Translate this page into:
Evaluation of key histologic variables in skin biopsies of patients of borderline leprosy with type 1 lepra reaction
2 Department of Dermatology and Sexually Transmitted Diseases, University College of Medical Sciences and Guru Teg Bahadur Hospital, University of Delhi, Delhi, India
Correspondence Address:
Nivedita Patnaik
Department of Pathology, University College of Medical Sciences and Guru Teg Bahadur Hospital, University of Delhi, Shahdara, Delhi - 110 095
India
| How to cite this article: Patnaik N, Agarwal S, Sharma S, Sharma S, Pandhi D. Evaluation of key histologic variables in skin biopsies of patients of borderline leprosy with type 1 lepra reaction . Indian J Dermatol Venereol Leprol 2014;80:402-408 |
Abstract
Background: Leprosy remains an important health problem mainly in the African and South-East Asia regions. Type 1 reaction is an immune-mediated phenomenon known to complicate at least 30% of patients of leprosy. Diagnosing type 1 reaction correctly is important for timely institution of therapy to prevent and treat neuropathy-associated disability and morbidity. There is paucity of literature on definitive criteria for histologic diagnosis of type 1 reaction. This study was conducted to determine the key histologic variables for diagnosing type 1 reaction. Methods: This was a prospective study recruiting 104 patients with borderline leprosy. Three pathologists blinded to the clinical diagnosis independently assessed the cases. The agreement between each histological variable and clinical diagnosis was then calculated by using Cohen's kappa (Κ) coefficient. Results: Histological diagnosis of type 1 reaction was given to 27 (67.5%) of 40 clinically diagnosed cases of type 1reaction cases. Histological variables chosen as key variables for histological diagnosis of type 1 reaction were presence of giant cells, dermal edema, intragranuloma edema, granuloma fraction 31-50%, and presence of medium to large giant cells. Conclusion: This study has shown that T1R are still underdiagnosed histologically in comparison with clinical assessments. The key variables for diagnosing type 1 reaction were proposed.INTRODUCTION
Leprosy is a disease caused by Mycobacterium leprae (M. leprae) that mainly affects skin and peripheral nerves resulting in disabling deformities. It is a significant problem mainly in the African and South-East Asian regions with a global total of 232,857 new cases reported in 2012. [1] Although India achieved the target of leprosy elimination (less than 1 case per 10,000 population) in 2005 [2] the country still continues to record the highest number of new leprosy cases in the world followed by Brazil and Indonesia. [1]
Type 1 reaction, a major complication seen in patients of borderline leprosy, is an immunologically mediated reaction which assumes great clinical importance because of the acute peripheral nerve damage that occurs during these episodes. The prevalence of type 1 reaction has been reported to vary from 8.9-35.7% in various prospective and retrospective studies. [3] It is believed to be caused by an increase in cell mediated immunity to mycobacterial antigen [4] and is associated with infiltration of CD4+ve lymphocytes in skin lesions and nerves which release interferon- γ (IFN-γ), interleukin-2 (IL-2), interleukin-12 (IL-12) and tumor necrosis factor- α (TNF-α) resulting in edema and painful inflammation. [5]
Type 1 reaction tends to occur in patients in the borderline spectrum of leprosy including borderline lepromatous (BL) leprosy, mid-borderline (BB) leprosy, and borderline tuberculoid (BT) leprosy. These reactions present with increased induration and erythema in existing lesions, along with new inflammatory lesions in the skin and nerves, prominent acral edema and often progressive neuritis, causing sensory and motor neuropathy. The clinical features of reactions in leprosy have been described in detail by Ridley, [6] Jopling, [7] and Sehgal. [8]
The histologic features of type 1 reaction have also been described by Ridley. [9],[10] However, using those histopathological criteria, a number of patients clinically diagnosed as type 1 reaction cannot be labeled as having a type 1 reaction on biopsy. For example, in the study done by Lockwood et al.,[11] only 32-62% with clinically diagnosed reactions received a histological diagnosis of lepra reaction.
There is a need for more inclusive histologic criteria for diagnosing type 1 reaction which would be beneficial to many pathologists who are still unfamiliar with the subtleties of leprosy reactions. This is important since appropriate diagnosis of type 1 reaction will result in timely treatment to patients and disabilities and morbidities can be prevented.
METHODS
This was a prospective study conducted on 104 patients with borderline leprosy. Patients were recruited from the Leprosy Clinic at the Department of Dermatology of Guru Tegh Bahadur Hospital, Delhi after obtaining clearance from the institutional ethics committee. These patients were divided in two groups, Group I (type I reaction) and Group II (non-reactional leprosy) as controls.
- Group I (cases) were defined as those with sudden appearance (within the previous 2 weeks) of new erythematous skin lesions and/or new development of erythema in existing skin lesions after a period of quiescence.
- Group II (controls) were defined as patients of borderline leprosy without any clinical evidence of lepra reactions as described above.
Patients with polar forms of leprosy (tuberculoid leprosy, lepromatous leprosy) and type 2 reaction were excluded from the study.
A detailed clinical history and examination was conducted for all patients and after informed consent, a biopsy was taken from the edge of a clinically active representative skin lesion.
The biopsies were fixed in 10% buffered formalin and routinely processed and embedded in paraffin. Three sections each of 4 μm thick were cut. Out of those, one was stained with hematoxylin and eosin (H and E) using Harris Hematoxylin, one with acid-fast bacteria (AFB) stain using modified Fite Faraco method and one lysinated slide was stained with monoclonal antibody to S-100 to highlight nerves. Two-step indirect technique of immunohistochemistry was used. [12]
Histological examination
A set of three slides for each case, stained by each of the three techniques was circulated among three pathologists. The slides were arbitrarily numbered by a technician who was not involved in biopsy readings. Each histopathologist, who was blinded to the clinical diagnosis, independently assessed the slides and recorded the findings on a separate sheet.
The basic characteristics noted by pathologists to diagnose type 1 reaction were those described by Ridley. [10] This study used the pre-agreed criteria used by Lockwood et al.[11] as follows: (1) Edema: dermal edema was defined as separation of collagen with pallor and dilated vasculature. Granuloma edema was said to be present when the granuloma was not compact and the inflammatory cells were separated by inter-cellular spaces. (2) Epidermal erosion: defined as presence of granulomatous inflammatory destruction of basal epidermis and (3) Spongiosis: defined as separation of keratinocytes by intercellular edema.
Each variable when present was given a score of 1 and each histopathologist scored the skin biopsies on the following features: (i) Epidermal variables: atrophy, spongiosis, exocytosis, and parakeratosis; (ii) Subepidermal zone: erosion of epidermis by granuloma; (iii) Dermis: edema [Figure - 1] and fibroplasias; (iv) Granuloma morphology: epithelioid cell (EC) granuloma compactness and edema [Figure - 2], epithelioid cell maturity, perigranuloma lymphocytes, intra-granuloma lymphocytes, and plasma cells; (v) Giant cells: presence and size [medium to large (taken as 1) or small (taken as 0)] [Figure - 3]; (vi) Macrophage apoptosis [Figure - 4], necrosis; (vii) Granuloma fraction (<10%, 11-30%, 31-50%, >50%); (viii) Bacterial index (0, 1-3, and 4-6); and (ix) Ridley-Jopling leprosy type. Each pathologist was finally asked to label whether the biopsy showed type 1 reaction.
 |
| Figure 1: Dermal edema with dilated dermal vessels. (H and E, ×100) |
 |
| Figure 2: Intragranuloma edema. (H and E, ×400) |
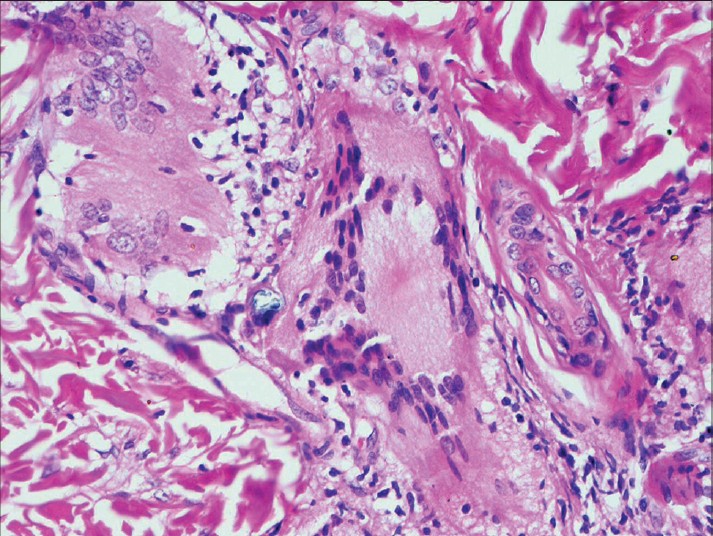 |
| Figure 3: Presence of medium to large giant cells in granuloma. (H and E, ×400) |
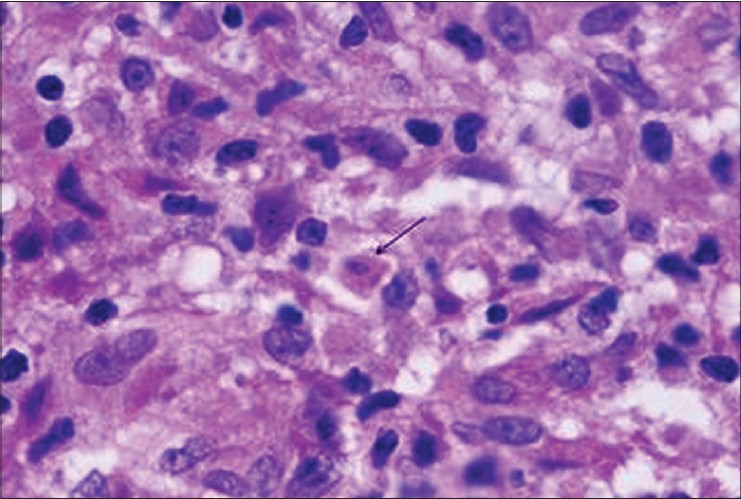 |
| Figure 4: Apoptotic body (arrow) inside granuloma. (H and E, ×1000) |
Besides this, the number of apoptotic bodies per 10 high power fields per sample was also recorded by each pathologist and an average of three values was taken for each patient. Apoptotic bodies in H and E sections were identified by the following features: nuclear condensation, round to ovoid bodies, eosinophilia of the cytoplasm, and karyorrhexis/karyolysis.
Histological observations of all the three pathologists were entered in a combined table. A histological feature was recorded to be present (or absent) if at least 2 histopathologists agreed on the finding.
Statistics
Statistical analyses was done by using SPSS version 20. Absence of a variable was taken as "0" and its presence as "1". Cohen′s kappa (ҡ) coefficient was calculated to evaluate the degree of agreement of each histological feature with a clinical diagnosis of type 1 reaction. The interpretation of the ҡ value was as follows: no agreement- less than 0; slight agreement- less than 0.20; fair agreement- 0.20-0.40; moderate agreement-0.40-0.60; substantial agreement- 0.60-0.80; perfect agreement- 0.80-1.00. Independent samples-t-test was used to calculate the statistical difference of the mean age of the two groups.
RESULTS
Group I
Fifty patients of borderline leprosy clinically in reaction were enrolled in this group. Ten cases were excluded from evaluation for various reasons: one showed type 2 reaction, two had lepromatous leprosy, two had indeterminate leprosy histologically and five cases were unsatisfactory for assessment. Thus, a total of 40 cases were assessed.
Group II
Fifty-four patients of borderline leprosy without clinical evidence of reaction were enrolled in this group. Four cases were excluded from evaluation for various reasons: in one, tissue was unsatisfactory for assessment, two had indeterminate leprosy and one had lepromatous leprosy. Thus, a total of 50 cases were assessed.
Demography
The mean ages of the patients in Group I and II was 33.03 ± 16.16 and 33.20 ± 17.13 respectively. There was no statistically significant difference between groups [P = 0.9].
Group I consisted of 26 (65%) males and 14 (35%) females while in group II there were 29 (58%) males and 21 (42%) females.
Clinical spectrum of leprosy
In group I, 23 (57.5%) cases had borderline tuberculoid (BT) leprosy, 3 (7.5%) cases had mid-borderline (BB) leprosy and 14 (35%) cases had borderline lepromatous (BL) leprosy. In group II, the number of BT, BB and BL cases were 28 (56%), 5 (10%), and 17 (34%) respectively.
Histological examination
Of 40 cases which were clinically in reaction, a histological diagnosis of type 1 reaction was made in 27 (67.5%) cases. There was moderate agreement (ҡ = 0.479) between histological and clinical diagnosis. In the group of patients who were clinically not in reaction, 10 patients received a histological diagnosis of type 1 reaction.
Each histological variable was compared between the group showing clinical type 1 reaction (group I) and the group that did not show clinical features of reaction (group II). The agreement between each histological variable and clinical diagnosis was then calculated by using Cohen′s ҡ coefficient.[Table - 1].

There was fair agreement with the following histological findings and the clinical diagnosis of type 1 reaction: presence of giant cells (ҡ = 0.391), dermal edema (ҡ = 0.334), presence of medium to large giant cells (ҡ = 0.278), granuloma fraction 31-50% (ҡ = 0.275), presence of intra-granuloma edema (ҡ = 0.213), and macrophage apoptosis (ҡ = 0.205). Slight agreement was observed for the following findings: epithelioid cell granuloma (ҡ = 0.171), macrophage necrosis (ҡ = 0.164), epithelioid cell maturity (ҡ = 0.163), intragranuloma lymphocytes (ҡ= 0.163), exocytosis (ҡ = 0.153), granuloma fraction 11-30% (ҡ = 0.143), perigranuloma lymphocytes (ҡ = 0.096), erosion by granuloma (ҡ = 0.093), granuloma fraction >50% (ҡ = 0.093), spongiosis (ҡ = 0.049), parakeratosis (ҡ = 0.038), atrophy (ҡ = 0.035), bacterial index 1-3 (k = 0.035), bacterial index 0 (ҡ = 0.015), and intragranuloma plasma cells (ҡ = 0.005). No agreement was observed for the remaining variables: bacterial index 4-6 (ҡ = −0.038), fibroplasias (ҡ = −0.038), and granuloma fraction <10% (ҡ = −0.326).
Since greater the ҡ value, more is the agreement between clinical and histological diagnosis, so the five variables with highest ҡ values were chosen as key variables for diagnosing type 1 reaction. They were presence of giant cells, dermal edema, medium to large giant cells, granuloma fraction 31-50%, and presence of intragranuloma edema.
The mean of the number of apoptotic bodies seen in group I was 2.77 ± 1.7 and in group II was 1.19 ± 0.93 (P < 0.001, t-test).
DISCUSSION
Based on a study of 12 patients with type 1 lepra reaction in borderline leprosy conducted in 1981, [9] Ridley described the histological features of type 1 lepra reaction in a subsequent publication. [10] A study undertaken by Lockwood et al. three decades later showed that clinically apparent leprosy reactions are under diagnosed histologically.[11] This study showed that only 32-62% of clinically diagnosed lepra reactions receive a histological diagnosis of lepra reaction. There are no published agreed diagnostic histological criteria of type 1 reaction. [11] This results in significant inter-observer variability and consequent under-diagnosis.
Our results showed that out of 40 cases of clinically diagnosed type 1 lepra reaction, the diagnosis could be histopathologically confirmed in only 27 (67.5%) cases. Similar results were reported by Lockwood et al. [11]
Another interesting finding of our study was that ten patients clinically not in reaction showed histological features of type 1 reaction. Ideally, this subgroup of patients should have been closely followed up for the subsequent development of clinical signs of type 1 reaction. However, we did not look into this aspect as this was a cross sectional study. Longitudinal studies may be planned to clarify whether or not such patients eventually develop clinical type 1 reaction.
The key histological variables which had a greater degree of agreement with clinical diagnosis were presence of giant cells, dermal edema, presence of medium to large giant cells, granuloma fraction 31-50% and presence of intragranuloma edema. Out of all these variables, presence of only one is not sufficient to make a diagnosis of type 1 reaction, for that presence of the all or most of the suggested variables should be present.
Lockwood et al.,[11] found that five histological findings, intra-granuloma edema, giant cell size, giant cell numbers, dermal oedema, and HLA-DR expression correlated with clinical type 1 reactions. Of these, we did not study HLA-DR expression analysis in our cases but the other four variables could be corroborated in our study. Our finding of a positive correlation with granuloma fraction of 31-50% was not mentioned in the Lockwood study. In contrast to the study by Lockwood et al.,[11] we found that the presence of intragranuloma plasma cells correlated poorly with the clinical diagnosis.
According to Ridley [10] early reactions were characterized by mild edema and proliferation of fibrocytes in interfascicular spaces of the dermis. He observed that an increase in the number of lymphocytes was more marked in upgrading than downgrading reactions. In the acute stage, necrosis was apparent in severe cases, giant cells of various types were frequently present and evolution of the granuloma cells depended on the type of reaction with clusters of mature epithelioid cells in upgrading reactions and macrophages in downgrading reactions. Of these features, fibroplasia, macrophage necrosis, and intragranuloma and perigranuloma lymphocytes were not found to be of objective value for diagnosis of type 1 lepra reaction in our study.
Apoptosis was quantitatively more seen in reactional cases compared to those who did not have reaction. But still macrophage apoptosis has not been suggested as a key variable for diagnosis of T1R, since its kappa value is less than that of the other 5 variables taken as key variables.
A limitation of the present study was the relatively small sample size and its cross sectional design. Larger studies with clinical and histopathological follow up may provide more definitive data on histopathological findings that help identify type 1 lepra reactions.
Based on the data in our study, we suggest that the diagnosis of type 1 lepra reaction should not be based on histopathology alone and clinically diagnosed cases, especially those with neural involvement, should be administered treatment for reaction irrespective of the histological findings.
ACKNOWLEDGEMENTS
We thank the Guru Teg Bahadur Hospital Staff, especially people working in the Department of Histopathology and Department of Dermatology.
| 1. |
Global leprosy: Update on the 2012 situation. Wkly Epidemiol Rec 2013;88:365-79.
[Google Scholar]
|
| 2. |
Announcement: India achieves National elimination of leprosy. Indian J Lepr 2006;78:101.
[Google Scholar]
|
| 3. |
Pandhi D, Chhabra N. New insights in the pathogenesis of type 1 and type 2 lepra reaction. Indian J Dermatol Venereol Leprol 2013;79:739-49.
[Google Scholar]
|
| 4. |
Britton WJ. The management of leprosy reversal reactions. Lepr Rev 1998;69:225-34.
[Google Scholar]
|
| 5. |
Little D, Khanolkar-Young S, Coulthart A, Suneetha S, Lockwood DN. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1(reversal) reactions before and during prednisolone treatment. Infect Immun 2001;69:3413-7.
[Google Scholar]
|
| 6. |
Ridley DS. Reactions in leprosy. Lepr Rev 1969;40:77-81.
[Google Scholar]
|
| 7. |
Jopling WH. Leprosy reactions (Reactional states). In: Jopling WH, editor. Handbook of leprosy. 2 nd ed. London: William Heinemann Medical books; 1978. p. 66-74.
[Google Scholar]
|
| 8. |
Sehgal VN. Reactions in leprosy; clinical aspects. Int. J. Dermatol. 26 (1987) 278-85.
[Google Scholar]
|
| 9. |
Ridley DS, Radia KB. The histological course of reactions in borderline leprosy and their outcome. Int J Lepr Other Mycobact Dis 1981;49:383-92.
[Google Scholar]
|
| 10. |
Ridley D. The pathogenesis of leprosy and related diseases. 1 st ed. London: Wright; 1988.
[Google Scholar]
|
| 11. |
Lockwood DN, Lucas SB, Desikan KV, Ebenezar G, Suneetha S, Nicholls P. The histological diagnosis of leprosy type 1 reactions: Identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol 2008;61:595-600.
[Google Scholar]
|
| 12. |
Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577-80.
[Google Scholar]
|
Fulltext Views
3,337
PDF downloads
1,788





