Translate this page into:
The reservoir effect of topical steroids in vitiliginous skin: A cross-sectional study
Correspondence Address:
Satyendra Kumar Singh
Department of Dermatology and Venereology, Institute of Medical Sciences, Banaras Hindu University, Varanasi - 221 005, Utter Pradesh
India
| How to cite this article: Singh SK, Nasir F. The reservoir effect of topical steroids in vitiliginous skin: A cross-sectional study. Indian J Dermatol Venereol Leprol 2015;81:370-375 |
Abstract
Background: Prolonged and frequent use of topical steroids may lead to decrease in efficacy as well as many local adverse effects. Stratum corneum has a unique property of reservoir effect. Aims: To study the reservoir effect of topical steroids in a steroid-responsive condition which may enable a decrease in the dosing frequency of topical steroids. Methods: A cross-sectional study design was used. Patients with at least three vitiliginous patches of more than 2 cm 2 present over the trunk or limbs were included. Exclusion criteria were topical or systemic corticosteroid use within the previous 4 weeks, antihistamine use within the previous 7 days, history of any allergy in the past and immunosuppression. Clobetasol propionate cream was applied on the first vitiliginous area (site A) and fluticasone propionate ointment was applied on the second vitiliginous area (site B). The third vitiliginous area, site C (control site) was left without applying any medication. Histamine-induced wheal suppression test was performed on each site, at the same time of the day, on every consecutive day following steroid application, until the values obtained at sites A and B approached those obtained at site C. SPSS software for Windows, version 16.0 was used for statistical analysis. The mean and standard deviation of the various studied parameters were calculated for various treatment groups and compared using analysis of variance (ANOVA) test. Results: Forty patients were included in the study. The average wheal volumes and average erythema sizes at sites A and B were significantly smaller than the corresponding values at site C for up to 5 days after applying medication (P < 0.001). Limitations: The presence of a cutaneous reservoir of topical steroid was confirmed based on its suppressive effect on the wheal and flare response to histamine. It is not certain that the concentration that suppresses histamine-induced wheal and flare is sufficient for therapeutic efficacy in vitiligo. Conclusion: The reservoir effect of topical clobetasol propionate and fluticasone propionate persisted for 5 days in vitiliginous skin. Hence, it may be possible to reduce the frequency of topical steroid application in vitiligo.INTRODUCTION
Topical corticosteroids have become the most frequently prescribed dermatological drugs. Many inflammatory, immunological and hyperplasic dermatological conditions which were previously resistant to treatment can now be treated effectively with these compounds. Despite a favorable efficacy-safety profile, proposed by earlier physicians and promoted by pharmaceutical companies, the use of topical corticosteroids is limited by their numerous side-effects. A landmark shift in the traditional concepts about topical corticosteroid therapy occurred when Vickers, in 1963, demonstrated that topically applied corticosteroid remains in the stratum corneum for a few days, forming a reservoir from which it is released to the deeper layers of the skin. [1] He demonstrated that topically applied corticosteroid forced into the stratum corneum by repeated occlusion for a few hours remained there for as long as 7-14 days as observed by the development of vasoconstriction which is a physiological marker. Because of this depot effect, it was possible to reduce the dosing frequency of topical glucocorticoids, while the effectiveness was preserved and the side-effects were reduced.
Vitiligo is a steroid-responsive autoimmune disorder of the skin. Topical steroids have to be used for a long period of time, without occlusion, in vitiligo. It is a condition associated with great cosmetic disfigurement and social taboo, especially in the poorer rural Asian communities. It is important to prevent atrophy of vitiliginous skin as a side effect of topical corticosteroids after which repigmentation may be difficult. Also, prolonged daily use of topical steroids may result in decrease in therapeutic efficacy due to tachyphylaxis and will increase the total cost of treatment. At present, no study is available that demonstrates the reservoir effect of topical corticosteroids applied without occlusion in human vitiliginous skin.
METHODS
This was a cross-sectional study, conducted at a tertiary health care centre. The study design was approved by the institutional ethics committee. Written informed consent was obtained from each patient. Vitiligo patients without any other associated medical conditions were included. The diagnosis of vitiligo was made on clinical grounds. Inclusion criteria were the presence of at least three patches of vitiliginous skin, each covering an area of more than 2 cm 2 , and located on the trunk and/or limb. Exclusion criteria were age below 12 and above 65 years, topical or systemic corticosteroid use within the last 4 weeks prior to study recruitment, antihistamine intake within the previous 7 days, history of any allergy in the past, presence of any systemic diseases, especially those with associated raised histamine levels (e.g., mastocytosis) or lowered threshold for histamine release (e.g., atopic dermatitis, urticaria) and immunosuppression (human immunodeficiency virus [HIV] infection). Patients with no new lesion or increase in size of previous lesions in the last 2 years were considered to have stable vitiligo.
The three areas of vitiliginous skin that were to be used as test sites were marked. In every patient, on day 0, clobetasol propionate 0.05% cream was applied on the first vitiliginous area designated as site A, and fluticasone propionate 0.005% ointment was applied on the second vitiliginous area designated as site B. The third vitiliginous area, site C (control site), was left without applying any medication. Medications were applied according to the finger-tip unit method and left un-occluded without wiping or washing for an hour. After 1 hour, patients were asked to continue their normal activities with the areas unprotected, and normal washing was permitted. Patients were advised not to rub or use soap on the studied areas.
Topical steroids suppress the wheal and flare response triggered by histamine. In our study, we used the histamine-induced wheal suppression test as designed by Reddy and Singh [2] and modified by Singh and Singh. [3] The histamine-induced wheal suppression test was performed at all 3 sites in each patient, at the same time of the day, on every consecutive day following steroid application, until the values measured at from sites A and B approached those measured at site C (control). A circular glass cup, open at both ends, was applied on each site and 1 ml of freshly prepared 1% histamine phosphate solution was poured into it with the help of an insulin syringe. A prick was made in the center using an automatic pricking needle. The length of the needle was kept constant at 2 mm throughout the study period. The solution was allowed to stay for 1 minute and then gently wiped away with cotton. Ten minutes after the prick, the minimum and maximum diameters of the wheal and the erythema produced were measured using a divider and a millimeter scale. Thickness of the wheals was measured using a spherometer (given by subtracting baseline curvature of site from height of wheal produced). Volume of the wheals was calculated using the formula V = πd 2h/4, where V = volume, d = average diameter and h = average height of the wheal.
Statistical analysis
The statistical analysis was done using SPSS software for Windows, version 16.0. For non-continuous data, Chi-square test was used. The mean and standard deviation of the parameters studied during the observation period were calculated for various treatment groups and compared using analysis of variance (ANOVA) test. For intra-group comparisons, paired t-tests were used. The critical value of ′P′ indicative of significant difference was taken as <0.05.
RESULTS
A total of 40 patients were included in this study in which 23 (57.5%) were females and 17 (42.5%) were males. Their age ranged from 13 to 56 years with a mean age of 28.60 ± 11.39 years. The mean duration of vitiliginous lesions was 6.45 ± 5.47 with a range of 1-25 years. In 26 (65%) patients, the disease was progressive, and the remaining 14 (35%) had stable disease. The sites of tested lesions were mainly on lower limbs followed by chest, abdomen, upper limb and back. The following sections present comparisons of the various measured parameters.
Control site C had the largest average wheal size followed by test sites B and A, in descending order. The differences in average wheal volume between sites A and C and sites B and C were highly significant for upto 5 days after steroid application (P < 0.001) [Table - 1] and significant (P < 0.05) on day 6. On Day 7 also, the difference in wheal volume was significant on comparing sites A and C (P < 0.05), but not on comparing sites B and C.
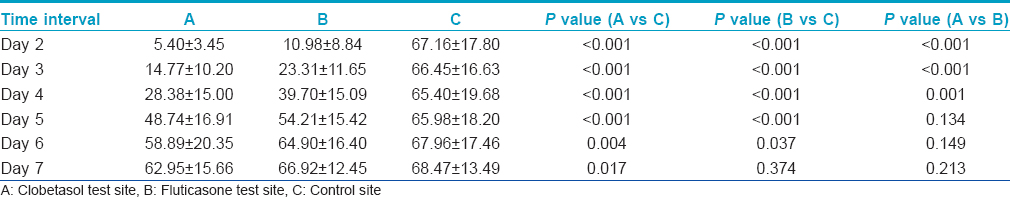
The average erythema sizes at site A and site B were significantly smaller when compared with the average erythema sizes at site C for upto 5 days after steroid application (P < 0.001) [Table - 2]. On the 6 th and 7 th day, erythema sizes at sites A and B showed no difference when compared with the control site C.
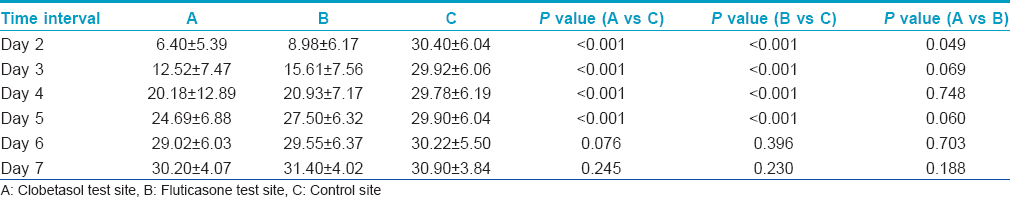
Inter-day comparisons of wheal and flare reactions are presented in [Table - 3] and [Table - 4], respectively. At sites A and B, average wheal size as well as erythema size grew significantly larger with each passing day as compared to the previous day, upto Day 7 after steroid application. These daily increases in size were highly significant on Days 3 through 6 (P < 0.001) and significant (P < 0.05) on Day 7. However, no such inter-day changes in wheal or erythema sizes could be appreciated at site C.
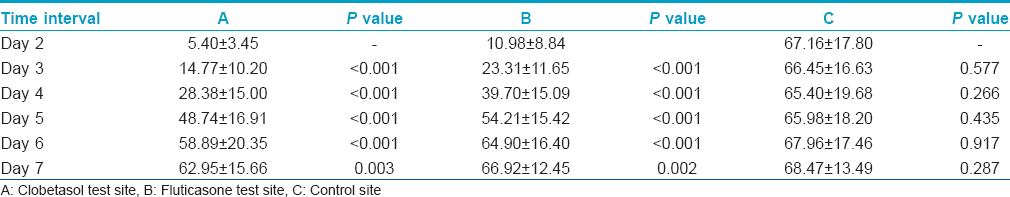
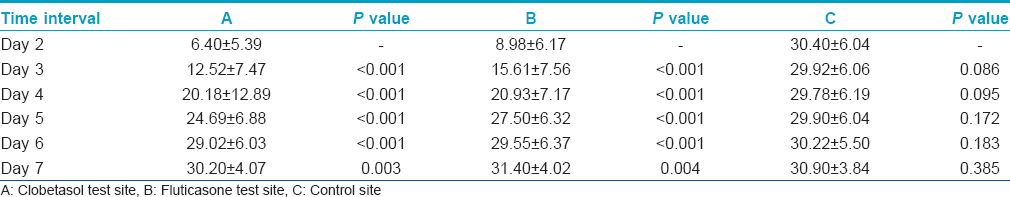
The average wheal sizes at site B (fluticasone propionate) were significantly larger than those at site A (clobetasol propionate) on Days 2 to 4. These differences were highly significant on Day 2 and Day 3 (P < 0.001) and significant on Day 4 (P < 0.05) [Table - 1]. On Days 5-7, there was no significant difference.
The average erythema size was significantly larger at site B compared with that at site A on Day 2 (P < 0.05) [Table - 2]. On Days 3-7, there was no significant difference.
DISCUSSION
Topical corticosteroids are the most frequently used drugs in dermatology. Since the introduction of topical hydrocortisone by Sulzberger and Witten, many different topical corticosteroids of various strengths have been synthesized till date. [4] The use of super-potent topical corticosteroids could cure many previously resistant dermatoses, bringing relief to several patients. Increasing efficacy of manufactured topical steroids was accompanied by increasing numbers of side effects, leading to the introduction of less potent steroids with more favorable benefit-risk ratios.
Due to their potent and rapid anti-inflammatory effects, topical corticosteroids have been widely misused by patients and even by untrained physicians. Their local side-effects include dermal thinning, striae, telengiectasiae, purpura, skin atrophy, acneiform eruptions, hypertrichosis, hypopigmentation, localized infections and tachyphylaxis. Prolonged application of steroids over large areas of skin, especially the more potent agents, causes systemic absorption followed by suppression of the hypothalamic-pituitary-adrenal axis, Cushing′s syndrome, stunting of growth, osteopenia and osteoporosis.
In the earlier years, topical corticosteroids were advised to be applied several times in a day. This thinking was revolutionized by the suggestion of the existence of a depot or reservoir effect for topically applied drugs by Malkinson and Ferguson. [5] This suggestion was based on the recovery of radioactivity in the 17-keto-steroid fraction of the urine in two patients for as long as 7 days after hydrocortisone-4- 14 C had been applied to the skin surface.
The presence of a reservoir effect of topically applied corticosteroids was confirmed by Vickers [1] using the vasoconstriction assay which was introduced by McKenzie and Stoughton. [6] Fluocinolone acetonide 1% solution and triamcinolone acetonide 1% solution were used under occlusion. Experiments were carried out on 19 healthy adults. The reservoir effect was seen in all, though its duration varied widely from 3 to 15 days. Three similar experiments were performed on two patients in whom the skin of the forearm was stripped of the stratum corneum. No vasoconstriction was seen in the stripped areas in contrast to control skin areas with intact stratum corneum which showed significant vasoconstriction. These experiments confirmed that the reservoir was in the stratum corneum.
Rimbau and Lleonart demonstrated reservoirs of corticosteroids in the skin at 400-700 μm depth. [7] Brickl et al. also confirmed the presence of a reservoir in the stratum corneum in an animal model. [8] They demonstrated that considerable proportions of radioactive corticosteroids may be recovered from stripped skin layers of the mini-pig on the second and fifth days after application of topical hydrocortisone. Clarys et al. demonstrated significant reservoirs of halcinonide using chromametry for upto 5 days following initial application. [9] Teichman et al. showed that the reservoir of drugs is present in the stratum corneum by using the tape stripping method. [10] They demonstrated that the saturation level depends upon the individual volunteer, the topically applied substance and the formulation used. Abidi et al., in 2010, demonstrated that a reservoir of topical clobetasol propionate 0.05% cream persisted for 4 days in albino rabbit skin. [11]
Vitiligo is a steroid-responsive autoimmune disorder with a chronic course which usually requires long-term application of steroids. One of the serious complications of treatment is atrophy of the skin after which the chances of repigmentation are reduced. Another aim of the study was to check if it is possible to reduce the side effects of topical steroids and also the cost of treatment with these agents without compromising their therapeutic efficacy.
In all the previous studies that were done to demonstrate the reservoir effect of topical corticosteroids, experiments were carried out in animal models or in healthy human volunteers and applications were done under occlusion. These conditions do not match the clinical setting where patients apply topical corticosteroid on vitiliginous skin, most often unoccluded.
In this present work, the aim was to study the reservoir effect of two different corticosteroids of different potencies, in human vitiliginous skin, after a single unoccluded application. Clobetasol propionate 0.05% cream and fluticasone propionate 0.005% ointment were used which belong to the American potency classification of topical corticosteroids, classes 1 and 3, respectively. The duration of topical steroid application on the lesions in our patients was only 1 hour. Singh et al. demonstrated that applications of topical steroids for half an hour and 24 hours are equally effective. [12] The histamine-induced wheal suppression test used in this study was introduced by Reddy and Singh [2] and modified by Singh and Singh. [3] It is a simple, reliable, non-invasive and reproducible method that has its advantages compared with other methods. More than two or three compounds can be tested on the same patient, allowing for a smaller number of subjects for the study. The pharmacological actions of histamine include an increase of capillary and post-capillary venular permeability. This vascular change leads to the wheal-flare response. Histamine is contraindicated in patients with a history of hypersensitivity to histamine products and in patients with hypotension, severe hypertension, vasomotor instability, severe cardiac, pulmonary or renal disease. Large doses of histamine may precipitate systemic reactions that may include flushing, dizziness, headache, bronchial constriction, urticaria, asthma, marked hypotension or hypertension, abdominal cramps, vomiting and metallic taste. Since we excluded those with associated systemic diseases, none of our patients developed any problem except minute local bleeding and local pruritus. As the test was conducted on depigmented vitiliginous skin, the wheals and erythema were easily appreciable. The wheal and erythema sizes at the test sites were compared with those at the control sites where no drug was applied. Comparison of erythema sizes produced on sites A and B with that produced at C indicates that the flare effect of histamine was inhibited by both the drugs until Day 5. Wheal size comparison between sites A and B shows that clobetasol propionate inhibited the wheals to a higher extent than fluticasone propionate, indicating the differences in their potencies.
Our study had limitations. The suppressive effect of topical steroids on the wheal and flare response triggered by histamine was used to detect the presence of steroid in this study. Whether this concentration of topical steroid is sufficient for therapeutic efficacy in vitiligo is a separate question which should ideally be answered by randomized controlled clinical trials.
This study showed that a significant amount of a reservoir of clobetasol propionate and fluticasone propionate persisted for at least 5 days in vitiliginous skin after a single application. The reservoir effect may be present for upto 7 days in case of clobetasol propionate, and for upto 6 days for fluticasone propionate because wheal suppression was maintained for upto 7 and 6 days, respectively.
The presence of a cutaneous reservoir of topical corticosteroids is an important factor which influences dosing frequency. As a result of the reservoir effect, it may be possible to reduce the frequency of application without compromising the efficacy. The benefits of less frequent applications include reduced local and systemic side-effects, reduced cost of treatment and better patient compliance. Less frequent applications may be advocated in vitiligo patients, such as once in every 4 or 5 days, but these dosing schedules need to be studied further. Our experiment demonstrates that the cutaneous topical steroid reservoir is capable of inhibiting the wheal and erythema induced by 1% histamine phosphate solution for upto 5 days; whether this reservoir is also sufficient for the treatment of steroid-responsive dermatoses is a question that cannot be answered definitively by our experiment. This issue needs to be further clarified by well-designed randomized controlled clinical trials.
| 1. |
Vickers CF. Existence of reservoir in the stratum corneum: Experimental proof. Arch Dermatol 1963;88:20-3.
[Google Scholar]
|
| 2. |
Reddy BS, Singh G. A new model for human bioassay of topical corticosteroids. Br J Dermatol 1976;94:191-3.
[Google Scholar]
|
| 3. |
Singh G, Singh PK. Tachyphylaxis to topical steroid measured by histamine induced wheal suppression. Int J Dermatol 1986;25:324-6.
[Google Scholar]
|
| 4. |
Sulzberger MB, Witten VH. Effect of topically applied compound F in selected dermatoses. J Invest Dermatol 1952;19:101-2.
[Google Scholar]
|
| 5. |
Malkinson FD, Ferguson EH. Percutaneous absorption of hydrocortisone-4-C14 in two human subjects. J Invest Dermatol 1955;25:281-3.
[Google Scholar]
|
| 6. |
McKenzie AW, Stoughton RB. Method for comparing percutaneous absorption of steroids. Arch Dermatol 1962;86:608-10.
[Google Scholar]
|
| 7. |
Rimbau V, Lleonart F. Kinetic study of the percutaneous absorption of 1,2,4-3H labeled flupamesone. Arzneimitteforschung 1975;25:1040-2.
[Google Scholar]
|
| 8. |
Brickl R, Koss FW, Roth W. Studies on pharmacokinetics of hydrocortisone 17-butyrate in the skin. Akt Dermatol 1980;6:13-9.
[Google Scholar]
|
| 9. |
Clarys P, Gabard B, Barel AO. A qualitative estimate of the influence of halcinonide concentration and urea on the reservoir formation in the stratum corneum. Skin Pharmacol Appl Skin Physiol 1999;12:85-9.
[Google Scholar]
|
| 10. |
Teichmann A, Jacobi U, Weigmann HJ, Sterry W, Lademann J. Reservoir function of the stratum corneum: Development of an in vivo method to quantitatively determine the stratum corneum reservoir for topically applied substances. Skin Pharmacol Physiol 2005;18:75-80.
[Google Scholar]
|
| 11. |
Abidi A, Ahmad F, Singh SK, Kumar A. Study of reservoir effect of clobetasol propionate cream in an experimental animal model using histamine-induced wheal suppression test. Indian J Dermatol 2010;55:329-33.
[Google Scholar]
|
| 12. |
Singh S, Singh SK, Pandey SS. Effect of duration of application and dosing frequency on the efficacy of topical 0.1% mometasone furoate ointment in psoriasis. J Dermatol Treat 1998;9:25-30.
[Google Scholar]
|
Fulltext Views
3,874
PDF downloads
2,663





