Translate this page into:
Metastatic porocarcinoma
2 Hinduja Hospitals, Mumbai, India
3 Consultant Dermatologist, Guntur, Andhra Pradesh, India
4 Consultant Dermatologist, Surya Skin Care and Research Center, Visakhapatnam, Andhra Pradesh, India
Correspondence Address:
G Raghurama Rao
Department of DVL, GSL Medical College, Rajanagaram, Rajahmundry - 533296, Andhra Pradesh
India
| How to cite this article: Rao G R, Joshi R, Seetharam K A, Amareswar A, Sridevi M. Metastatic porocarcinoma. Indian J Dermatol Venereol Leprol 2015;81:210-213 |
Sir,
Malignant sweat gland tumours are extremely rare, especially so in the upper extremities. Amongst these, eccrine porocarcinoma occurs most frequently, representing 0.005-0.01% of all cutaneous neoplasms. [1] First described by Pinkus and Mehregan in 1963, [1] Mishisma and Moriko introduced the term ′eccrine porocarcinoma in 1969. [1] Approximately 250 cases have been reported, of which only 2 cases were documented as having occurred on the fingers. [2] About 20% of cases recur locally, and about 20% metastasize to the regional lymph nodes. Although rare, distant metastases have been reported. Multiple cutaneous metastases have been reported uncommonly. [1],[2],[3],[4] We describe a case of a diabetic woman with metastatic porocarcinoma of the left upper limb with lymphedema and axillary lymphadenopathy.
In November 2012, a 52-year-old diabetic female was referred for evaluation of cutaneous lesions on her left upper limb associated with edema of the entire limb of 11 months duration. In November 2011, her left ring finger had been amputated for a non-healing, foul smelling, necrotic ulcer of a year′s duration. Unfortunately, the specimen had not been sent for histopathological examination. Twenty days after surgery, she developed edema of the entire left limb which was treated with physiotherapy and compressive bandages. Two months later, she developed multiple, pigmented, raised lesions, initially on her left palm, and increasing in number to involve the hand and forearm bilaterally. She also developed reddish, raised lesions on the inner side of the left arm.
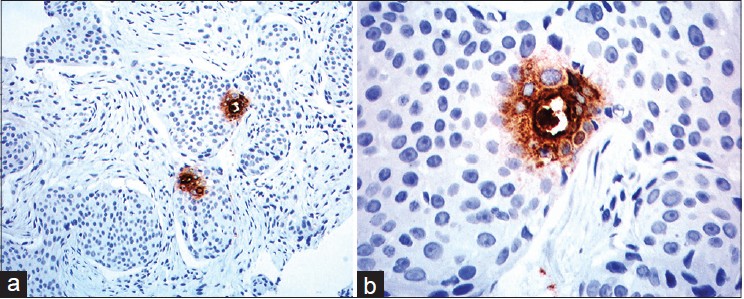
On presentation to our department, the entire left limb was edematous with multiple, pigmented, hyperkeratotic papules arranged linearly on both sides of hand and forearm. Some of these lesions were ulcerated and bleeding. In addition, there were erythematous, non-tender, indurated plaques on the medial aspect of left arm [Figure - 1]a and b. Left axillary lymph nodes were palpable. No systemic abnormalities were found. Differential diagnoses included cutaneous metastases and malignant melanoma. Hematological and biochemical investigations were within normal limits except for a fasting blood sugar of 130 mg/dl. Chest X-ray, CT scan of chest (plain and contrast) and ultrasound abdomen were all normal. Fine needle aspiration cytology (FNAC) of left axillary lymph node revealed a few clusters of atypical cells arranged in sheets and having an acinar pattern, with mild-to-moderate nuclear atypia. Two biopsies were taken for histopathological examination, one from the hyperkeratotic pigmented lesion and another from the indurated plaque Both sections revealed infiltration of the entire dermis by numerous, variably sized epithelial aggregates of atypical poroid cells, with stromal retraction and ductal structures. No connection to the overlying epidermis was seen [Figure - 2]a-c and [Figure - 3]. Sections from the pigmented, hyperkeratotic lesions additionally showed a few nests of atypical poroid cells with melanin and dendritic melanocytes in the epidermis, giving an epidermal hidracanthoma-like picture. Immunohistochemical studies were positive for pancytokeratin markers and Ki-67 positivity was noted in 15-18% of cells [Figure - 4]a and b. The ductal structures were identified by carcinoembryonic antigen (CEA) staining [Figure - 5]a and b. A final diagnosis of metastatic porocarcinoma was made. She was referred to a surgical oncologist who performed amputation of the entire left limb with left axillary dissection. At one year of follow-up, there were no local recurrences and there was no evidence of distant metastases.
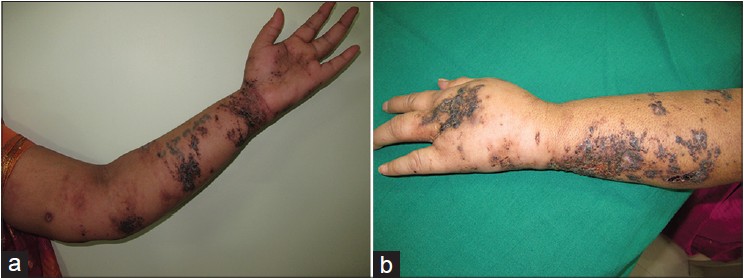 |
| Figure 1: (a) Groups of multiple, pigmented, hyperkeratotic papules along the left upper limb (b) Close-up view of hyperkeratotic, pigmented papules and ulcerated lesions with edema of left hand and forearm |
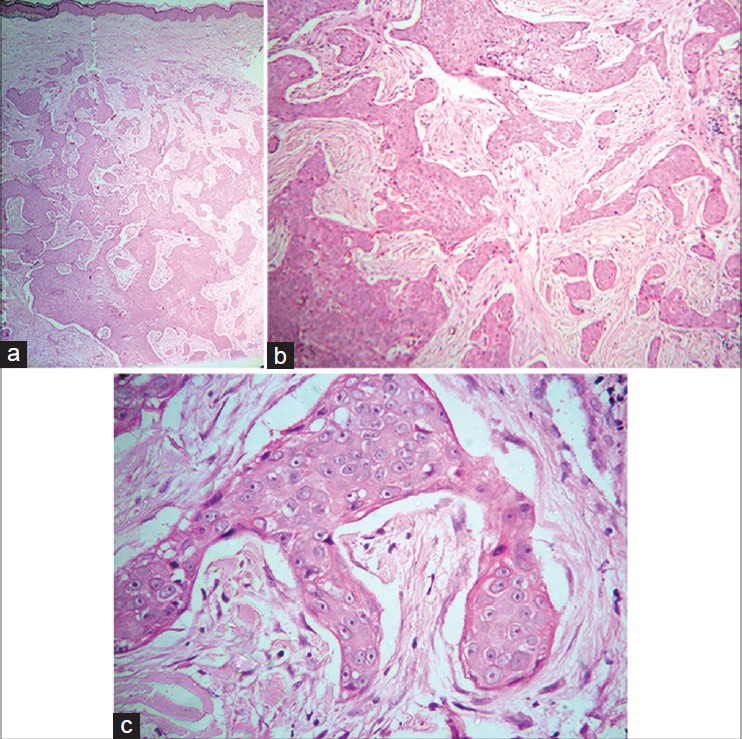 |
| Figure 2: (a) Infiltration of entire dermis by numerous variably sized epithelial aggregates with irregular jagged outlines and infiltrative pattern. No connection to overlying epidermis is seen (H and E, 40×) (b) Infiltrating irregular epithelial aggregates of atypical poroid cells with stromal retraction artifacts (H and E, 100×) (c) Close-up view of atypical cells with prominent pink nucleoli (H and E, 400×) |
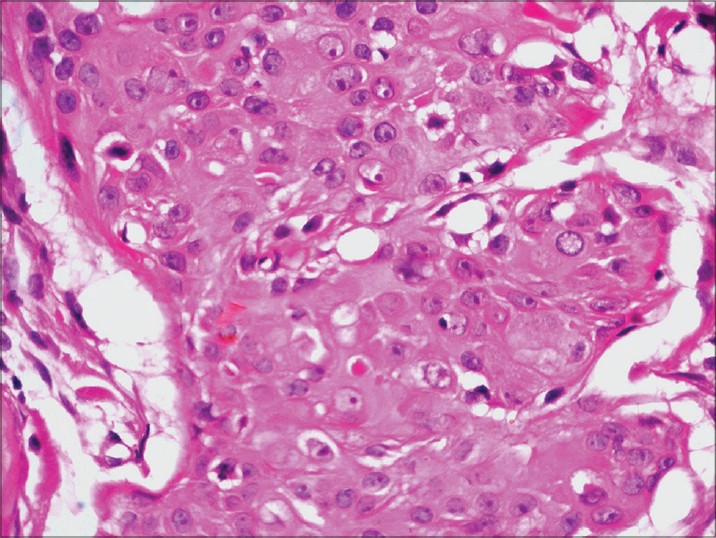 |
| Figure 3: Ductal structures in the aggregate of atypical poroid cells (H and E, 400×) |
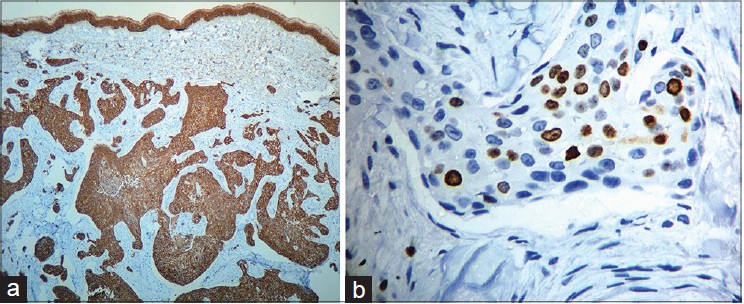 |
| Figure 4: (a) The infiltrating neoplasm is uniformly positive for cytokeratin (Immuno-peroxidase, 40×) (b) Many Ki 67 positive cells (Immuno-peroxidase, 40×) |
 |
| Figure 5: (a) Nodular aggregates of poroid cells, two ductal structures seen in adjacent nodules stained brown (CEA, 100×) (b) Ductal structure within the nodule of poroid cells showing duct and surrounded cuticular cells stained brown (CEA, 400×) |
Like poroma, porocarcinoma may originate from apocrine or eccrine sweat glands and it is generally impossible to determine the exact lineage of any given tumor. Eccrine porocarcinoma, a malignant tumor with metastatic potential arises from intra-epidermal eccrine duct epithelium or acrosyringium. [5],[6] It may develop either de novo, or from a pre-existing benign eccrine poroma in 18% of cases. [1] It mainly occurs in the elderly, with equal incidence in both sexes. Eccrine porocarcinoma may present as a single ulcerated nodule or plaque, or as a polypoid or verrucous lesion. Multiple lesions are uncommon. The most common sites are lower extremities (50%), trunk (24%), head and neck (24%), while it is uncommon in the upper extremities, nail bed, vulva, breast, and penis. [5],[6] The differential diagnoses include squamous cell carcinoma, basal cell carcinoma, Paget′s disease, amelanotic melanoma, and metastatic adenocarcinoma. [6] Eccrine porocarcinoma is challenging to diagnose based on clinical presentation alone. Histologic analysis with special immunohistochemical staining is almost always required in establishing the diagnosis. [1],[4],[6] The tumour may be associated with immunocompromised states such as human immunodeficiency virus (HIV) infection, diabetes, sarcoidosis, and organ transplantation. [6] The prognosis is variable, although it normally grows slowly and the initial surgical treatment is usually curative in the majority of cases. Local recurrences are seen in 17%, regional lymph node metastases in 19% and systemic metastasis in 11% of patients. [5] With lymph node metastases, there is a high mortality rate. [1] Our patient presented with multiple cutaneous lesions with lymphedema of the entire upper limb with regional lymph node involvement. The histological findings of the lesions revealed striking infiltration of the entire dermis by numerous epithelial aggregates of atypical poroid cells with ductal differentiation. These cells were positive for pancytokeratins, immunoperoxidase, and Ki-67 with immunohistochemical staining and the ductal structures were highlighted by CEA special stains. The final diagnosis of metastatic porocarcinoma was confirmed by histopathology and immunohistochemistry. Our patient developed the cutaneous lesions nearly one year after the amputation of her ring finger for an undiagnosed non-healing ulcer. From the subsequent clinical course and histopathology of present lesions, we presume that the ulcerated lesion of her ring finger was a primary eccrine porocarcinoma and the subsequent lesions were metastases. The tumour can spread by lymphovascular invasion or by pagetoid extension of the neoplastic cells. In this case, although lymphovascular involvement could not be found in histological sections, the distribution of cutaneous lesions and lymphedema of the entire limb with axillary lymphadenopathy were suggestive of the the primary tumour of the ring finger having metastasized to the skin and lymph nodes. Bhat et al. reported a similar case of an eccrine porocarcinoma of the left ring finger with metastasis to the forearm and axilla in an 80-year-old male. [2]
Wide local surgical excision or Mohs micrographic surgery is the treatment of choice for localized lesions. No standard therapeutic protocols for metastatic eccrine porocarcinoma exist. However, a variety of chemotherapeutic agents have been used with varying degrees of responsiveness. [4],[7] Metastatic porocarcinoma is very resistant to adjunctive chemotherapy or radiation. In this case, however, the patient was free of recurrence at follow up after a year.
| 1. |
Kim JW, Oh DJ, Kang MS, Lee D, Hwang SW, Park SW. A case of metastatic eccrine porocarcinoma. Acta Derm Venereol 2007;87:550-2.
[Google Scholar]
|
| 2. |
Bhat W, Akhtar S, Khotwal A, Platt AJ. Primary eccrine porocarcinoma of the finger with transit forearm and axillary metastasis. Ann Plast Surg 2011;66:344-6.
[Google Scholar]
|
| 3. |
Marone U, Caracò C, Anniciello AM, Di Monta G, Chiofalo MG, Di Cecilia ML, et al. Metastatic eccrine porocarcinoma: Report of a case and review of the literature. World J Surg Oncol 2011;9:32.
[Google Scholar]
|
| 4. |
González-López MA, Vázquez-López F, Soler T, Gómez-Diéz S, Garcia YH, Manjón JA, López-Escobar M, Pérez-Oliva N. Metastatic eccrine porocarcinoma: A 5.6-year follow-up study of a patient treated with a combined therapeutic protocol. Dermatol Surg 2003;29:1227-32.
[Google Scholar]
|
| 5. |
Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, et al. Eccrine porocarcinoma (malignant eccrine poroma): A clinicopathologic study of 69 cases. Am J Surg Pathol 2001;25:710-20.
[Google Scholar]
|
| 6. |
Brown CW Jr, Dy LC. Eccrine porocarcinoma. Dermatol Ther 2008;21:433-8.
[Google Scholar]
|
| 7. |
Zeidan YH, Zauls AJ, Bilic M, Lentsch EJ, Sharma AK. Treatment of eccrine porocarcinoma with metastasis to the parotid gland using intensity-modulated radiation therapy: A case report. J Med Case Rep 2010;4:147
[Google Scholar]
|
Fulltext Views
3,357
PDF downloads
2,037





