Translate this page into:
Hormone therapy in acne
Correspondence Address:
Chembolli Lakshmi
Professor, Department of Dermatology, PSG Hospitals and PSGIMSR, Coimbatore 641 004, Tamil Nadu
India
| How to cite this article: Lakshmi C. Hormone therapy in acne. Indian J Dermatol Venereol Leprol 2013;79:322-337 |
Abstract
Underlying hormone imbalances may render acne unresponsive to conventional therapy. Relevant investigations followed by initiation of hormonal therapy in combination with regular anti-acne therapy may be necessary if signs of hyperandrogenism are present. In addition to other factors, androgen-stimulated sebum production plays an important role in the pathophysiology of acne in women. Sebum production is also regulated by other hormones, including estrogens, growth hormone, insulin, insulin-like growth factor-1, glucocorticoids, adrenocorticotropic hormone, and melanocortins. Hormonal therapy may also be beneficial in female acne patients with normal serum androgen levels. An understanding of the sebaceous gland and the hormonal influences in the pathogenesis of acne would be essential for optimizing hormonal therapy. Sebocytes form the sebaceous gland. Human sebocytes express a multitude of receptors, including receptors for peptide hormones, neurotransmitters and the receptors for steroid and thyroid hormones. Various hormones and mediators acting through the sebocyte receptors play a role in the orchestration of pathogenetic lesions of acne. Thus, the goal of hormonal treatment is a reduction in sebum production. This review shall focus on hormonal influences in the elicitation of acne via the sebocyte receptors, pathways of cutaneous androgen metabolism, various clinical scenarios and syndromes associated with acne, and the available therapeutic armamentarium of hormones and drugs having hormone-like actions in the treatment of acne.Introduction
Acne, a disease of the pilosebaceous unit is common in adolescence and may persist into adulthood, and affecting about with 33% of individuals between the ages 15 and 44 years. [1]
Clinically, hormone-related acne is characterized by lesions along the jawline and chin, new-onset adult acne, pre-menstrual acne, cystic acne with/without menstrual irregularities and hirsutism. Fluctuations in the levels of androgens could result in cyclical flares of acne, including pre-menstrual aggravation.
Involvement of androgens and other hormones in acne pathogenesis is inferred from clinical evidence [Table - 1]. [2] Androgen-insensitive persons do not develop acne as they don′t produce sebum; and high androgen states are associated with acne. [3] Sebaceous gland hypersensitivity to circulating androgens is believed to be the main factor in the development of acne. Testosterone and dihydrotestosterone (DHT) bind nuclear androgen receptors (AR), which then interact with deoxyribonucleic acid in the nucleus of sebaceous cells and ultimately regulate genes involved in cell proliferation and lipogenesis. [4] Peroxisome proliferator-activated receptor (PPAR) ligands are thought to play a role in the regulation of lipid metabolic genes. [5] There are several syndromes associated with hormonal imbalances and presenting with acne. [6],[7]
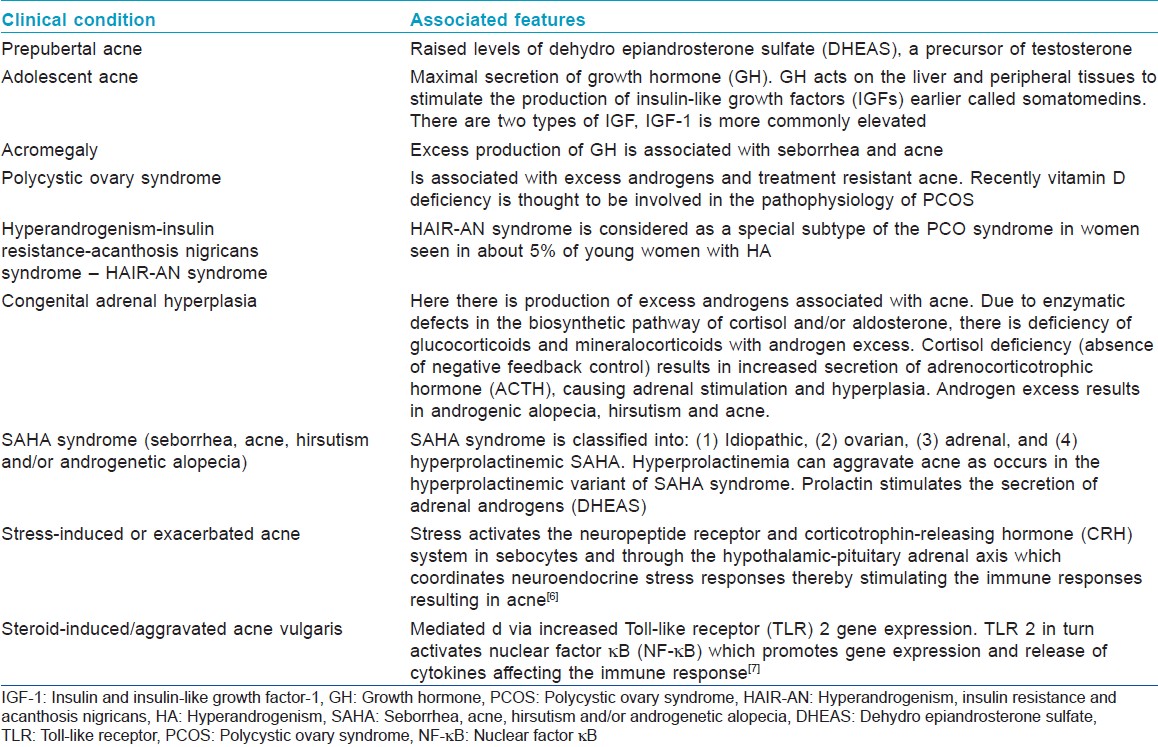
Various syndromes associated with hormonal imbalances and acne are listed below:
- Polycystic ovary syndrome (PCOS)
- Cushing′s syndrome
- Congenital adrenal hyperplasia (CAH)
- Hyperandrogenism, insulin resistance and acanthosis nigricans (HAIR-AN) syndrome
- Seborrhea, acne, hirsutism and/or androgenetic alopecia (SAHA) syndrome
- Acromegaly
- Synovitis-acne-pustulosis-hyperostosis-osteitis syndrome (SAPHO)
- Pyogenic arthritis, pyoderma gangrenosum, acne conglobata syndrome (PAPA)
- Apert syndrome-acrocephalosyndactyly type I
Polycystic Ovary Syndrome
PCOS is the commonest hormonal disorder in young women. The Rotterdam Consensus Group Criteria requires the presence of two out of the three following criteria: [8]
- Oligomenorrhoea or amenorrhea;
- Hyperandrogenism (HA) (clinical evidence of androgen excess) or hyperandrogenemia (biochemical evidence of androgen excess);or
- Polycystic ovaries as demonstrated by ultrasound. (Defined as the presence of 12 or more follicles in either ovary measuring 2-9 mm in diameter, and/or increased ovarian volume greater than 10 mL.) If a follicle >10 mm in diameter is present, the scan should be repeated at a time of ovarian quiescence in order to calculate the ovarian volume.
Other conditions with HA that mimics PCOS (e.g., CAH (late-onset), Cushing syndrome, syndromes of severe insulin resistance like HAIR-AN syndrome, androgen or ovarian androgen-secreting tumors, drugs) should be excluded. [9],[10] Other conditions mimicking PCOS include thyroid dysfunction and hyperprolactinemia.
In 2006, the Androgen Excess Society recommended an evidence-based definition for PCOS. [11] This set of criteria highlighted PCOS as primarily an androgen-excess disorder and proposed that PCOS be diagnosed by all the following:
- Androgen excess (clinical or biochemical)
- Ovarian dysfunction (anovulation/oligo-ovulation and/or polycystic ovaries)
- Exclusion of other androgen-excess and ovulatory disorders.
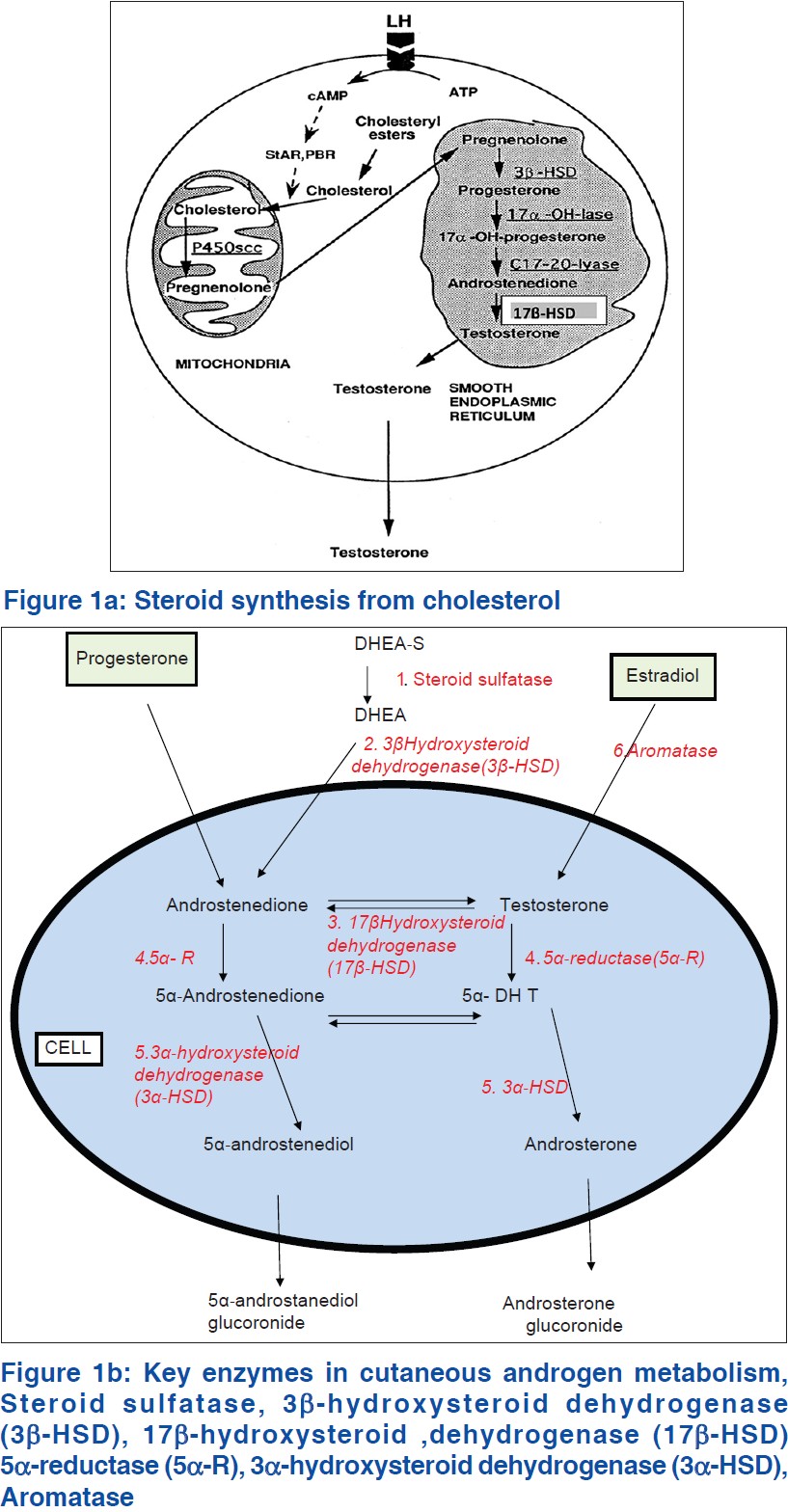
In PCOS, there is increased ovarian production of androgen, due to dysregulation of the rate limiting enzyme for the androgen biosynthesis, 17 α-hydroxylase [Figure - 1]a. Locally, these androgens cause ovarian dysfunction and peripherally acne, hirsutism and alopecia. In peripheral adipose tissue which possesses aromatase, excess androgens are converted to estrogens (androstenedione to estrone and testosterone to estradiol), stimulating release of leutenizing hormone (LH) from the pituitary gland. Peripheral insulin resistance seen with PCOS causes hyperinsulinemia, which also causes ovarian hypersecretion of androgens and release of LH from the pituitary. [11] The excess of adipose tissue in obese patients is associated with the combination of excess androgens (causing hirsutism and virilization) and excess estrogens (inhibits follicle stimulating hormone [FSH] by feedback inhibition).
 |
| Figure 1 |
There is abnormal secretion of gonadotrophins LH and FSH with an elevated LH: FSH ratio of ≥2 . Elevation of LH is pathognomonic of but not diagnostic of PCOS. [9]
Cushing syndrome
Cushing syndrome arises due to excess glucocorticoid secretion, and may be due to pituitary hypersecretion of adrenocorticotrophic hormone (ACTH), ectopic secretion of ACTH by non-pituitary tumors, corticotrophin-releasing hormone (CRH) secreting tumors, bilateral adrenal hyperplasia, and cortisol secretion by adrenocortical adenoma or carcinomas. [12] Acne lesions are mainly papules and pustules. Comedones
and deep cysts as in adolescent acne is uncommon. Abnormalities in CRH, ACTH and cortisol levels may be contributory as they increase testosterone levels, lipogenesis, and sebum production. [11]
Genetic deficiency of the enzymes (21-hydroxylase, 11-hydroxylase) in steroid synthesis results in CAH.
Congenital adrenal hyperplasia (CAH)
As a result, steroids are shunted from the cortisol biosynthetic pathway to the androgen biosynthetic pathway. Cortisol levels are normal, but adrenal androgens are increased. [13]
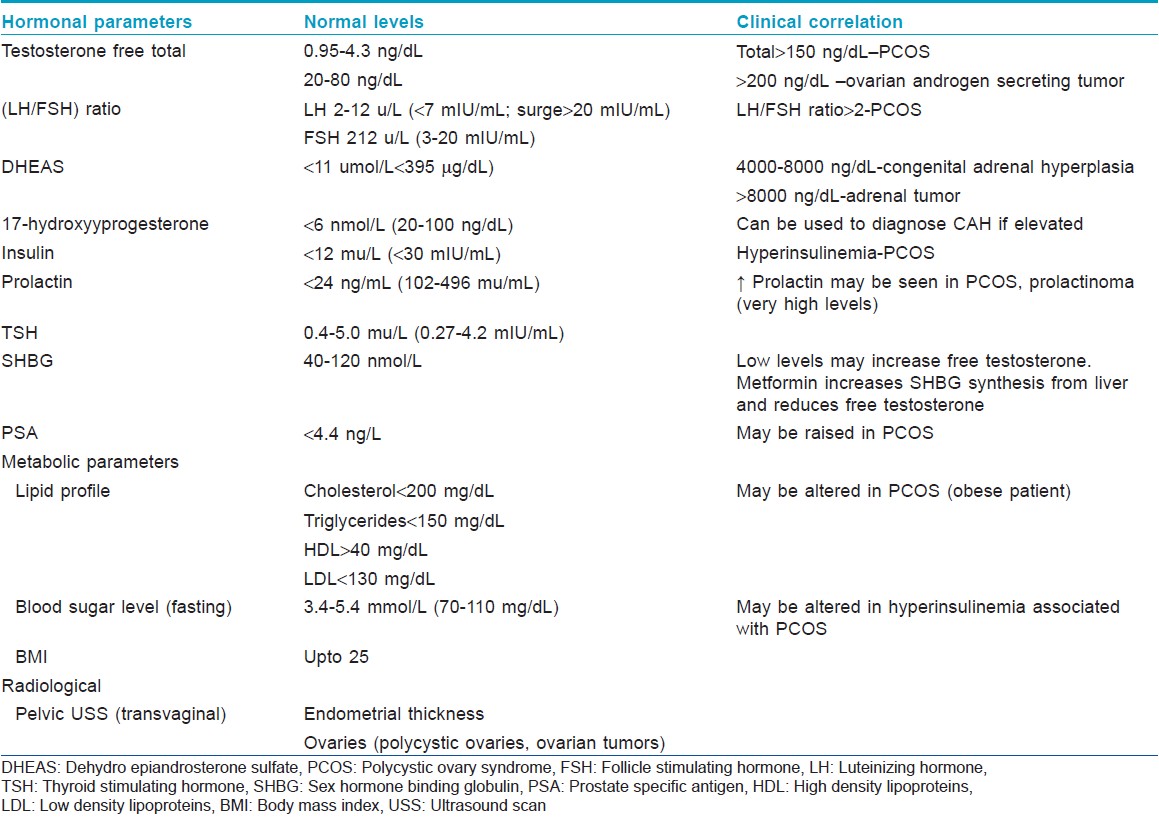
Early and a severe onset of acne, pubic hair, rapid growth, amenorrhea, or any signs of androgen excess, in children and adolescents should raise the suspicion of CAH and be screened. Serum dehydroepiandrosterone sulfate (DHEAS), a marker of adrenal androgens is raised (4000-8000 ng/mL). A high concentration of 17-hydroxyprogesterone (>242 nmol/L, >3 ng/mL) is diagnostic for complete deficiency of 21-hydroxylase (classic CAH). In partial deficiency of 21-hydroxylase (non-classic CAH), a corticotropin stimulation test with measurement of 17-hydroxyprogesterone at 60 min is the gold standard. [14] A stimulated 17-hydroxyprogesterone concentration higher than 45 nmol/L is diagnostic. [14]
Glucocorticoid replacement corrects the deficiency and HA, by suppressing the inappropriately activated hypothalamic-pituitary-adrenal axis. Low-dose prednisone 2.5-5 mg or dexamethasone 0.25-0.5 mg daily at bedtime, is used.
Adrenocortical carcinomas present as Cushing′s syndrome with virilization. Pure androgen producing (without cortisol) tumors are very rare and present only with virilization. Ovarian androgen secreting tumors present with features of HA. Laboratory investigations and radiology helps differentiating them. [15]
Androgen secreting tumors (adrenal, ovarian, or testicular origin) are very rare and only account for 0.2% of the causes for increased androgens. The diagnostic work-up is listed in [Table - 2]. A serum level of DHEAS greater than 8000 ng/dL is suggestive of an adrenal tumor. Ovarian or testicular tumors show marked elevation of total testosterone (greater than 200 ng/dL). [6]
Hyperandrogenism, Insulin Resistance and Acanthosis Nigricans Syndrome
The HA, insulin resistance and AN syndrome is unique subtype of PCOS, which presents more severely and runs in families. [16.17] HAIR-AN occurs in about 1-3% of women with HA and in 5% of women with PCOS. HAIR-AN can be divided into type A or type B. Type A is due to insulin receptor mutations and is characterized by severe insulin resistance. Type B is an autoimmune, acquired condition resulting from antibodies to the insulin receptor and is not as severe. Other autoimmune endocrine disorders may also be present. HAIR-AN syndrome is associated with elevated levels of insulin, testosterone, and androstenedione with other parameters being within normal limits. [9]
Seborrhea, Acne, Hirsutism and/or Androgenetic Alopecia Syndrome
The SAHA may be associated with PCOS, obesity and infertility. [18] It can be classified into four types (idiopathic, ovarian, adrenal, and hyperprolactinemic SAHA. The HAIR-AN syndrome associated with endocrine abnormalities has been designated the fifth variant.
Acromegaly
In acromegaly, where there is excess secretion of growth hormone (GH) is associated with acne and sebum production has been shown to be increased in patients with acromegaly. Both GH and insulin-like growth factor-1 (IGF-1) which are increased may be contributory to sebaceous lipogenesis induced by androgens. [6]
Synovitis-Acne-Pustulosis-Hyperostosis-Osteitis Syndrome
The acronym SAPHO, first described in 1987 is a disease predominantly afflicting children and young adults. [19],[20]
Three diagnostic criteria for this syndrome have been proposed: (i) chronic recurrent multifocal osteomyelitis with or without skin manifestations; (ii) acute or chronic sterile arthritis associated with either pustular psoriasis or palmoplantar pustulosis, or severe acne; and (iii) sterile osteitis in the presence of one of the skin manifestation. Any of these three presentations is sufficient for diagnosis of this syndrome. Other inflammatory bowel disorders culcerative colitis, Crohn′s disease) have been associated. Skin lesions in SAPHO syndrome are characterized by severe acne fulminans or acne conglobata, palmoplantar pustulosis, or pustular psoriasis. [19],[20]
Pyogenic Arthritis, Pyoderma Gangrenosum, Acne Syndrome
This refers to the triad of pyogenic sterile arthritis, pyoderma gangrenosum, and acne conglobata. [6] The genetic defect has been located on of CD2-binding protein 1 (CD2BP1; also called proline/serine/threonine phosphatase-interacting protein 1, PSTPIP1) on chromosome 15q24-25.1.
Aperts Syndrome (Acrocephalosyndactyly Type I)
Aperts syndrome is a rare autosomal dominant congenital disorder caused by a mutation in the gene encoding the fibroblast growth factor receptor. [6] Clinically, these patients present with different craniofacial deformities, hypertelorism, dental abnormalities, and proptosis of the eyes. The dermatologic hallmark of Apert syndrome is severe inflammatory and comedonal acne involving the face, chest, back, and unusual sites such as the forearms, buttocks, and thighs.
Hormones which directly or indirectly contribute to the pathogenetic lesions of acne are listed below.
- Testosterone
- DHT: 10 times more potent in binding the AR than testosterone.
- DHEA-S
- Estrogens
- Insulin and IGF-1
- GH
- Glucocorticoids, ACTH,
- CRH and other neuroendocrine regulators (melanocortins, b-endorphin, vasoactive intestinal polypeptide, neuropeptide Y and calcitonin gene-related peptide).
- Prolactin
- Vitamin D
Cutaneous Androgen Metabolism
The pilosebaceous unit of the skin (sebaceous glands and hair follicles) can synthesize androgens de novo from cholesterol or by local conversion of weak androgens to potent ones. [21],[22],[23] Cholesterol synthesized by the sebaceous skin is utilized for cell membrane formation, epidermal barrier, in sebum and also is a substrate for steroid hormone synthesis. [22]
All steroid hormones, including androgens share the preliminary step in the biosynthesis, which is the conversion of cholesterol to pregnenolone. [23] Cholesterol is released from cholesterol esters by the action of cholesterol esterase [Figure - 1]a. Free cholesterol is transferred from the outer mitochondrial membrane to the inner mitochondrial membrane by steroid acute regulatory protein. This transport is followed by the conversion of cholesterol to pregnenolone by the cytochrome P450 side-chain cleavage enzyme (P450scc), present in the inner mitochondrial membrane of all steroidogenic cells. This conversion is the first step in the synthesis of steroid hormones. Pregnenolone undergoes 17 α-hydroxylation by 17 α-hydroxylase which is the rate limiting step for androgen biosynthesis. The product is 17 α-hydroxypregnenolone, which is converted to 17 α-OH-progesterone by 3 β-hydroxysteroid dehydrogenase (3 β-HSD). Conversion of 17 α-hydroxyprogresterone to 11-deoxycortisol is effected by the enzyme 21-hydroxylase and finally, cortisol is produced from 11-deoxycortisol by the action of the enzyme 11 β-hydroxylase.
There are six major enzyme systems involved in cutaneous androgen metabolism [Figure - 1]b.
- Steroid sulfatase
- 3 β-HSD
- 17 β-HSD
- 5 α-reductase (5 α-R)
- 3 α-HSD
- Aromatase
The classic steroidogenic organs, (gonads and adrenal glands) produce testosterone. The skin and appendages (hair follicles, sebaceous glands, eccrine/apocrine glands) also express the enzymes for the synthesis and metabolism of androgens. Testosterone, a potent androgen is produced in the skin from the conversion of circulating DHEA-S, the adrenal androgen precursor which is a weak androgen, present in abundance.
In the skin of post-menopausal women, all sex steroids are made from adrenal precursors. 3 β-HSD converts it to androstenedione in the adrenal gland and sebaceous gland. Androstenedione can be reversibly converted to testosterone by 17 β-HSD, which also interconverts weak and potent estrogens (estrone and estradiol). 17 β-HSD is the regulatory enzyme in the androgen/estrogen metabolism in skin. Androstenedione appears to be the major precursor hormone for DHT formation in women. [9]
Testosterone can be activated to DHT by 5 α-R or inactivated to estradiol by aromatase. There are 2 isoenzymes for 5 α-R. Type 1 is active in the sebaceous gland and Type 2 in the prostate gland.
Estrogens
It is important to know that women cannot make any estrogen without first making testosterone. LH stimulates the theca cells of the ovary to produce androstenedione which is converted to testosterone. Some of this is released into circulation and some is converted into estrogens by aromatase elaborated by the ovarian follicles. Estradiol is the major active estrogen produced from testosterone, the reaction being mediated by aromatase (active in ovaries, adipose tissue and peripheral tissues). Estrogens in supraphysiological doses will suppress sebum production. This dose is higher than the ovulation suppression dose. [3],[24] The dose of ethinyl estradiol commonly found in current oral contraceptive pills (OCPs) is not sufficient to demonstrate a reduction in sebum secretion.
These OCPs are effective in acne and act by mechanisms other than reduction in sebum secretion. They act by [3],[24] inhibition of gonadal testosterone production through negative feedback suppression of pituitary gonadotropin release, [2] increasing sex hormone binding globulin (SHBG) production by the liver, thereby decreasing free serum testosterone, [3] direct opposition of androgen within the sebaceous gland and [4] regulate genes in sebaceous gland growth and suppressing lipid production.
Insulin-Like Growth Factor-1
Stimulates synthesis of androgens from the adrenal gland and inhibits hepatic SHBG production increasing free androgens. IGF-1 receptors are expressed hair follicles and the sebaceous gland. IGF-1 stimulates sebum production and androgen-induced sebaceous lipogenesis. [6]
Insulin
It is structurally similar to the IGF-1 receptor and binds to it. In very high doses, insulin up-regulates GH receptor expression on sebocytes. [25] In addition, insulin stimulates ovarian and adrenal androgen production, inhibits SHBG production from the liver.
Insulin may act as a key regulator of lipid biosynthetic enzymes by stimulating ovarian and adrenal androgen production and inhibiting hepatic SHBG production.
Insulin decreases IGF binding protein, which maximizes free IGF-1 concentrations to act on target tissues and increases testosterone bioavailability and DHEAS concentrations. [6],[25] The role of diet in acne is controversial, but recent studies support the association. It has been proposed that high foods with a high glycemic load elevate plasma insulin concentrations, which regulates levels of androgen, IGF-1 and IGF binding protein, promotes unregulated tissue growth, and enhances androgen synthesis. [26]
Insulin and IGF-1 stimulate sebaceous lipogenesis. [27] This has been demonstrated in SEB-1 sebocytes, and is by the induction of sterol response element-binding protein-1. [26]
Serum IGF-1 levels are increased in the PCOS and acromegaly. [27],[28] Recombinant human IGF-1, used in the treatment of the short child, results in androgenesis and acne. [29] Diets with a high glycaemic index (carbohydrate-rich diets) are associated with hyperglycemia, reactive hyperinsulinemia and increased levels of IGF-1. [30] Cow′s milk contains active IGF-1 and IGF-2, which are not destroyed by pasteurization. Due to structural similarity between bovine IGF-1 and human IGF-1, both bind to the human IGF-1R. [31] Thus milk has an insulinotropic effect which resides predominantly within the whey fraction (80%); while casein (20%) has a stronger IGF-1 stimulating effect. [32]
Growth Hormone
Acts both directly and through IGF-1 stimulation. [6] Receptors for GH are found in hair follicles and sebaceous gland acini. Acne and increased sebum production is seen in acromegaly where GH secretion is increased. Both GH and IGF-1 contribute to these effects.
Glucocorticoids, Adrenocorticotrophic Hormone, and Corticotrophin-Releasing Hormone
Cortisol, the prinicipal stress hormone is under the direct regulation of ACTH. It is well known that use of topical or systemic glucocorticoids promotes an acneiform eruption. Mediated via increased Toll-like receptor (TLR) 2 gene expression. TLR 2 in turn activates nuclear factor κB (NF-κB) which promotes gene expression and release of cytokines affecting the immune response. [33],[34],[35]
CRH secreted by the hypothalamus causes the synthesis of pro-opiomelanocortin (POMC). [35]
POMC is broken down into the melanocortins, ACTH and melanocyte stimulating hormone (α-MSH). Sebaceous gland is the main target of CRH in the skin, where it enhances sebaceous lipogenesis. In addition, it stimulates conversion of DHEA to testosterone.
Sebocytes express melanocortin receptors MC-1R and MC-5R. MSH suppresses the secretion of IL-8, a major inflammatory mediator in acne. MC-5R is involved in sebaceous differentiation and lipogenesis and is increased in lesional skin of acne patients. [36]
Prolactin
Since prolactin receptors are expressed by the adrenal gland, hyperprolactinemia may be associated with increased secretion of adrenal androgens by the zona reticularis, which is corrected with bromocriptine. [13],[12]
Vitamin D (1,25-dihydroxyvitamin D3)
Vitamin D receptor gene regulates about 3% of the human genome, including genes that are crucial for glucose and lipid metabolism and blood pressure regulation. [37] Vitamin D deficiency may be linked to the pathogenesis of insulin resistance and the metabolic syndrome in PCOS. [38]
Clinical Features of Androgen Excess
The clinical features of HA in female patients are listed below: [9],[39],[40],[41]
- Acne/seborrhea/oily skin
- Hirsutism
- Irregular menstrual periods
- AN
- Androgenetic alopecia
- Cushingoid facies as some adrenal tumors secrete large amounts of androgens and cortisol and symptoms of glucocorticoid excess may be the clinical presentation
- Clitoromegaly, which may be associated with increased libido, deepening of voice
- Associated diabetes mellitus due to the insulin resistance
- Obesity.
Androgens may be produced from three sources in women:
- In the ovary, under the influence of FSH and LH from the pituitary gland. It is important to know that women cannot make any estrogen without first making testosterone. LH stimulates the theca cells of the ovary to produce androstenedione, which is converted to testosterone. Some of this is released into circulation and some is converted into estrogens by aromatase elaborated by the ovarian follicles. Hyperinsulinemia associated with raised insulin levels result in stimulation of IGF-1 receptors resulting in androgen production in the ovarian stroma. [42]
- In the adrenal gland under the influence of ACTH secreted by the pituitary resulting in the production of DHEA, which can be converted to potent androgens like androstenedione and testosterone. [41]
- In the skin, which possesses the enzymatic machinery for the production of DHT from DHEA.
Elevated testosterone suggests an ovarian source. The LH/FSH ratio is increased (more than 2), in association with irregular menstrual periods, hirsutism and obesity in the PCO syndrome. When testosterone is markedly elevated, an ovarian tumor has to be considered. Salivary testosterone is a good indicator of circulating free testosterone and correlates well with androgen excess. [43]
An elevated level of DHEAS suggests an adrenal source.
An elevated 17-hydroxyprogesterone is seen in CAH, pointing to an adrenal source for the androgens.
If oral contraceptives are being taken any underlying HA may be masked, which is why OCP are to be discontinued 6 weeks prior to an endocrinological work-up. Most women having a pre-menstrual flare of acne with normal serum androgens also respond to hormonal treatment.
The various drugs having an anti-androgenic effect are classified into different groups based on the mechanism of action [Table - 3].

Androgen Receptor Blockers (Anti-Androgens)
They block the effect of androgens on the sebaceous gland and the infundibulum of the hair follicle.
Androgens act through the AR located in the nucleus. AR belongs to the steroid/nuclear receptor superfamily. It is present in epidermal and follicular keratinocytes, sebocytes, sweat gland cells, dermal papilla and fibroblasts, endothelial cells and genital melanocytes. [44] Dysfunction of the AR is associated with hirsutism and androgenetic alopecia. Androgens cause enlargement of the hair follicles in androgen dependent areas (beard, axillae, and pubic area in male adolescents), but paradoxically over the scalp the same androgens cause miniaturization of hair. Inhibition of wound healing in the skin is also mediated through the AR.
They include spironolactone, cyproterone acetate (CPA), drospirenone, flutamide, and anti-sense siRNA oligonucleotides. [40],[45]
Spironolactone
It is an aldosterone antagonist (potassium sparing diuretic), an anti-androgen and weak progestin. [46],[47] It may be more effective in adult women with acne as compared to OCPs. It competitively inhibits the AR at higher doses, and inhibits 5 α-R activity to a lesser extent. In a Cochrane review, (2003) spironolactone 100 mg/day was found to be superior to finasteride 5 mg/day and low dose CPA 12.5 mg/day (first 10 days of cycle). [46] Oral contraceptives and spironolactone are synergistic and response rates can increase by 75% with the use of this combination. [46] It decreases ovarian and adrenal androgen production. It competes with DHT for AR in the skin thus inhibiting the binding of testosterone and DHT. SHBG levels are increased decreasing circulating free testosterone levels, and increases clearance of testosterone by a combination of increased activity of hepatic hydroxylase and reduced 5-α-reductase activity. [47] Orally, the recommended dose is 50-100 mg after food, but many patients show the response with a lower dose of 25 mg once or twice daily. It inhibits type 2 17-β HSD and also reduces sebum secretion. Side effects include breast tenderness, irregular menstrual cycles, and electrolyte imbalance (hyperkalemia seen with high doses). Dietary withholding of excessive amounts of bananas and diet soda is advised. Periodic monitoring of serum potassium levels early in therapy is recommended. It is contraindicated in pregnancy since it has been reported to cause hypospadias in the male fetus. It can induce gynecomastia and is contraindicated in women with risk of breast cancer. Topical 5% spironolactone has been used in Europe in acne and seborrhea. [48]
Cyproterone acetate
It is both an anti-androgen and progestin. The dosage ranges from 2 to 100 mg daily. It is reported to be effective as monotherapy in more than 75% of women. It is commonly combined with estrogens in the OCP (ethinyl estradiol 50.35 μg/CPA, Diane® , Dianette® ). Its use in acne, androgenetic alopecia and hirsutism is established. [49],[50],[51] It is very effective in recalcitrant acne associated with PCOS. [49] CPA can be used either with OCPs in a combined formulation or as separate preparation, which may be used with OCP or spironolactone When used singly it is administered from day 1 to 10 of the menstrual cycle. [52] However, it is safer to administer it in combination with an OCP.
Krimson35® which also contains cyproterone acetate and ethinyl estradiol in the same concentration as in Diane® has to be administered between 3-5 days of the menstrual cycle or about 5 days after withdrawal bleed (in cases with prolonged amenorrhoea where progestins are administered for a short period - Meprate® -medroxyprogesterone acetate -10 mg once or twice daily for 5 days- and withdrawn, resulting in a withdrawal bleed).
Topical CPA in a novel vehicle (solid lipid nano particles) having enhanced follicular penetration may be useful when systemic medication is unacceptable and can be used in both men and women. [53]
Drospirenone
It is a novel progestin derived from 17 α-spironolactone having both anti-androgenic and anti-mineralocorticoid activity. [54] In combination with lower doses of estrogen (drospirenone 3 mg/ethinyl estradiol 20, 30 μg), it is reported to have a beneficial effect in acne vulgaris, and hirsutism and the OCP induced fluid retention caused by the estrogen component. Ethinylestradiol and drospirenone (EE-DR) show comparable efficacy to ethinylestradiol and norgestimate. [55] The brand names include Yasmin® , Yasminelle® , Yaz® , Beyaz® , Ocella® , Zarah® , and Angeliq® . Side effects include thromboembolic episodes, and hyperkalemia. It is contraindicated in hepatic and renal dysfunction, and in adrenal insufficiency. Drugs, which can induce hyperkalemia (angiotensin converting enzyme [ACE] inhibitors, angiotensin-II receptor agonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, nonsteroidal anti inflammatory drungs [NSAIDs]), are contraindicated, while on drospirenone.
Flutamide
Flutamide is a non-steroidal androgen-receptor blocker used in prostate cancer and is used to treat hirsutism and acne in women at doses of 250-500 mg/day. Serious hepatotoxicity limits its use in acne. [9]
Anti-sense oligonucleotides
Anti-sense RNA oligonucleotides in combination with penetration enhancers may prove a novel therapeutic option. It acts by inhibiting the expression of the AR. [56]
Drugs Acting on Adrenal Androgen Production
Low-dose glucocorticoids (prednisolone 2.5-5 mg daily at bedtime) or low-dose dexamethasone is useful in patients with late onset CAH (21-hydroxylase or 11-hydroxylase deficiency). As a result of enzyme deficiency, steroid precursors accumulate and are shunted into the pathway for androgen biosynthesis. [40] The risk of adrenal suppression is higher with dexamethasone. Serum DHEAS can be monitored for evaluating the effect of steroids. The levels of DHEAS are normalized following treatment. Adrenal suppression can be monitored with the adreno corticotrophin hormone (ACTH) stimulation test. Here plasma cortisol is measured 30 min after injecting ACTH.
Drugs Acting on Ovarian Androgen Production
Gonadotrophin-release hormone agonists
Gonadotrophin-release hormone (GnRH) agonists (buserelin, nafarelin, and leuprolide, triptorelin,) cause continuous stimulation of FSH and LH, blocking the cyclical release of these hormones from the pituitary necessary for ovulation.
They cause anovulation and suppress both the ovarian androgen and estrogen production and are effective in acne and hirsutism. Signs and symptoms of hypoestrogenism (menopausal symptoms, headache, osteoporosis) may occur. Formulations available include injections, nasal spray or implant.
Combined oral contraceptives
They are combinations of an estrogen and a progestin. [57],[58],[59] The estrogenic component is usually ethinyl estradiol and rarely mestranol. Although some progestins have androgen-like effects, when combined with EE, the net result is overall anti-androgenic.
Most of the combined oral contraceptives (COCs) in the market today contain lower doses of estrogens (20-50 μg) and common ones are listed in [Table - 4]. They used to contain high doses of estrogen (100 μg). Although estrogens have sebosuppressive action only at high doses, COCs block the androgen production in acne by four mechanisms.
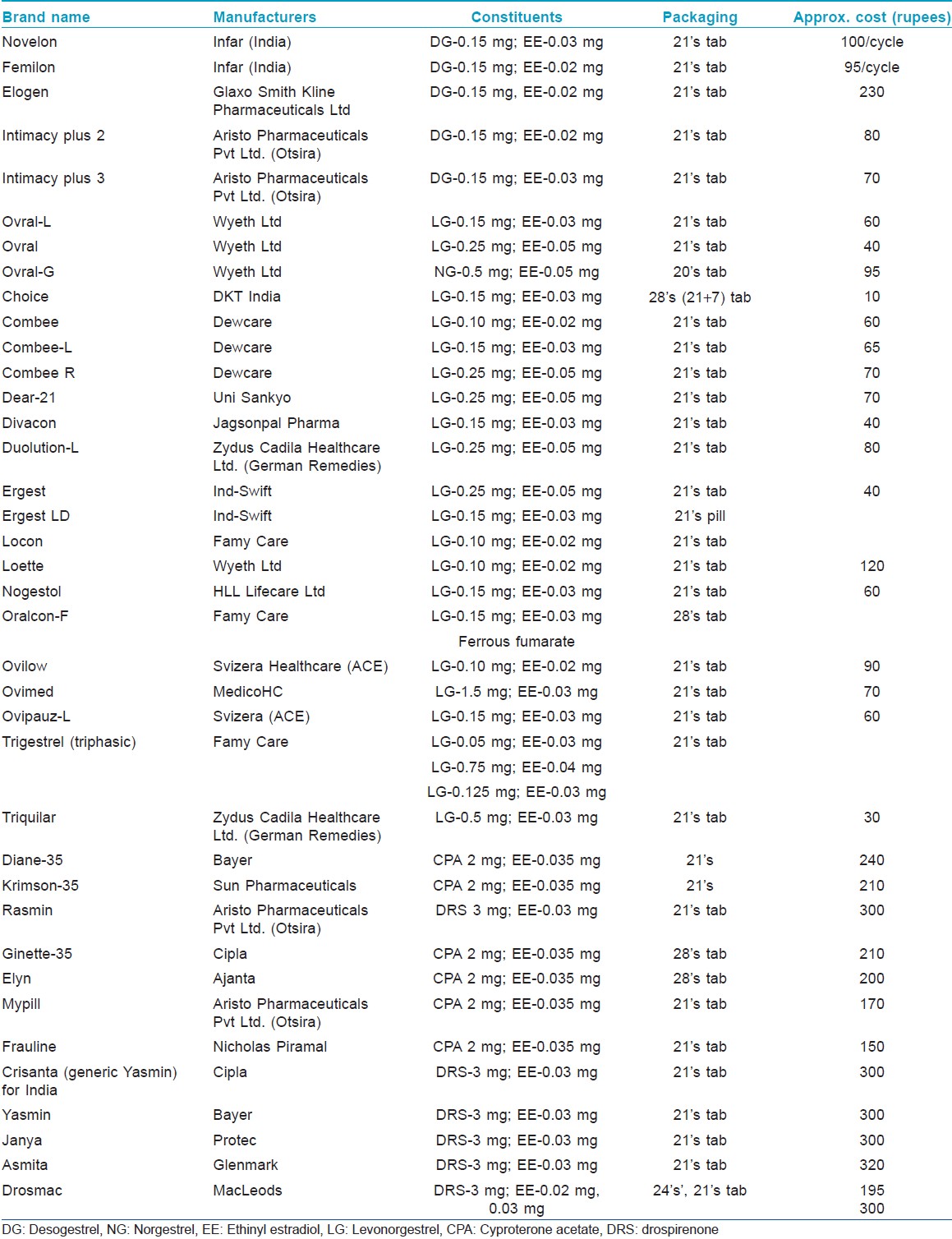
- Decrease gonadal androgen production. They cause decreased secretion of the gonadotropins (LH and FSH). This blocks ovulation and blocks LH-induced ovarian androgen production
- Block the AR
- Estrogens increase the synthesis of SHBG synthesis from the liver, decreasing the serum levels of free bioavailable testosterone
- Some progestins also inhibits 5 α-R and inhibits conversion of weaker androgens to the potent ones.
Drospirenone is the only progestin which is food and drugs administration (FDA) approved in the United States that blocks the AR and is truly anti-androgenic, even without the addition of EE (Yaz® , Yasmin® ) Unlike endogenous progesterone, synthetic progestins have androgenic activity that may exacerbate or trigger acne, and thus the treatment of acne necessitates choosing an OCP that contains a progestin with low androgenic properties.
Among the progestins, norgestimate, and desogestrel are least androgenic whereas norgestrel and levonorgestrel have more androgenic potential. [9] Thus COCs containing these progestins with EE would be preferred.
Side-effects include nausea, headache, breast tenderness, bloating, breakthrough bleeding, acne, decreased sexual desire and depression, weight gain. Thromboembolic episodes were much higher with COCs having a high estrogen dose and these events have decreased with current low estrogen formulations. Low-dose COCs alone may increase the incidence of MI in women. Those women with a history of hypertension, smoking or migraine, associated with an aura have an increased risk. The frequency of venous thromboembolism is also about three times higher in COC users as compared to non-users. Apart from the treatment of acne, the other beneficial effects of OCPs include protection against ovarian and endometrial cancer, benign breast disease, ectopic pregnancy, dysmenorrhea, iron-deficiency anemia, and pelvic inflammatory disease.
Progestins
These are 19-Nortestosterone derivatives and may cross react with the AR causing aggravation of acne, hirsutism, or androgenetic alopecia. [40] Third generation progestins (norgestimate, desogestrel, and gestodene) are more selective for the progesterone receptor than for the AR.
Newer contraceptive forms such as contraceptive patches, vaginal rings, injectable combination hormones have not been studied in the treatment of acne. Transdermal delivery of OCPs is now available and can avoid liver metabolism and decrease needed effective dose and risks associated with OCPs.
Transdermal contraceptive patch: The Ortho Evra_transdermal contraceptive patch was approved for birth control in 2001. It is applied to the buttock, abdomen, upper torso, or lateral portion of the upper arm and lasts for 7 days, and is replaced by a fresh patch at weekly intervals for 3 weeks. A 7-day patch-free week follows, at which time withdrawal bleeding occurs. [57]
Inhibitors of Peripheral Androgen Metabolism
Peripheral androgen metabolism is inhibited by the 5 α -reductase inhibitors. [22],[60]
5 α- DHT is formed from testosterone by the action of the enzyme 5 α -reductase. 5 αR is the key enzyme in androgen metabolism. DHT has 2-10 times more potent androgenic action as compared to testosterone and plays a pathogenetic role in androgen-dependent diseases like acne, hirsutism, androgenetic alopecia, benign prostatic hyperplasia, and prostatic carcinoma. Approximately, 70-80% of serum DHT in men is produced by the type 2 isoenzyme, and 20-30% by the type 1 isoenzyme.
5-αR exists as 2 isoenzymes with different localization. Type 1 isoenzyme is predominantly localized to the sebaceous glands where it may exert a regulatory role in sebum production. Other sites for the type 1 isoenzyme includes epidermis, eccrine sweat glands, apocrine sweat glands, hair follicles (outer root sheath cells, dermal papilla cells, matrix cells), endothelial cells of small vessels and in the Schwann cells of myelinated nerves in the skin. Type 2 isoenzyme is localized predominantly to the prostate and genital skin. In the hair follicle type 2 isoenzyme is seen in the inner layer of outer root sheaths, inner root sheaths, infundibulum, and the sebaceous ducts. Both isoenzymes are expressed in the liver. Type 1 is not seen in the fetus but is expressed in skin and the scalp at puberty. The Type 2 isoenzyme does not seem to have a definite role but may have a priming effect on the skin, prostate and the scalp, for action of the Type 1 isoenzyme later in life.
5 α-R inhibitors may be classified based on:
- Chemical structure (steroidal/non-steroidal)
- Competitive/Non-competitive
- Specificity (type 1/type 2/dual) inhibitors.
Structural inhibitors: 4-azasteroid derivatives
Finasteride is a specific, competitive, type 2 5 α-R inhibitor effective for benign prostatic hyperplasia and androgenetic alopecia. However, there is no reduction of sebum secretion, possibly because it does not affect the type 1 isoenzyme in the sebaceous gland.
Dutasteride also a 4-azasteroid inhibits both the isoenzymes. These molecules have not been found to be effective in women. Besides dutasteride is contraindicated in women.
4-methyl-4 azasteroids e.g., turosteride is a potent inhibitor of type 2 isoenzyme.
Other medications with 5 α-R inhibitor action include zinc, azelaic acid, saw palmetto, and various phytotherapeutic agents. [60]
Zinc, inhibits the type 1 isoenzyme. Azelaic acid (1,9-non-anedioic acid) occurring in wheat, rye, sorghum and barley is a competitive inhibitor of 5 α-R. Saw palmetto berries (Serenoa repens) have dual 5 α-R inhibitor action and contain phytosterols (β-sitisterol, stigmasterol, lupeol, lupenone, and cycloartenol).
Other dual phytotherapeutic inhibitors include Pygeum africanum, Thuja orientalis, Laminaria saccharina, Arnica Montana, Cinchona succiruba, Eugenia caryophyllata, Humulus lupulus, Hypericum perforatum, Mentha piperata, Rosmarinus officinalis, Salvia officinalis, Thymus officinalis, isoflavonoids (genistein, curcumin, daidzein), unsaturated fatty acids (g-linolenic acid, a-linolenic acid, linoleic acid, palmitoleic acid, oleic acid, myristoleic acid).
Dopamine Agonists in Hyperprolactinemia
Bromocriptine, cabergoline, quinagolide are the most frequently used dopamine agonists. [61] Cabergoline and quinagolide are newer dopamine agonists with improved efficacy and lower incidence of adverse effects. Quinagolide can be used until the point of confirmation of pregnancy test and so is now considered a first-line agent in the treatment of hyperprolactinemia. Unlike bromocriptine, which is ergot derived and acts against both D1 (antagonist) and D2 (agonist) receptors, quinagolide is a non-ergot derived dopamine agonist specific against the D2 receptor with minimal action against the D1 receptor. Initial dosing is with 0.025 mg/day gradually increased to 0.075 mg/day (Norprolac, Ferring Pharmaceuticals, Lausanne, Switzerland).
Insulin Sensitizing Agents
Insulin resistance is characterized by reduced cellular uptake of glucose and normal or increased levels of insulin. In IR, the intracellular pool of the insulin-responsive glucose transporter 4(GLUT 4) is markedly reduced. Metformin, a biguanide reverses this effect by delaying GLUT 4 endocytosis and by increasing GLUT 4 gene expression, both resulting in increased glucose uptake and revering the insulin resistance. Metformin and thizolidinediones (rosiglitazone/pioglitazone) can decrease both fasting and stimulated plasma insulin levels and thus reduce insulin resistance, this action being mediated through interaction with PPAR - PPAR γ. [48] There are three isotypes (PPAR: α, δ, and γ). [34] PPAR are present in mitochondria, peroxisomes and microsomes of sebocytes and regulate multiple lipid metabolic genes. PPAR α and γ predominate in human sebocytes.
PPAR γ usually repress the GLUT 4 promoter. By decreasing PPAR γ induced repression of the GLUT 4 promoter, insulin resistance is reduced with improved glucose uptake. [27] Insulin resistance and compensatory hyperinsulinemia may play a very important role in the development of acne, by inducing HA. [62] The effect of androgens on sebaceous lipids is mediated by the PPAR ligands. Increased activity of the PPAR γ represses the GLUT4 promoter and results in insulin resistance.
Serious side effects to metformin treatment are very rare. It is important to note that metformin does not cause hypoglycaemia. In the 1 st week, an upset stomach or diarrhea is common and this side effect can be reduced by taking it after food and by starting with a very low dose (250 mg), increasing slowly by 250 mg per week until the full dose of 1500-2000 mg is achieved. Metformin works much better if combined with a strict regime of diet and exercise. There are no recommendations on how long to continue the drug. A beneficial effect should be seen within 6 months for continuation. Metformin restores regular menses in approximately 62% of predominately obese PCOS women with oligomenorrhea or amenorrhea. [63]
Serious cardiovascular and liver toxicity precludes the use of the thizolidinediones for acne. Metformin is a safer drug for use in acne in women with hirsutism and acne associated with the PCOS. [64] It is also more effective than placebo in hormonal acne.
Antibiotics and Anti-Fungals
Limecycline, a second generation tetracycline and the macrolide roxithromycin have both anti-androgenic and anti-inflammatory actions. [45]
Ketoconazole exerts its anti-androgenic effect by 2 mechanisms.
High oral doses (400 mg thrice daily) blocks testicular and adrenal androgen synthesis, decreasing serum testosterone levels. [9],[65] This action is mediated by inhibition of cytochrome P450 and 17, 20 lyase enzyme systems involved in steroid metabolism (synthesis and degradation of testosterone precursors). It also acts as an AR antagonist competing with testosterone and DHT, in high oral doses.
Recent Advances
Vitamin D deficiency has been reported to aggravate menstrual irregularities, insulin resistance, hirsutism, HA associated with the PCOS. Supplementation of vitamin D may prove beneficial. [66]
Recently the sebosuppressive effect of isotretinoin has been explained by reduction in formation of DHT and androstenedione as isotretinoin competitively inhibits 3 α-hydroxysteroid oxidation by retinol dehydrogenase. [67]
Screening for Hormonal Factors in Acne
I. History and physical examination.
- Menstrual history (menarche, regular cycles, infertility). Amenorrhea or oligomenorrhea (characterized by less than 8 menstrual cycles per year).
- Severe acne
- Hirsutism is the commonest manifestation in women (70-80%); associated with increased levels of free testosterone. 70% of hirsute females have HA
- AN
- Androgenetic alopecia
- Cushingoid facies as some adrenal tumors secrete large amounts of androgens and cortisol and symptoms of glucocorticoid excess may be the clinical presentation.
- Signs of virilization (clitoromegaly which may be associated with increased libido, deepening of voice, increased muscle mass, decreased breast size).
- Associated diabetes mellitus due to the insulin resistance.
- Other dermatological disorders: Rosacea and seborrheic dermatitis, acne related to cosmetics and pomades, acne venenata
- History of drug intake: Phenytion, INH, vitamins (B2, B6, B12), lithium, danazol, halogens, epidermal growth receptor inhibitors, selective serotonin reuptake inhibitor
- History of administering hormone therapy: testosterone, progestins, glucocorticoids.
II. Investigations are listed in [Table - 2].
Since hormones exhibit diurnal rhythm, an early morning fasting sample would be ideal. The timing of the blood test should not coincide with ovulation, which is associated with a surge of hormones. The ideal time would be during the menstrual period (day 1-3). In patients with amenorrhea, fasting sample would suffice. OCP can interfere with hormonal testing and should be discontinued at least 4-6 weeks prior to testing.
In conclusion, hormonal treatment should be considered in female patients whose acne is refractory to conventional lines of treatment. HA is a definite indication for therapy; hormonal therapy should also be considered in women who have clinical signs of HA but have normal serum androgen levels. Antibiotics and anti-fungals with anti-androgenic effect may potentiate hormonal therapy. Since PCOS is the most common menstrual disorder in teenage girls, in addition to OCPs, metformin should be considered in these patients, especially the obese teenagers as it can restore ovulation and improve symptoms of HA like acne, hirsutism, and weight gain. In patients who have contraindication to OCPs and who are unwilling, metformin is more effective than placebo in restoring the normal menstrual cycle and thus reducing acne.
| 1. |
Stern RS. The prevalence of acne on the basis of physical examination. J Am Acad Dermatol 1992;26:931-5.
[Google Scholar]
|
| 2. |
George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Semin Cutan Med Surg 2008;27:188-96.
[Google Scholar]
|
| 3. |
Thiboutot D. Acne: Hormonal concepts and therapy. Clin Dermatol 2004;22:419-28.
[Google Scholar]
|
| 4. |
Liang T, Hoyer S, Yu R, Soltani K, Lorincz AL, Hiipakka RA, et al. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J Invest Dermatol 1993;100:663-6.
[Google Scholar]
|
| 5. |
Rosenfield RL, Deplewski D, Kentsis A, Ciletti N. Mechanisms of androgen induction of sebocyte differentiation. Dermatology 1998;196:43-6.
[Google Scholar]
|
| 6. |
Lolis MS, Bowe WP, Shalita AR. Acne and systemic disease. Med Clin North Am 2009;93:1161-81.
[Google Scholar]
|
| 7. |
Chen W, Obermayer-Pietsch B, Hong JB, Melnik BC, Yamasaki O, Dessinioti C, et al. Acne-associated syndromes: Models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol 2011;25:637-46.
[Google Scholar]
|
| 8. |
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41-7.
[Google Scholar]
|
| 9. |
Lowenstein EJ. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. Dermatol Ther 2006;19:210-23.
[Google Scholar]
|
| 10. |
Maharaj S, Amod A. Polycystic ovary syndrome. JEMDSA 2009:14:86-96.
[Google Scholar]
|
| 11. |
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237-45.
[Google Scholar]
|
| 12. |
Braverman IM. Endocrine and metabolic disorders. In: Braverman IM, editor. Skin Signs of Systemic Disease. Philadelphia: WB Saunders Company; 1998. p. 452-7.
[Google Scholar]
|
| 13. |
Lin-Su K, Nimkarn S, New MI. Congenital adrenal hyperplasia in adolescents: Diagnosis and management. Ann N Y Acad Sci 2008;1135:95-8.
[Google Scholar]
|
| 14. |
Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet 2005;365:2125-36.
[Google Scholar]
|
| 15. |
Cordera F, Grant C, van Heerden J, Thompson G, Young W. Androgen-secreting adrenal tumors. Surgery 2003;134:874-80.
[Google Scholar]
|
| 16. |
Elmer KB, George RM. HAIR-AN syndrome: A multisystem challenge. Am Fam Physician 2001;63:2385-90.
[Google Scholar]
|
| 17. |
Amesse LS, Ding X, Pfaff-Amesse T. From HAIR-AN to eternity. J Pediatr Adolesc Gynecol 2002;15:235-40.
[Google Scholar]
|
| 18. |
Orfanos CE, Adler YD, Zouboulis CC. The SAHA syndrome. Horm Res 2000;54:251-8.
[Google Scholar]
|
| 19. |
Chamot AM, Vion B, Gerster JC. Acute pseudoseptic arthritis and palmoplantar pustulosis. Clin Rheumatol 1986;5:118-23.
[Google Scholar]
|
| 20. |
Kahn MF, Khan MA. The SAPHO syndrome. Baillieres Clin Rheumatol 1994;8:333-62.
[Google Scholar]
|
| 21. |
Chen W, Thiboutot D, Zouboulis CC. Cutaneous androgen metabolism: Basic research and clinical perspectives. J Invest Dermatol 2002;119:992-1007.
[Google Scholar]
|
| 22. |
Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, et al. Human skin is a steroidogenic tissue: Steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J Invest Dermatol 2003;120:905-14.
[Google Scholar]
|
| 23. |
Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am 2005;34:293-313.
[Google Scholar]
|
| 24. |
Strauss JS, Pochi PE. Effect of cyclic progestin-estrogen therapy on sebum and acne in women. JAMA 1964;190:815-9.
[Google Scholar]
|
| 25. |
Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000;21:363-92.
[Google Scholar]
|
| 26. |
Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol 2008;128:1286-93.
[Google Scholar]
|
| 27. |
Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol 2009;18:833-41.
[Google Scholar]
|
| 28. |
Thierry van Dessel HJ, Lee PD, Faessen G, Fauser BC, Giudice LC. Elevated serum levels of free insulin-like growth factor I in polycystic ovary syndrome. J Clin Endocrinol Metab 1999;84:3030-5.
[Google Scholar]
|
| 29. |
Rosenbloom AL. The role of recombinant insulin-like growth factor I in the treatment of the short child. Curr Opin Pediatr 2007;19:458-64.
[Google Scholar]
|
| 30. |
Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: A randomized, investigator-masked, controlled trial. J Am Acad Dermatol 2007;57:247-56.
[Google Scholar]
|
| 31. |
Blum JW, Baumrucker CR. Insulin-like growth factors (IGFs), IGF binding proteins, and other endocrine factors in milk: role in the newborn. In: Bosze Z, editor. Biactive Components of Milk. Advances in Experimental Medicine and Biology. vol. 606. New York: Springer; 2008. p. 397-422.
[Google Scholar]
|
| 32. |
Hoppe C, Mølgaard C, Michaelsen KF. Cow's milk and linear growth in industrialized and developing countries. Annu Rev Nutr 2006;26:131-73.
[Google Scholar]
|
| 33. |
Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids enhance Toll-like receptor 2 expression in human keratinocytes stimulated with Propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol 2009;129:375-82.
[Google Scholar]
|
| 34. |
Bhambri S, Del Rosso JQ, Bhambri A. Pathogenesis of acne vulgaris: Recent advances. J Drugs Dermatol 2009;8:615-8.
[Google Scholar]
|
| 35. |
Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol 2009;160:345-52.
[Google Scholar]
|
| 36. |
Ganceviciene R, Graziene V, Böhm M, Zouboulis CC. Increased in situ expression of melanocortin-1 receptor in sebaceous glands of lesional skin of patients with acne vulgaris. Exp Dermatol 2007;16:547-52.
[Google Scholar]
|
| 37. |
Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81.
[Google Scholar]
|
| 38. |
Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 2009;161:575-82.
[Google Scholar]
|
| 39. |
Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology 2003;206:57-67.
[Google Scholar]
|
| 40. |
Thiboutot D. Hormonal influences in acne. In: Webster GF, Rawlings AV, editors. Acne and its Therapy: Acne Treatments. Part 2, Ch. 7. New York (NY), USA: Informa Health Care Inc.; 2007. p. 83-95.
[Google Scholar]
|
| 41. |
Junkins-Hopkins JM. Hormone therapy for acne. J Am Acad Dermatol 2010;62:486-8.
[Google Scholar]
|
| 42. |
Rager KM, Omar HA. Androgen excess disorders in women: The severe insulin-resistant hyperandrogenic syndrome, HAIR-AN. Sci World J 2006;6:116-21.
[Google Scholar]
|
| 43. |
Karrer-Voegeli S, Rey F, Reymond MJ, Meuwly JY, Gaillard RC, Gomez F. Androgen dependence of hirsutism, acne, and alopecia in women: Retrospective analysis of 228 patients investigated for hyperandrogenism. Medicine (Baltimore) 2009;88:32-45.
[Google Scholar]
|
| 44. |
Zouboulis CC, Degitz K. Androgen action on human skin - From basic research to clinical significance. Exp Dermatol 2004;13:5-10.
[Google Scholar]
|
| 45. |
Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol 2009;18:821-32.
[Google Scholar]
|
| 46. |
Farquhar C, Lee O, Toomath R, Jepson R. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev 2003;CD000194.
[Google Scholar]
|
| 47. |
Inal MM, Yildirim Y, Taner CE. Comparison of the clinical efficacy of flutamide and spironolactone plus Diane 35 in the treatment of idiopathic hirsutism: A randomized controlled study. Fertil Steril 2005;84:1693-7.
[Google Scholar]
|
| 48. |
Sawaya M. Antiandrogens and androgen inhibitors. In: Se W, editor. Comprehensive Dermatologic Drug Therapy. Philadelphia: W.B. Saunders Co.; 2001. p. 385-401.
[Google Scholar]
|
| 49. |
Eden JA. The polycystic ovary syndrome presenting as resistant acne successfully treated with cyproterone acetate. Med J Aust 1991;155:677-80.
[Google Scholar]
|
| 50. |
Dorrington-Ward P, McCartney AC, Holland S, Scully J, Carter G, Alaghband-Zadeh J, et al. The effect of spironolactone on hirsutism and female androgen metabolism. Clin Endocrinol (Oxf) 1985;23:161-7.
[Google Scholar]
|
| 51. |
Erenus M, Yücelten D, Durmuþoðlu F, Gürbüz O. Comparison of finasteride versus spironolactone in the treatment of idiopathic hirsutism. Fertil Steril 1997;68:1000-3.
[Google Scholar]
|
| 52. |
Haider A, Shaw JC. Treatment of acne vulgaris. JAMA 2004;292:726-35.
[Google Scholar]
|
| 53. |
Stecová J, Mehnert W, Blaschke T, Kleuser B, Sivaramakrishnan R, Zouboulis CC, et al. Cyproterone acetate loading to lipid nanoparticles for topical acne treatment: Particle characterisation and skin uptake. Pharm Res 2007;24:991-1000.
[Google Scholar]
|
| 54. |
Thorneycroft IH. Evolution of progestins. Focus on the novel progestin drospirenone. J Reprod Med 2002;47:975-80.
[Google Scholar]
|
| 55. |
Thorneycroft lH, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis 2004;74:123-30.
[Google Scholar]
|
| 56. |
Fimmel S, Saborowski A, Térouanne B, Sultan C, Zouboulis CC. Inhibition of the androgen receptor by antisense oligonucleotides regulates the biological activity of androgens in SZ95 sebocytes. Horm Metab Res 2007;39:149-56.
[Google Scholar]
|
| 57. |
Chan CS, Harting M, Rosen T. Systemic and barrier contraceptives for the dermatologist: A review. Int J Dermatol 2009;48:795-814.
[Google Scholar]
|
| 58. |
Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther 2009;22:452-7.
[Google Scholar]
|
| 59. |
Kamangar F, Shinkai K. Acne in the adult female patient: A practical approach. Int J Dermatol 2012;51:1162-74.
[Google Scholar]
|
| 60. |
Voegeli R, Zouboulis CC, Elsner P, Schreier T. 5 α-reductase and its inhibitors. In: Webster GF, Rawlings AV, editors. Acne and its Therapy. Ch. 14. New York (NY), USA: Informa Health Care Inc.; 2007. p. 167-201.
[Google Scholar]
|
| 61. |
Barlier A, Jaquet P. Quinagolide: A valuable treatment option for hyperprolactinaemia. Eur J Endocrinol 2006;154:187-95.
[Google Scholar]
|
| 62. |
Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5alpha-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol 2007;156:428-32.
[Google Scholar]
|
| 63. |
Costello MF. Polycystic ovary syndrome: A management update. Aust Fam Physician 2005;34:127-33.
[Google Scholar]
|
| 64. |
Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol 2002;147:217-21.
[Google Scholar]
|
| 65. |
Eil C. Ketoconazole binds to the human androgen receptor. Horm Metab Res 1992;24:367-70.
[Google Scholar]
|
| 66. |
Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012;77:343-50.
[Google Scholar]
|
| 67. |
Downie MM, Guy R, Kealey T. Advances in sebaceous gland research: Potential new approaches to acne management. Int J Cosmet Sci 2004;26:291-311.
[Google Scholar]
|
Fulltext Views
15,858
PDF downloads
4,581





