoma with unusual manifestation and spontaneous regression. Indian J Dermatol Venereol Leprol 2020;86:428-431 |
Sir,
An otherwise healthy 78-year-old Chinese man presented to the department of dermatovenereology with slowly expanding nodules on his forehead for more than a year and a tiny nodule on his left cheek for about half a year. On physical examination, clustered erythematous nodules were noted on his forehead [Figure - 1], with a small nodule on his left cheek measuring 0.5 cm in diameter.
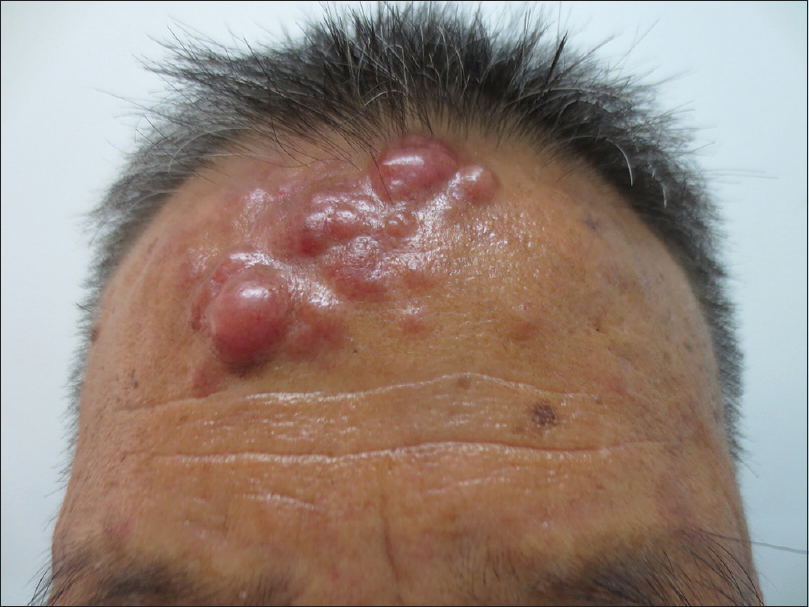 |
| Figure 1: Clustered erythematous nodules on the forehead |
A skin biopsy performed on the forehead lesion showed normal epidermis, diffuse infiltration of lymphoid cells in the dermis, composed of medium-sized lymphocytes with abundant cytoplasm, in the interfollicular compartment, and reactive follicles with partial destruction. Immunohistochemically, the tumor cells were stained positively for CD20, CD79a and partial Bcl-2, and negatively for CD3, CD5, Bcl-6, CyclinD-1 and Mum-1 [Figure - 2]. The expansile and irregular networks of follicular dendritic cells were highlighted by CD21 and CD10 [Figure - 2]. The percentage of Ki-67 positively stained cells was about 10%. Both monoclonal immunoglobulin heavy chain and immunoglobulin κ light chain gene rearrangements were detected.
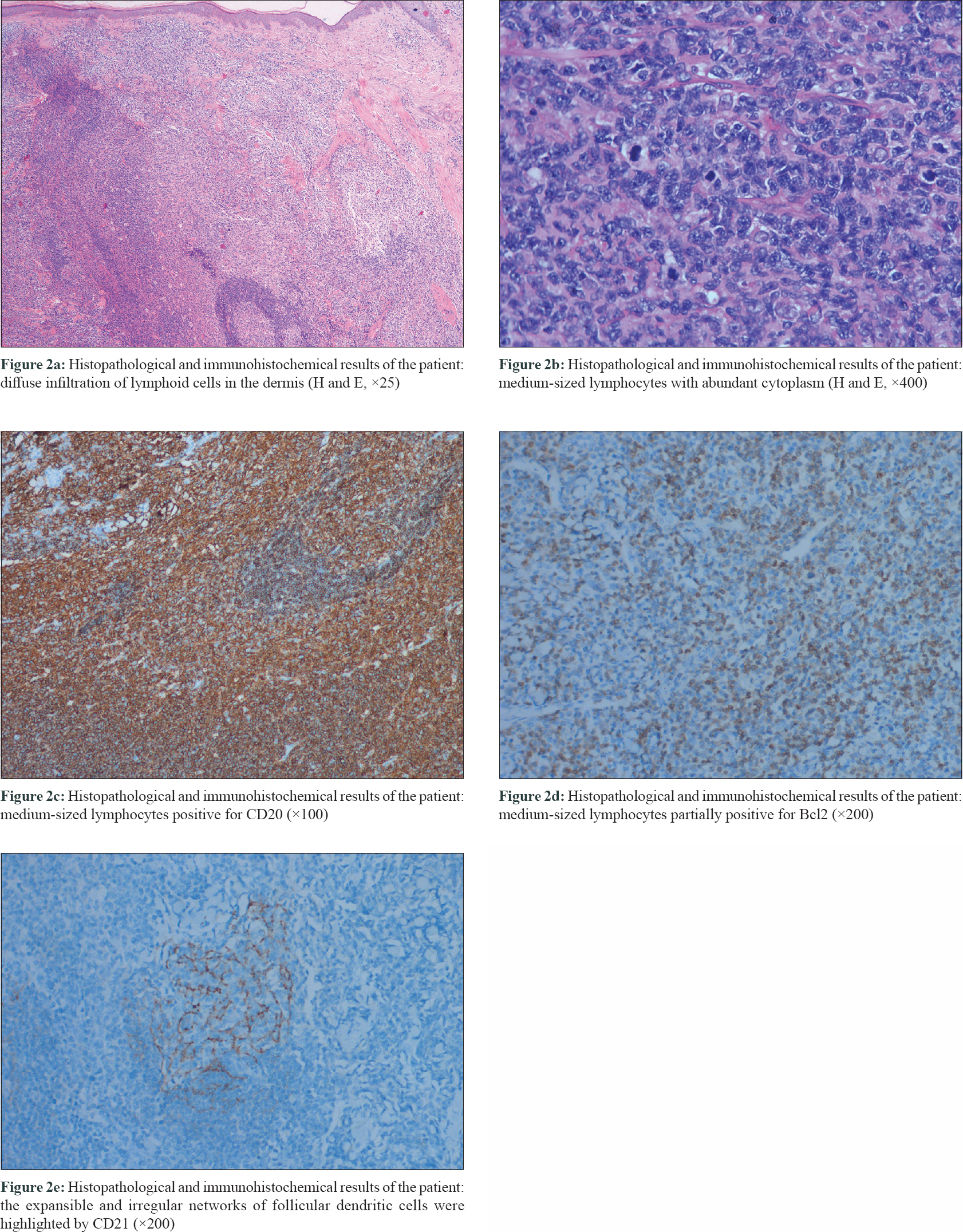 |
| Figure 2: |
Routine laboratory examinations included complete blood count, serum chemistry panel with renal and hepatic function were unremarkable. Chest X-ray examination and abdominal ultrasonography were carried out and did not demonstrate any visceral manifestations. Based on these findings, a diagnosis of primary cutaneous marginal zone B-cell lymphoma was made. The patient refused any treatment. Meanwhile, most of his lesions showed a spontaneous regression after 2 months [Figure - 3]. After 8 months, the lesions almost completely regressed. Unfortunately, half a month later, he died of a massive cerebral hemorrhage.
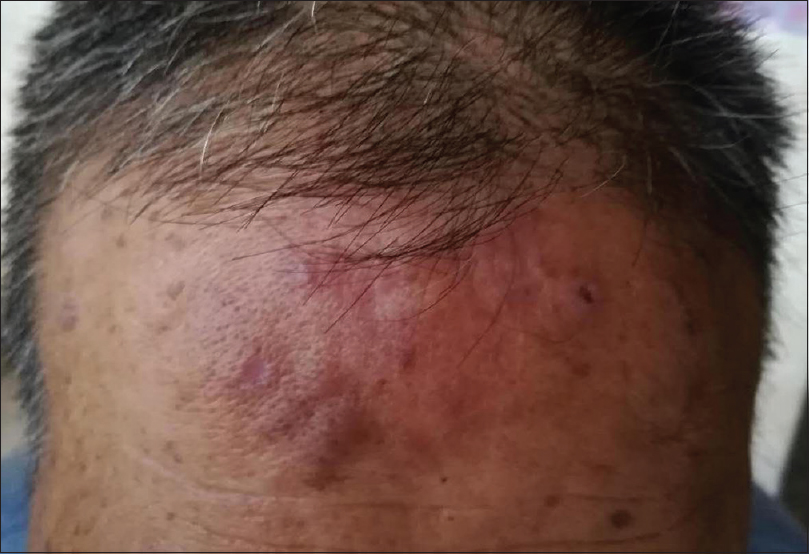 |
| Figure 3: The clinical manifestation of the patient after 2 months of the first visit (photo provided by the son of the patient) |
Primary cutaneous marginal zone B-cell lymphoma is a low-grade B-cell lymphoma and runs an indolent course.1 Most patients present with red to violaceous papules, plaques or nodules localized preferentially on the trunk or extremities, especially the arms.[1],[2],[3] Skin lesions present on head, neck and genitals are also reported.[4],[5],[6] This is the first reported case presenting with cluster of nodules on the forehead. Moreover, his lesions showed spontaneous regression. The histological examination usually reveals dermal confluent nodular or diffuse lymphocytic infiltrates composed of small lymphocytes, lymphoplasmacytoid cells, mature plasma cells and reactive germinal centers with tingible body macrophages.[4] The marginal zone B cells express CD20, CD79a and Bcl-2, and are negative for CD5, CD10 and Bcl-6, which may be useful in differentiating it from primary cutaneous follicle center lymphoma. Reactive germinal centers are typically positive for Bcl-6 and CD10, and negative for Bcl-2 markers. Immunoglobulin heavy chain genes are clonally rearranged.[1]
The differential diagnosis of primary cutaneous marginal zone B-cell lymphoma includes primary cutaneous follicle center lymphoma and cutaneous lymphoid hyperplasia. These diseases involve both the head and face and are manifested as red to violaceous, slow-growing cutaneous nodules. Thus making clinical features rarely discriminatory. Diagnosis mainly depends on histopathology and immunohistochemistry for identification.
Primary cutaneous marginal zone B-cell lymphoma and primary cutaneous follicle center lymphoma are low-grade lymphoma with indolent clinical course and excellent prognosis, with a survival rate in 5 years exceeding 95% and 90%, respectively. Radiotherapy and surgery are the most frequently used therapies in primary cutaneous marginal zone B-cell lymphoma, with no difference in relapse rate and long-term disease-free survival in patients initially treated with surgery or radiotherapy.[4],[7] There have been numerous reports of spontaneous resolution of cutaneous T-cell lymphoma, especially primary cutaneous CD30+ anaplastic large-cell lymphoma in literature. Complete or partial spontaneous regression is reported to occur in 10–42% of these tumors.[8] In addition, other cutaneous T-cell lymphomas such as mycosis fungoides, subcutaneous panniculitis-like T-cell lymphoma and natural killer cell lymphoma have also been reported to undergo spontaneous regression.[9],[10] A few data are available about the spontaneous remission rate of cutaneous B-cell lymphoma in the absence of treatment. Gerami et al. reported 30 cases of primary cutaneous marginal zone B-cell lymphoma. Of these, 12 patients who did not receive any treatment, had persistent lesions.[11] Hoefnagel et al. studied 50 cases of primary cutaneous marginal zone B-cell lymphoma. Of these, 4 patients did not receive any treatment, among which one patient achieved partial remission, one patient achieved complete remission and other two cases had persistent lesions.[4] Ferrara et al. described spontaneous regression of primary cutaneous marginal zone B-cell lymphoma associated with anetoderma and hepatitis B virus-related chronic active hepatitis, hypothesizing that, the chronic infection may be related to the spontaneous regression.[12] In addition, cases of primary cutaneous follicle center lymphoma and primary cutaneous diffuse large B-cell lymphoma, leg type with spontaneous regression have also been reported.[13],[14],[15]
The mechanism of spontaneous resolution of cutaneous lymphoma is unclear. Factors associated with spontaneous regression primarily include apoptosis, the host's immune response and particular conditions of the tumor microenvironment, such as the presence of inhibitors of metalloproteinases and angiogenesis and the absence or scarcity of specific proteins.[16] Recently, Fujii et al. reported that numbers of forkhead box P3-positive cells and programmed cell death 1-positive cells may be associated with spontaneous regression.[17] Other probable factor includes an inadequate T-cell immune response.[13] Recent reports revealed that some microRNAs can be used as prognostic markers.[18] Poor prognostic factors of primary cutaneous follicle center lymphoma include skin lesions located on the leg, expression of FOXP1 and evidence of bone marrow infiltration.[3],[19]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published, and due efforts will be made to conceal the identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85.
[Google Scholar]
|
| 2. |
Lima M. Cutaneous primary B-cell lymphomas: From diagnosis to treatment. An Bras Dermatol 2015;90:687-706.
[Google Scholar]
|
| 3. |
Nicolay JP, Wobser M. Cutaneous B-cell lymphomas – Pathogenesis, diagnostic workup, and therapy. J Dtsch Dermatol Ges 2016;14:1207-24.
[Google Scholar]
|
| 4. |
Hoefnagel JJ, Vermeer MH, Jansen PM, Heule F, van Voorst Vader PC, Sanders CJ, et al. Primary cutaneous marginal zone B-cell lymphoma: Clinical and therapeutic features in 50 cases. Arch Dermatol 2005;141:1139-45.
[Google Scholar]
|
| 5. |
Martorell M, Gaona Morales JJ, Garcia JA, Manuel Gutierrez Herrera J, Grau FG, Calabuig C, et al. Transformation of vulvar pseudolymphoma (lymphoma-like lesion) into a marginal zone B-cell lymphoma of labium majus. J Obstet Gynaecol Res 2008;34:699-705.
[Google Scholar]
|
| 6. |
Tang J, Chen J, Liu H, Wang L. Primary cutaneous marginal zone B-cell lymphoma of vulva in a pregnant woman. Indian J Dermatol Venereol Leprol 2019;85:211-4.
[Google Scholar]
|
| 7. |
Servitje O, Muniesa C, Benavente Y, Monsálvez V, Garcia-Muret MP, Gallardo F, et al. Primary cutaneous marginal zone B-cell lymphoma: Response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol 2013;69:357-65.
[Google Scholar]
|
| 8. |
Miyagawa F, Ogawa K, Asada H. A case of CD4/CD8+double-positive primary cutaneous anaplastic large cell lymphoma of the lip involving spontaneous regression after biopsy. Eur J Dermatol 2017;27:68-9.
[Google Scholar]
|
| 9. |
Johnston EE, LeBlanc RE, Kim J, Chung J, Balagtas J, Kim YH, et al. Subcutaneous panniculitis-like T-cell lymphoma: Pediatric case series demonstrating heterogeneous presentation and option for watchful waiting. Pediatr Blood Cancer 2015;62:2025-8.
[Google Scholar]
|
| 10. |
Isobe Y, Aritaka N, Sasaki M, Oshimi K, Sugimoto K. Spontaneous regression of natural killer cell lymphoma. J Clin Pathol 2009;62:647-50.
[Google Scholar]
|
| 11. |
Gerami P, Wickless SC, Rosen S, Kuzel TM, Ciurea A, Havey J, et al. Applying the new TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome in primary cutaneous marginal zone lymphoma. J Am Acad Dermatol 2008;59:245-54.
[Google Scholar]
|
| 12. |
Ferrara G, Cusano F, Robson A, Stefanato CM. Primary cutaneous marginal zone B-cell lymphoma with anetoderma: Spontaneous involution plus de novo clonal expansion. J Cutan Pathol 2011;38:342-5.
[Google Scholar]
|
| 13. |
Graham PM, Richardson AS, Schapiro BL, Saunders MD, Stewart DM. Spontaneous regression of primary cutaneous diffuse large B-cell lymphoma, leg type with significant T-cell immune response. JAAD Case Rep 2018;4:305-9.
[Google Scholar]
|
| 14. |
Marrero-Alemán G, Montenegro-Dámaso T, Peñate Y. Primary cutaneous diffuse large B-Cell lymphoma, leg type, with spontaneous regression after biopsy. Am J Dermatopathol 2017;39:785-7.
[Google Scholar]
|
| 15. |
Cuellar A, Lozano AK, Gomez M, Weish O, Ocampo J. Primary cutaneous follicular center B-cell lymphoma with spontaneous regression in a Hispanic woman. J Am Acad Dermatol 2018;79:AB228.
[Google Scholar]
|
| 16. |
Ricci SB, Cerchiari U. Spontaneous regression of malignant tumors: Importance of the immune system and other factors (review). Oncol Lett 2010;1:941-5.
[Google Scholar]
|
| 17. |
Fujii K, Uchida Y, Hatanaka M, Jimura N, Okubo A, Sakanoue M, et al. Numbers of forkhead box P3-positive cells and programmed cell death 1-positive cells were associated with spontaneous regression in a patient with primary cutaneous diffuse large B-cell lymphoma with multiple lesions. J Dermatol 2019;46:e410-2.
[Google Scholar]
|
| 18. |
Monsálvez V, Montes-Moreno S, Artiga MJ, Rodríguez ME, Sanchez-Espiridion B, Lozano M, et al. MicroRNAs as prognostic markers in indolent primary cutaneous B-cell lymphoma. Mod Pathol 2013;26:171-81.
[Google Scholar]
|
| 19. |
Senff NJ, Hoefnagel JJ, Jansen PM, Vermeer MH, van Baarlen J, Blokx WA, et al. Reclassification of 300 primary cutaneous B-Cell lymphomas according to the new WHO-EORTC classification for cutaneous lymphomas: Comparison with previous classifications and identification of prognostic markers. J Clin Oncol 2007;25:1581-7.
[Google Scholar]
|
Fulltext Views
4,818
PDF downloads
2,475





