Translate this page into:
Drug advertisements in two dermatology journals: A critical comparison of IJDVL and JAAD
2 Department of Pediatrics, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly, Uttar Pradesh, India
Correspondence Address:
Pratik Gahalaut
69, Silver Estate, Bareilly - 243 006, Uttar Pradesh
India
| How to cite this article: Gahalaut P, Chauhan S, Mishra N, Rastogi M, Thakur R. Drug advertisements in two dermatology journals: A critical comparison of IJDVL and JAAD. Indian J Dermatol Venereol Leprol 2014;80:115-121 |
Abstract
Background: Though drug promotion regulations exist worldwide, low quality of journal drug advertising is a global issue. Medical journals are regarded as a leading source of information for new drugs. They may also modulate prescribing behavior of physicians without their knowledge. A comparative analysis of advertisements from different countries may provide insights regarding strengths and weaknesses of different regulating systems. Aims: Prescription drug advertisements from the Indian Journal of Dermatology, Venereology, and Leprology (IJDVL) and Journal of American Academy of Dermatology (JAAD) were compared to check their compliance with criteria of World Health Organization (WHO) and International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Methods: All the prescription drug advertisements of at least one page length appearing in all the issues of IJDVL and JAAD from January 2012 till December 2012 were included in this study. The contents of both advertisements were compared for compliance regarding different criteria of ethical codes for drug advertising of WHO and IFPMA. Statistical analysis was done using Fisher's exact test. Results: Compared to IJDVL, more advertisements in JAAD complied with WHO and IFPMA codes. On the whole, advertisements in IJDVL had significantly less information regarding the approved usage, dosage, abbreviated prescribing information (API), summary of scientific information, safety information regarding the drug, and references to the scientific literature to support various claims. However, JAAD had more advertisements with multiple claims than IJDVL, and many advertisements interspersed between scientific articles while IJDVL had none. Conclusion: The complex issue of ethical drug advertising in dermatology journals requires constant review and discussion. Dermatologists should be cautious in assessing any advertisement or claim even if it seems evidence-based. The results from our study highlight the need for a global, proactive and effective regulatory system to ensure ethical medicinal drug advertising in medical journals.Introduction
Peer-reviewed medical journals are generally considered to be a source of unbiased and reliable information about drugs and they are the third leading source of information about new prescription drugs for physicians. [1],[2] They might influence prescription behavior of the prescribing physician in both developed and developing countries without necessarily benefitting the patient. [3],[4],[5],[6],[7],[8] Pharmaceutical companies value print advertisements in medical journals because they increase sales effectively and may hasten introduction of new drugs. [9] The monetary involvement in drug promotion is quite high. [10] Pharmaceutical companies spend 20-30% of their sales turnover or about two to three times the average expenditure on research and development, for promotion and marketing. [11] In 2001, around 2% of the total pharmaceutical promotional budget, that is, approximately $400 million, was spent on advertising in various medical journals in USA. [12]
Despite various drug promotional regulations available worldwide, low quality of journal advertising is a global issue. [13] Internationally, important guidelines for regulation of drug promotional activities are "Ethical criteria for medicinal drug promotion" by World Health Organization (WHO), "Code of Pharmaceutical Marketing Practices" by International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), and the code prepared by Health Action International. [14],[15],[16]
Presently in India, there are no constitutional guidelines which deal with drug promotion in various medical journals though the Magic Remedies (Objectionable Advertisement) Act 1954 prohibits false or misleading advertisements related to drugs. [17]
Though there are a few descriptive studies about journal drug advertising from India, codes of conduct and regulations on pharmaceutical advertising have not been updated since long. [18],[19],[20],[21],[22] Besides, worldwide, such a comparative analysis in dermatology is long overdue. [23] Latest information regarding comparative data is required which would provide policy makers with recent evidence of strengths and weaknesses of different regulatory systems. [13] We decided to analyze the advertisements in official dermatology journals of the respective national associations of dermatology for USA and India, that is, Journal of American Association of Dermatology (JAAD) and Indian Journal of Dermatology, Venereology, and Leprology (IJDVL) based on various criterion of WHO and IFPMA codes.
Methods
We included all the advertisements for prescription drugs which appeared in all the issues of JAAD and IJDVL from January 2012 till December 2012. To rule out any discrepancy, supplement issues if any were excluded. Printed copies of the journals were extracted from the central library of Shri Ram Murti Smarak Institute of Medical Sciences (Bareilly), a medical college in northern India. Any advertisement of a prescription drug of at least A4 paper size appearing in JAAD or IJDVL during the study period was included. We excluded advertisements describing more than one drug/formulation/brand in one advertisement and advertisements referring to medical equipment, surgical appliances, nutritional supplements, medications of alternative therapies, or nonprescription medicines. To rule out any duplication, only distinct advertisements were included and subsequent identical advertisements, repeated in different issues, were excluded. However, if two different advertisements described the same drug but had different graphic or written content, then both were included in the study.
Pictures were analyzed separately and grouped into three categories as living/nonliving/combination. Advertisements were also analyzed for their graphic content including tables and graphs. Abbreviated prescribing information (API) was searched for and its surface area was recorded as a percentage of total surface area for that particular print ad.
Number of claims in all advertisements was determined. Cited references were searched in our college library or on the internet using various electronic databases like PubMed, IndMED, Google search, etc. A reference was considered ′available′ if a softcopy or hardcopy of the cited article or its abstract was available in the public domain. This study did not evaluate the authenticity of the claims which were made in the study advertisements.
Data analysis was done with the help of GraphPad software. Fisher′s exact test was used at a significance level of two-tailed P < 0.05 to compare percentages. Analysis of variance (ANOVA) was used to compare significant differences among the sample means. The study was approved by our institutional ethical committee.
All advertisements were independently assessed by a single experienced dermatologist (PG) to maintain consistency. This information was rechecked by another author (MKR). In case of any disagreement, the opinion of a third researcher (NM) was sought and the consensus prevailed. Kappa tests were done to assess the consistency between two observers. The rate of agreement between PG and MKR ranged from k = 0.85 to 0.96 for most of the parameters.
Results
In 2012, JAAD published 12 issues and one supplement whereas IJDVL published six issues with one supplement. The detailed statistical information about the two journals and their advertisements is mentioned in [Table - 1], [Table - 2], [Table - 3], [Table - 4]. The proportion of pages occupied by these advertisements, compared to the total number of pages with academic content was similar in both the journals. Out of a total of 285 advertisements for prescription drugs, 76 advertisements were distinct and included in the present analysis.
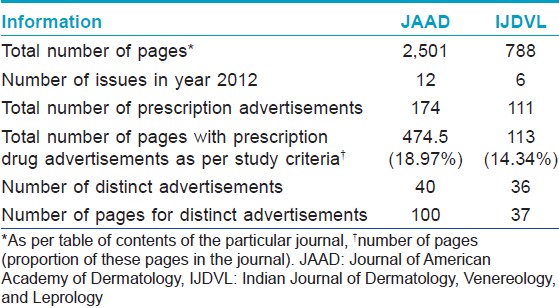
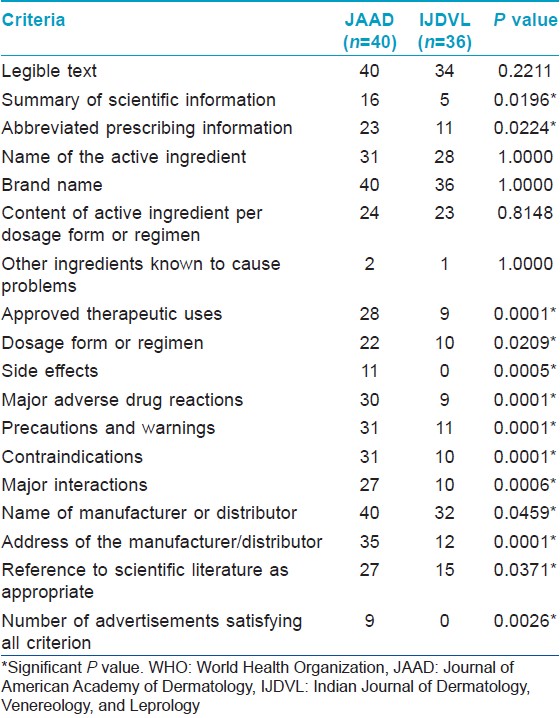
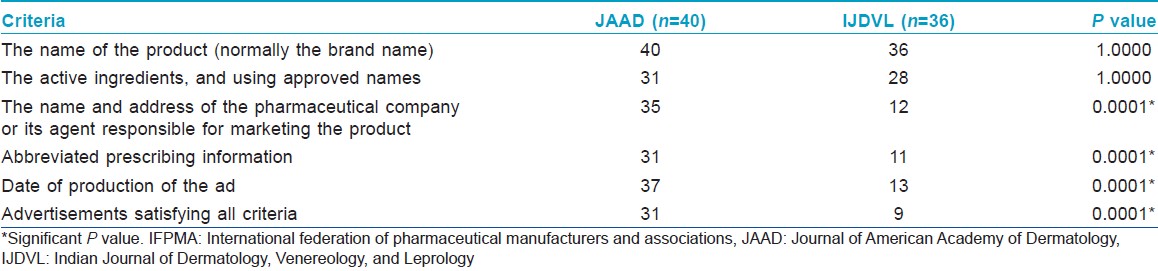
Compared to IJDVL, more advertisements in JAAD complied with WHO and IFPMA codes. On the whole, advertisements in IJDVL had significantly less information regarding the approved usage, dosage, API, summary of scientific information, safety information, and references to scientific literature to support the various claims made. Many advertisements in IJDVL mentioned company data or data on file to support claims, but the address of the manufacturing or marketing company was missing in significantly more advertisements in IJDVL compared to JAAD.
Discussion
This study was done with the intent of comparing the quality of advertisements in leading dermatology journals of a developed and developing country, based on various ethical codes for journal drug advertising. Only 25% of advertisements in JAAD fulfilled all the WHO criteria and 77.5% met the requirements of the IFPMA code. Advertisements in IJDVL performed worse, where none of the advertisements fulfilled WHO criterion and only 25% of advertisements complied with the IFPMA code. In the past, poor quality of advertising has been observed in both developing and developed countries. [13],[24] Our findings for drug advertisements in IJDVL are similar to earlier studies from developing countries. [13],[25],[26],[27],[28]
Contrary to the guidelines of the International Committee of Medical Journal Editors (ICMJE), JAAD had many advertisements interspersed between the academic articles compared to IJDVL, which had none. [29]
More advertisements satisfied criteria for the IFPMA Code of Practice than the WHO criteria in both journals. This is noteworthy because the IFPMA code allows less medicinal information to be presented in advertisements. [15] It does not require information on warnings, major interactions, content of active ingredient per dosage form or regimen and names of other ingredients known to cause problems. [28] In India, drug promotional activities are largely governed by the Organization of Pharmaceutical Producers of India (OPPI) that has adopted a self-regulatory code of ethics for drug promotion, which is based on the IFPMA code. [16] This code has to be followed by all member companies. [15],[16],[30] In many OPPI member companies, promotional material has to be approved by a medical advisor. Interestingly, qualified medical advisors are missing in most of the companies in India and the marketing department takes overriding decisions on promotional materials. [30]
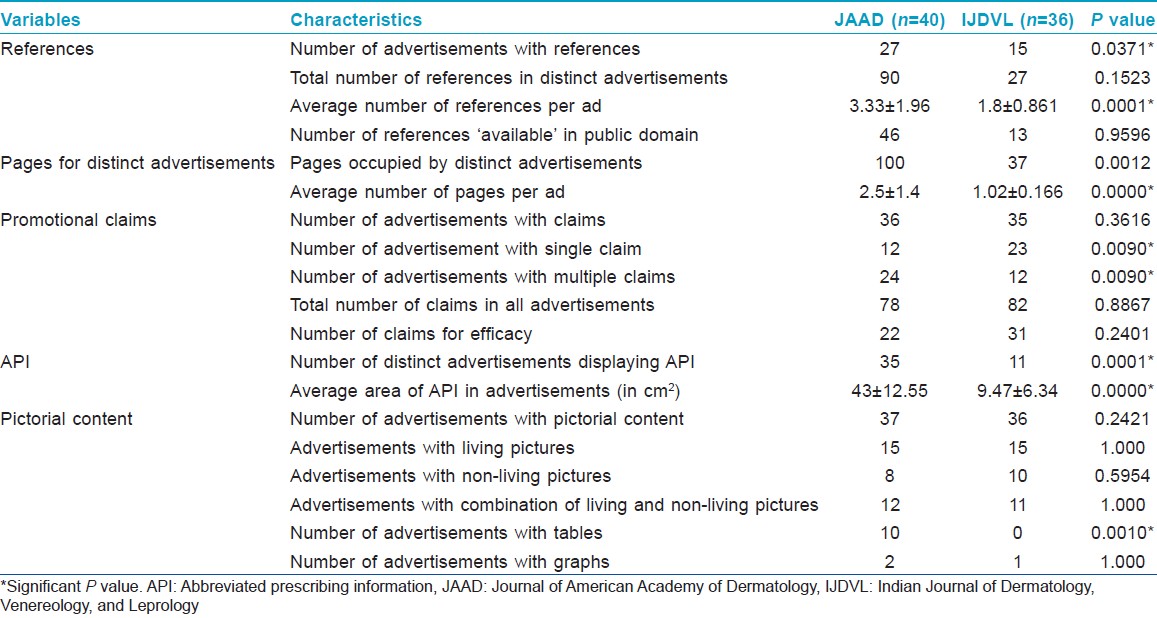
Though JAAD had significantly more references in total and for every advertisement compared to IJDVL, both journals had a similar percentage of references available in the public domain. As shown in [Table - 4], JAAD had significantly more advertisements with multiple claims, while IJDVL had significantly fewer advertisements displaying abbreviated prescribing information.
Nearly all the advertisements in this study had some pictorial content to make them more attractive, though pictures should not have a role in medical decision-making. [10],[31],[32] The total area devoted to abbreviated prescribing information was significantly lower in IJDVL and hence information was difficult to read. [32],[33],[34]
Various strategies have been proposed and implemented to ensure compliance of journal drug advertisements to ethical guidelines. These consist of tightening of existing government regulations and the need for stringent measures to promote ethical pharmaceutical publications. [10] Past studies have also recommended reinforcement of review procedures by the journals′ editors, including peer review of advertisements. [13],[23] Voluntary implementation of codes of good practices and self-regulation by the pharmaceutical industry has been stressed upon. [35],[36] Even lobbying by medical bodies for appropriate marketing of pharmaceuticals has been tried in the past. [37] Public reporting of violations of these codes has also been suggested as another remedial measure. [28] Unfortunately, none of these measures have had the desired effect. Journals are reluctant to take control activities for obvious reasons ranging from the already heavy workload involved in publishing a journal, to fear of losing financial support, reluctance to endorse advertisements with the journal′s seal of approval, and awareness that a promotional advertisement is not a systematic review, and that physicians are a target audience capable of distinguishing advertisements from scientific evidence. [38] Editors of some journals have rightly argued that journal resources should be dedicated toward improving editorial content and not for reviewing advertisements. [39],[40] Formal training in the critical appraisal of drug advertisements during undergraduate medical teaching in pharmacology needs to be addressed. [25] Several authors have advocated other sources of revenue for medical journals, and that medical journals should accept advertisements for products other than those supplied by the healthcare or pharmaceutical industry. [2] Results from our study and earlier studies show that self-regulatory measures are insufficient. [41],[42],[43] Finally, despite the stir caused by some campaigns, lobbying activities have very little overall effect on improving advertisements. [38]
In India, regional ethics committees collect complaints against unethical drug promotional advertisements and forward them to drug controller authorities to take necessary steps to discipline guilty companies. [33],[44] There is evidence that many violations of marketing codes go unreported. [41] Besides, the sheer volume of data may make this strategy unviable. The Food and Drug Administration (FDA) has acknowledged that it cannot review all submissions because of the volume that it receives. [45] Hence, only the future will tell how beneficial these activities may be in India. Government regulatory bodies must play a proactive role where a code of ethics is failing. [33] Again, this is easier said than done. Regulating promotional activities involves monetary concerns and various possible models to generate revenue have their own pitfalls. [45] The availability of information on complaints, code breaches, and sanctions in the public domain may discourage repeated breaches. [46]
Our study has many limitations; viz. small sample size, identification of advertisements by convenience sampling, accuracy/quality of claims or references were not determined, and results cannot be generalized to other journals or medical specialties or countries. Besides, we might have been unable to trace all the references. Research on journal advertisements is frustrating. [2]
Publishing is expensive and medical journals obviously need a business model to be sustainable. [47] Journal advertisements generate profits not only for pharmaceutical companies and medical journals, but also for the physician organizations that publish the journals. [2] The reduced cost of medical journals is a positive but harsh reality of medical advertising. [32] Without the revenue from medical advertisements, medical journals would either be more expensive for the physician or would contain fewer pages. [47],[48]
Conclusion
The available regulatory codes provid a useful guideline for drug promotion, but they are still considered the beginning rather than the end of this debate. [30] Overall, the complex issue of ethical drug advertising in dermatology medical journals requires constant review and discussion. Dermatologists should be cautious in assessing any advertisement or claim even if it seems evidence-based. Ongoing efforts including complaints and recommendations by researchers, health professionals, and policy makers to improve the quality of advertisements in medical journals are crucial. [13] In future, interventional research should be planned to determine the awareness of physicians that drug advertisements in medical journals may not comply with the ethical code of conduct, its effects on their prescribing behavior, standards by which advertisements should be reviewed by editors, and journals′ advertising policies. Reliable data on these aspects will lead to informed discussion because some investigators argue that too much attention has already been focused on regulating advertisements that they claim are never read. [49],[50]
| 1. |
Krupka LR, Vener AM. Prescription drug advertising: Trends and implications. Soc Sci Med 1985;20:191-7.
[Google Scholar]
|
| 2. |
Fugh-Berman A, Alladin K, Chow J. Advertising in medical journals: Should current practices change? PLoS Med 2006;3:e130.
[Google Scholar]
|
| 3. |
Roy N, Madhiwalla N, Pai SA. Drug promotional practices in Mumbai: A qualitative study. Indian J Med Ethics 2007;4:57-61. Available from http://www.issuesinmedicalethics.org/pdfs/152oa57.pdf [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 4. |
Liebman M. Listen up, publishers say journal advertising sells! Med Marketing Media 2000;35:89-94.
[Google Scholar]
|
| 5. |
Avorn J, Chen M, Hartley R. Scientific versus commercial sources of influence on the prescribing behavior of physicians. Am J Med 1982;73:4-8.
[Google Scholar]
|
| 6. |
Spiller LD, Wymer WW Jr. Physicians' perceptions and uses of commercial drug information sources: An examination of pharmaceutical marketing to physicians. Health Mark Q 2001;19:91-106.
[Google Scholar]
|
| 7. |
Wolfe S. Drug advertisements that go straight to the hippocampus. Lancet 1996;348:632.
[Google Scholar]
|
| 8. |
Walton H. Ad recognition and prescribing by physicians. J Advert Res 1980;20:39-40.
[Google Scholar]
|
| 9. |
Mackowiak JI, Gagnon JP. Effects of promotion on pharmaceutical demand. Soc Sci Med 1985;20:1191-7.
[Google Scholar]
|
| 10. |
Wilkes MS, Doblin BH, Shapiro MF. Pharmaceutical advertisements in leading medical journals: Experts' assessments. Ann Intern Med 1992;116:912-9.
[Google Scholar]
|
| 11. |
Drug promotion: Stealth, wealth and safety. Lancet 1993;341:1507-8.
[Google Scholar]
|
| 12. |
US General Accounting Office. Prescription drugs: FDA oversight of direct-to-consumer advertising has limitations. Washington, DC. 2002 Dec 04. Report No.:GAO-03-177. Available from: http://www.gao.gov/assets/240/236205.html. [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 13. |
Othman N, Vitry A, Roughead EE. Quality of Pharmaceutical advertisements in medical journals: A Systematic Review. PLoS One 2009;4:e6350.
[Google Scholar]
|
| 14. |
World Health Organization. Ethical criteria for medical drug promotion. Available from: http://apps.who.int/medicinedocs/documents/whozip08e/whozip08e.pdf [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 15. |
International Federation of Pharmaceutical Manufacturers Associations (IFPMA) [Internet]. IFPMA Code of Practice 2012. Available from: http://www.ifpma.org/fileadmin/content/Publication/2012/IFPMA_Code_of_Practice_2012_new_logo.pdf [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 16. |
Organization of Pharmaceutical Producers of India [Internet]. OPPI code of pharmaceutical practices 2012. Available from: http://www.indiaoppi.com/OPPI%20Code%20of%20Pharmaceutical%20Practices%20%20-%202012.pdf [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 17. |
The Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954 (Act No 21 of 1954) [Internet]. 1954. Available from: http://www.accmumbai.gov.in/aircargo/pdf/drug_objectional_advertisement_act.pdf [Last accessed on 2013 Sep 5].
[Google Scholar]
|
| 18. |
Lal A. Information contents of drug advertisements: An Indian experience. Ann Pharmacother 1998;32:1234-8.
[Google Scholar]
|
| 19. |
Lal A, Moharana AK, Srivastava S. Comparative evaluation of drug advertisements in Indian, British and American medical journals. J Indian Med Assoc 1997;95:19-20.
[Google Scholar]
|
| 20. |
Lal A, Sharma ML. Drug advertisements in Indian medical journals. Indian J Physiol Pharmacol 1992;36:139-40.
[Google Scholar]
|
| 21. |
Tandon V, Gupta BM, Khajuria V. Pharmaceutical drug advertisements in national and international journals. Indian J Pharmacol 2004;36:313-5.
[Google Scholar]
|
| 22. |
Charan J, Yadav P, Saxena D, Kantharia ND. Drug advertisements published in Indian Medical Journals: Are they ethical? J Pharm Bioallied Sci 2011;3:403-6.
[Google Scholar]
|
| 23. |
Bartus CL, Katz KA. Advertising in dermatology journals: Journals' and journal editors' policies, practices and attitudes. J Am Acad Dermatol 2006;55:116-22.
[Google Scholar]
|
| 24. |
Breckenridge AM. The pharmaceutical industry and developing countries. Br J Clin Pharmacol 1986;22 Suppl 1:27-9S.
[Google Scholar]
|
| 25. |
Oshikoya KA, Senbanjo IO, Soipe A. Adequacy of pharmacological information provided in pharmaceutical drug advertisements in African medical journals. Pharmacy Practice [Internet]. 2009;7:100-7. Available from http://pharmacypractice.org/vol07/pdf/100-107.pdf [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 26. |
Dhanaraj E, Nigam A, Bagani S, Singh H, Tiwari P. Supported and unsupported claims in medicinal drug advertisements in Indian medical journals. Indian J Med Ethics 2011;8:170-4.
[Google Scholar]
|
| 27. |
Vlassov V, Mansfield P, Lexchin J, Vlassova A. Do drug advertisements in Russian medical journals provide essential information for safe prescribing? West J Med 2001;174:391-4.
[Google Scholar]
|
| 28. |
Othman N, Vitry A, Roughead EE. Medicines information in medical journal advertising in Australia, Malaysia and the United States: A comparative cross sectional study. South Med Rev 2010;3:11-8.
[Google Scholar]
|
| 29. |
International Committee of Medical Journal Editors [Internet]. Publishing and editorial issues related to publication in medical journals. Available from www.icmje.org/publishing_h.html [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 30. |
Bhatt AD. Drug promotion and doctor: A relationship under change? J Postgrad Med 1993;39:120-3.
[Google Scholar]
|
| 31. |
Cooper RJ, Schriger DL, Wallace RC, Mikulich VJ, Wilkes MS. The quantity and quality of scientific graphs in pharmaceutical advertisements. J Gen Intern Med 2003;18:294-7.
[Google Scholar]
|
| 32. |
Hogan DJ, Sarel D, Canas A, Bellman B, Eaglstein W, Hogan LA, et al. An analysis of advertisements in the Journal of the American Academy of Dermatology, 1980 and 1990. J Am Acad Dermatol 1993;28:993-7.
[Google Scholar]
|
| 33. |
Mali SN, Dudhgaonkar S, Bachewar NP. Evaluation of rationality of promotional drug literature using World Health Organization guidelines. Indian J Pharmacol 2010;5:267-72.
[Google Scholar]
|
| 34. |
Rahman MS, Begum M, Haque Z, Akhter N. Drug advertisements in Medical Journals: A Commentary. Bangladesh J Physiol Pharmacol [Internet]. 1999;15:31-6. Available from: http://www.researchgate.net/publication/207422895_Drug_Advertisements_in_Medical_Journals_A_Commentary [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 35. |
Perman E. Voluntary control of drug promotion in Sweden. N Engl J Med 1990;323:616-7.
[Google Scholar]
|
| 36. |
Lexchin J. Code of marketing practices. Voluntary self-regulation for the Pharmaceutical Manufacturers Association of Canada. Can Fam Physician 1997;43:1997.
[Google Scholar]
|
| 37. |
Mansfield PR. MaLAM, a medical lobby for appropriate marketing of pharmaceuticals. Med J Aust 1997;167:590-2.
[Google Scholar]
|
| 38. |
Villanueva P, Peiró S, Librero J, Pereiró I. Accuracy of pharmaceutical advertisements in medical journals. Lancet 2003;361:27-32.
[Google Scholar]
|
| 39. |
Rennie D. Editors and advertisements. What responsibility do editors have for the advertisements in their journals? JAMA 1991;265:2394-6.
[Google Scholar]
|
| 40. |
Smith R. Medical journals and pharmaceutical companies: Uneasy bedfellows. BMJ 2003;326:1202-5.
[Google Scholar]
|
| 41. |
Herxheimer A, Collier J. Promotion by the British pharmaceutical industry, 1983-8: A critical analysis of self regulation. BMJ 1990;300:307-11.
[Google Scholar]
|
| 42. |
Lexchin J. Canadian marketing codes: how well are they controlling pharmaceutical promotion. Int J Health Serv 1994;24:91-104.
[Google Scholar]
|
| 43. |
Roughead EE, Gilbert AL, Harvey KJ. Self-regulatory codes of conduct: Are they effective in controlling pharmaceutical representatives' presentations to general medical practitioners? Int J Health Serv 1998;28:269-79.
[Google Scholar]
|
| 44. |
Gopalkrishnan S, Murali R. India: Campaign to tackle unethical promotion. World Health Orgnization. Essential Drugs Monitor [Internet]. 2002;31:22. Available from http://www.healthyskepticism.org/files/WHOEDM/31/Page22.pdf [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 45. |
Lexchin J. Models for financing the regulation of pharmaceutical promotion. Globalization and Health [Internet]. 2012 [cited 2013 Sep 10];8:24. doi: 10.1186/1744-8603-8-24. Available from: http://www.globalizationandhealth.com/content/8/1/24 [Last accessed on 2013 Sep 10].
[Google Scholar]
|
| 46. |
Lexchin J. Enforcement of codes governing pharmaceutical promotion: What happens when companies breach advertising guidelines? CMAJ 1997;156:351-6.
[Google Scholar]
|
| 47. |
Morgan PP. Pharmaceutical advertising in medical journals. Can Med Assoc J 1984;130:1412.
[Google Scholar]
|
| 48. |
Moser RH. Advertising and our journal. Ann Intern Med 1977;87:114-5.
[Google Scholar]
|
| 49. |
Doctors, drug companies and gifts. JAMA 1990;263:2177-9.
[Google Scholar]
|
| 50. |
Narendran R, Narendranathan M. Influence of pharmaceutical marketing on prescription practices of physicians. J Indian Med Assoc 2013;111:47-50.
[Google Scholar]
|
Fulltext Views
3,971
PDF downloads
2,953





