Translate this page into:
Interface dermatitis
Correspondence Address:
Rajiv Joshi
14 Jay Mahal, A Road, Churchgate, Mumbai - 400 020
India
| How to cite this article: Joshi R. Interface dermatitis. Indian J Dermatol Venereol Leprol 2013;79:349-359 |
Abstract
Interface dermatitis includes diseases in which the primary pathology involves the dermo-epidermal junction. The salient histological findings include basal cell vacuolization, apoptotic keratinocytes (colloid or Civatte bodies), and obscuring of the dermo-epidermal junction by inflammatory cells. Secondary changes of the epidermis and papillary dermis along with type, distribution and density of inflammatory cells are used for the differential diagnoses of the various diseases that exhibit interface changes. Lupus erythematosus, dermatomyositis, lichen planus, graft versus host disease, erythema multiforme, fixed drug eruptions, lichen striatus, and pityriasis lichenoides are considered major interface diseases. Several other diseases (inflammatory, infective, and neoplastic) may show interface changes.Introduction
Interface dermatitis includes conditions in which the primary pathology involves the "interface," i.e., the dermo-epidermal junction. The components of this "interface" include the basal layer of the epidermis, the dermo-epidermal junction, the papillary dermis, and the adventitial dermis around the adnexal structures. These constituents function as a single anatomico-physiological unit and pathological alterations of any of these results in changes that affect all the individual constituents.
Patho-physiologically, [1] apoptosis/individual cell necrosis of keratinocytes is the most prominent feature in primary interface dermatitis and although specific antigens have not yet been indentified, T-cell-mediated cytokine damage is the most likely mechanism resulting in these diseases. The morphological changes seen are primarily due to keratinocyte (basal cell) damage, remodeling of the basement membrane, and the effects of the inflammatory infiltrate. Secondary changes follow the primary events and may affect the epidermis, the papillary dermis, or both resulting in varying histological findings that characterize the morphological findings in individual interface dermatitis.
Morphological changes seen in interface dermatitis include some or all of the following:
Primary changes
- Basal cell vacuolization (Vacuolar alteration) [Figure - 1]: This may be the most prominent feature and includes small basal or sub-basal vacuoles. There is expansion of the cytoplasm leading to partial or complete destruction of the basal cell with the appearance of either a ballooned cell or total absence of the basal cell with the spinous keratinocytes abutting the papillary dermis (squamatization of basal layer). Confluent basal cell damage results in the formation of clefts and subepidermal vesicles.
- Apoptotic keratinocytes (syn. Colloid bodies, Civatte bodies, dyskeratotic keratinocytes, individually necrotic keratinocytes) [Figure - 2]: These are seen as small rounded eosinophilic hyaline, anucleate structures, and slightly smaller than the size of a basal keratinocyte. These may be seen in the basal layer, in the upper papillary dermis, individually or in clumps, or in the mid- and upper spinous layers, sometimes as whorls in acrosyringia and also in the stratum corneum.
- Obscuring of the dermo-epidermal junction by inflammatory cells [Figure - 3]: The lymphocyte is the most common of inflammatory cells participating in interface reactions, but other cell types, namely, eosinophils, neutrophils, mast cells, and histiocytes may also be seen. Ingress of lymphocytes across the basement membrane and into the basal cell layer or in the lower spinous layers obliterates the clear distinction between the epidermis and the papillary dermis seen in normal skin. The density of the inflammatory infiltrate is variable, and depends on the disease and its stage of evolution, it can vary from sparse (pauci-inflammatory) to dense band-like (lichenoid) infiltrate.
Secondary changes
- Epidermal changes: Epidermal reactions to the basal cell damage can be diametrically opposite, i.e., a thin flat atrophic epidermis is typical for lupus erythematosus (LE), whereas epidermal acanthosis with hypergranulosis and a thick compact orthokeratotic stratum corneum is usual for lichen planus (LP). Different diseases within the spectrum of "interface dermatitis" show variable and different epidermal findings depending on the disease, time of biopsy in the course of evolution or devolution of the disease, and the site of the biopsy. For example, although the usual epidermal reaction in LP is broad acanthosis with jagged irregular rete ridges, a long-standing atrophic lesion shows a thin flat atrophic epidermis. Likewise, cutaneous LE is usually associated with a thin and atrophic epidermis, at least focally, however, the epidermis may attain remarkable irregular hyperplasia in verrucous discoid lupus erythematosus (DLE).
- Papillary dermal changes: The papillary dermis (also the adventitial dermis surrounding adnexal structures especially hair follicles) also shows changes secondary to basal cell damage. The papillary dermis undergoes expansion to accommodate the inflammatory infiltrate, more so, when the infiltrate is dense and arranged in a band-like manner (lichenoid). The effect of long-standing lymphocytic infiltrate in the papillary dermis results in fibrosis or sclerosis of the papillary dermis, which persists long after the inflammatory infiltrate has resolved. Second, basal cell damage leads to "incontinence" of melanin into the papillary dermis and melanophages are seen scattered in the thickened and often fibrotic papillary dermis [Figure - 4] and [Figure - 5].
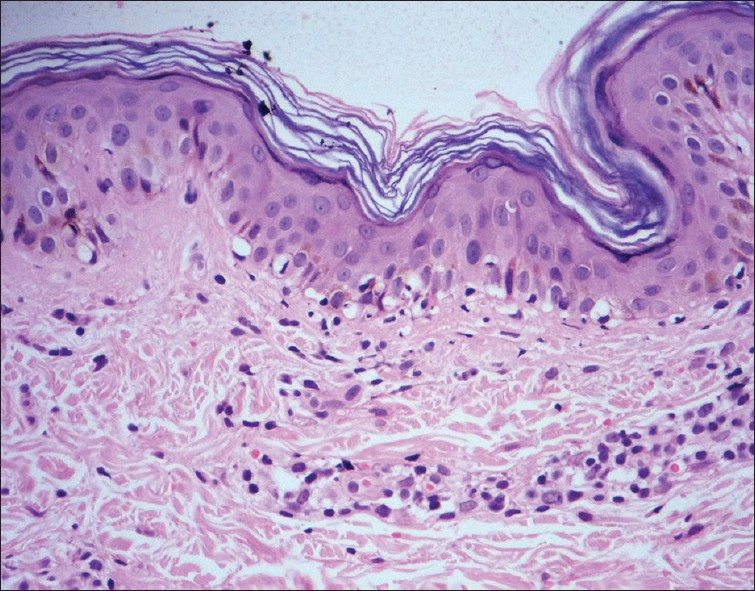 |
| Figure 1: Vacuolar changes of basal cells with sparse perivascular lymphocytic infiltrate. Morbilliform drug eruption (H and E, ×100) |
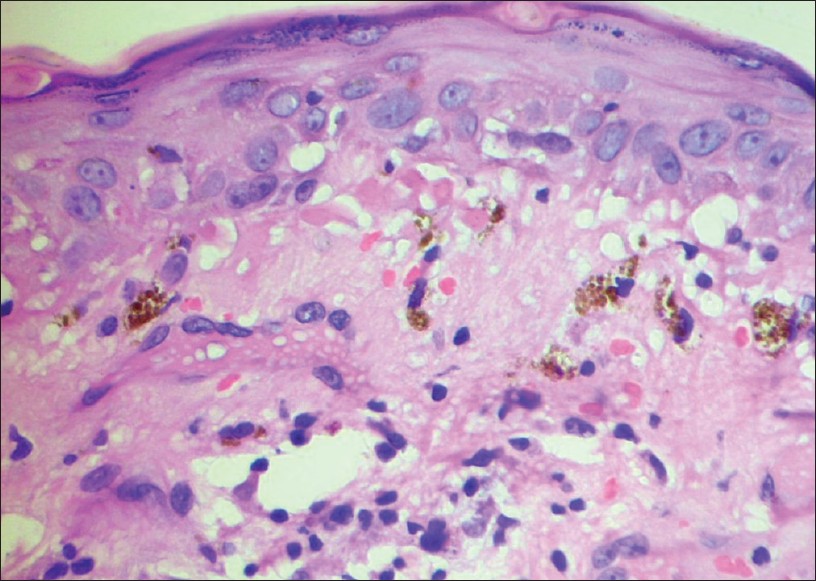 |
| Figure 2: Colloid (Civatte) bodies at the dermo-epidermal junction with basal cell vacuolization with melanophages in the upper papillary dermis. Lupus erythematosus (H and E, ×400) |
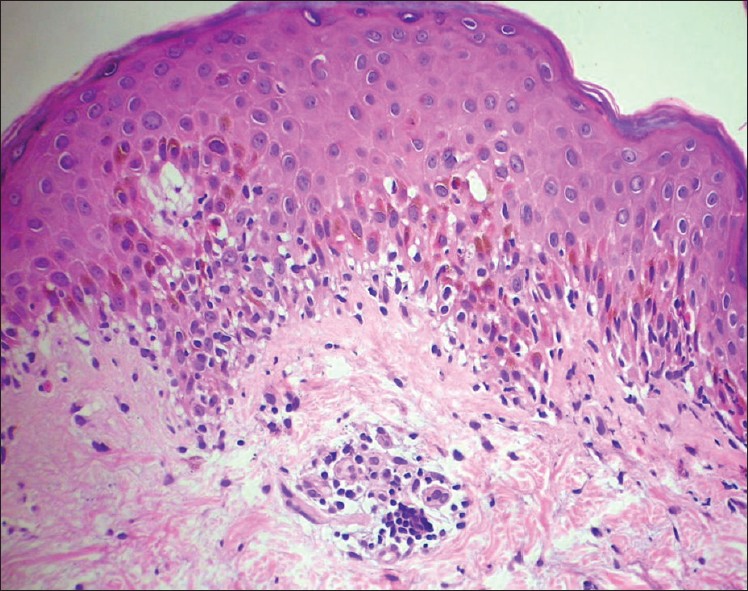 |
| Figure 3: Several lymphocytes in the basal cell layer obscuring the dermo-epidermal junction with basal cell vacuolization and few apoptotic keratinocytes in the lower spinous zone. Erythema multiforme (H and E, ×400) |
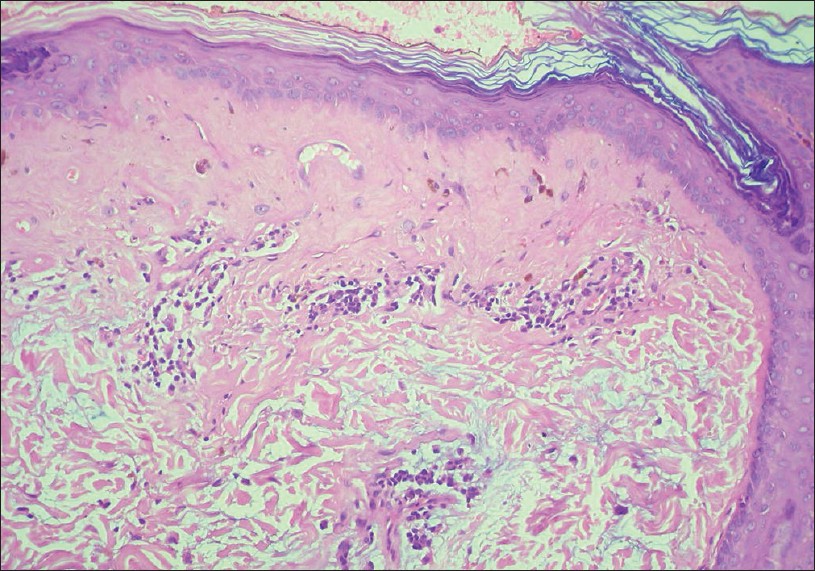 |
| Figure 4: Sclerosis of thickened papillary dermis with smudging of dermo-epidermal junction. Note the bluish-grey mucin in the upper reticular dermis. Lupus erythematosus (H and E, ×100) |
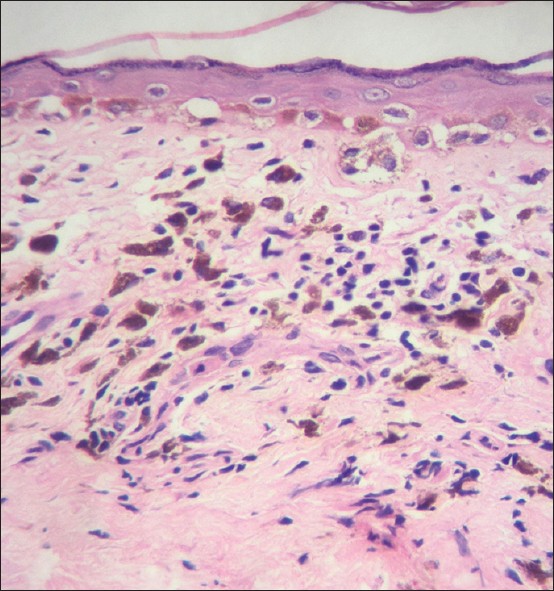 |
| Figure 5: Thickened papillary dermis with sparse lymphocytic infiltrate and numerous melanophages. Note the persisting basal cell vacuolization. Lichen planus pigmentosus (H and E, ×100) |
Other changes
Involvement of other anatomical components of the skin may be seen and include mucin deposits in the reticular dermis in cutaneous LE, perivascular and periadnexal infiltrates of lymphocytes in the mid- and deep reticular dermis, and lymphocytic lobular panniculitis in lupus profundus.
Classification of Interface Dermatitis
Before we get to the current schemas for classification of interface dermatitis, a caveat is in order. It is important to realize that all classification systems are for the convenience of grouping of various diseases with a similar presentation. It is an artificial system and the novice is often confronted with situations where the histological sections or case under consideration does not "fit into" any known category. Most inflammatory diseases may be classified under various schemas, depending on which least common factor (clinical, histological, immunological, and etiological) has been considered for the classification.
Histologically, interface dermatitis has been classified as: [2],[3]
- Prominent basal cell vacuolization (Vacuolar-interface dermatitis): Wherein basal cell vacuolization is the most prominent pathological finding and is accompanied by variably dense perivascular and interstitial infiltrates of lymphocytes. Examples of this pattern are erythema multiforme (EM), viral exanthems, early cutaneous LE, and acute graft vs host reaction.
- Prominent infiltrate in the papillary dermis aligned in lichenoid pattern (Lichenoid-interface dermatitis): In which the striking histological finding is a dense band-like infiltrate in the papillary dermis which often overshadows basal cell vacuolization which may be inconspicuous or even absent.
Parenthetically, a true lichenoid infiltrate is by definition restricted to the papillary dermis and is seen typically in interface dermatitis, whereas a band-like infiltrate may be seen in several other conditions including granulomatous conditions like tuberculosis verrucosa cutis (TBVC), lupus vulgaris, benign, premalignant, and malignant conditions like lichenoid keratosis, Paget′s disease (both mammary and extramammary), Bowen′s disease, and resolving malignant melanoma in which the infiltrate often extends into the reticular dermis without any interface changes.
Le Boit [4] has used epidermal changes as a more consistent and easier way of classifying interface dermatitis. This system is predicated on observations that the epidermal changes seen in interface dermatitis are different for various diseases and reflect patho-physiological differences. Also the pattern and density of inflammatory cells in the papillary dermis do not correlate well with the epidermal changes.
In this classification, five categories described are as follows:
- Acute cytotoxic type: This is characterized by basal cell vacuolization with lymphocytes infiltrating the lower epidermis with scatter of individually necrotic keratinocytes at various levels in the epidermis. The entire process is a rapid one and as it does not interfere with epidermal keratinization, the horny layer is unaffected and maintains its normal basket weave arrangement. EM is the prototype of this category. The other conditions described with this change include, fixed drug eruptions (FDE), acute graft versus host reaction, early lesions of pityriasis lichenoides et varioliformis acuta (PLEVA), morbilliform viral and drug eruptions, eruption of lymphocyte recovery, skin reactions secondary to chemotherapeutic agents, and acute radiation-induced dermatitis. Early lesions of acute cutaneous LE and photodermatitis also show these changes.
- Premature terminal differentiation: This refers to an early development of a thick granular layer and compact stratum corneum that resembles acral skin and is usually associated with dense lichenoid infiltrates of lymphocytes. LP is the prototype with focal hypergranulosis and a thick compact orthokeratotic stratum corneum. Other conditions in which this pattern may be seen are DLE, lichenoid drug eruptions, and lichenoid graft vs. host disease (GVHD). Dermatomyositis may show similar changes of the epidermis, albeit with a paucity of dermal infiltration.
- Irregular epidermal hyperplasia: This is a variation of the above pattern in which there is a marked irregular epidermal hyperplasia and is seen in hypertrophic LP, verrucous DLE, and some long-standing lichenoid drug eruptions.
- Interface dermatitis with psoriasiform hyperplasia: Some of the conditions in this group are diseases which show interface changes as a secondary pathological feature and are therefore not classified as primary interface dermatitis. These include lichenoid variants of pigmented purpuric dermatitis, mycosis fungoides, porokeratosis, and secondary syphilis. True interface dermatitis which show this pattern are lichen striatus and fully developed lesions of pityriasis lichenoides.
- Interface dermatitis with epidermal atrophy: This represents the late atrophic phase of several dermatoses like atrophic LP, LE, and dermatomyositis, acrodermatitis chronic atrophicans, lichen sclerosus et atrophicus, center of old lesions of porokeratosis, and poikiloderma due to various causes.
Diseases with Interface Changes as Primary Pathology
Cutaneous LE
Pattern of inflammation
A superficial and deep perivascular and periadnexal lymphocytic infiltrate is the most common finding in all lesions of LE [Figure - 6]. The density of the infiltrate is variable and can be sparse to moderately dense. Occasionally, nodular collections of lymphocytes may be seen which resemble a lymphocytoma. Epidermal changes almost always accompany the dermal lymphocytic infiltrate which helps in the diagnosis; however, in tumid LE, the epidermis is normal and the differential diagnoses then includes the classical 5 Ls, i.e., tumid LE, Jessner′s lymphocytic infiltrate, plaque type of PMLE, lymphocytoma cutis, and a true cutaneous lymphoma.
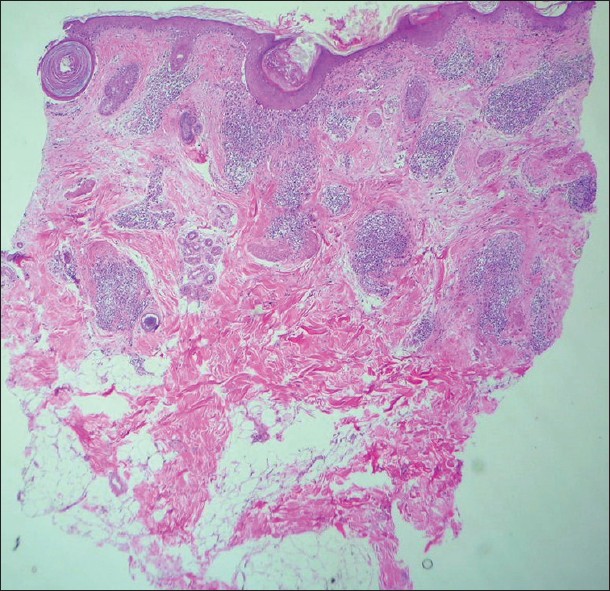 |
| Figure 6: Superficial and deep perivascular and periadnexal lymphocytic infiltrates. Note the thin epidermis, basal cell vacuolization with subepidermal clefts that involve follicular infundibular epithelium, follicular plugging at one end of the sections. Lupus erythematosus (H and E, ×20) |
A dense lichenoid infiltrate may occasionally be seen in cases of lichenoid LE when differentiation from LP may be difficult. The findings of a deep perivascular lymphocytic infiltrate, dermal mucin, and a thickened basement membrane suggest LE; however, in their absence, differentiation from LP can be very difficult and a positive DIF may resolve the issue.
Verrucous epidermal hyperplasia with prominent follicular infundibular dilatation and plugging is seen in verrucous DLE, where the epidermal changes may resemble prurigo nodularis or hypertropic LP.
In early lesions of LE, including the rash associated with systemic lupus erythematosus (SLE), neutrophils are often seen in the upper dermal infiltrate, close to the epidermis. Nuclear dust may be seen and is usually due to karryorrhexis of lymphocytes without any vasculitis. [3]
Epidermal changes
The epidermis typically shows vacuolar-interface dermatitis with basal cell vacuolization, smudging of the dermo-epidermal junction, and a few individually necrotic (apoptotic) keratinocytes (colloid bodies) at the dermo-epidermal junction or in the uppermost papillary dermis. A finding that is very typical of long-standing lesions of cutaneous LE, especially in lesions clinically described as DLE is marked thickening of the basement membrane which appears as a homogenous pink band at the dermo-epidermal junction and stains bright red with PAS stain. Similar thickening of the basement membrane may be seen in dermatomyositis, and may be considered to be a specific histological finding for LE and dermatomyositis.
Atrophy of the nucleated epidermis, along with a thick laminated horny layer, is a common finding in skin lesions of LE and may be used to differentiate LE from other interface dermatitis like PLEVA and erythema multiforme (EM). Dilated follicular infundibula with keratotic plugs are sometimes seen but cannot be considered a specific finding of LE.
Uncommon changes of the epidermis that may be seen in LE include, focal parakeratosis, numerous apoptotic keratinocytes scattered throughout the epidermis, confluent basal cell vacuolization resulting in subepidermal clefting, and confluent epidermal necrosis that resembles epidermal changes seen in toxic epidermal necrolysis (TEN).
Dermal changes
Mucin deposits in the upper and mid-reticular dermis, seen as stringy bluish-grey material between the collagen bundles are very common findings in cutaneous LE [Figure - 7]. At times, large amounts of mucin may be seen, which present as small pools of bluish-grey material in the reticular dermis along with a variable lymphocytic infiltrate. Dermal mucin deposits in the absence of typical epidermal changes can be used to make a definitive histological diagnosis of LE.
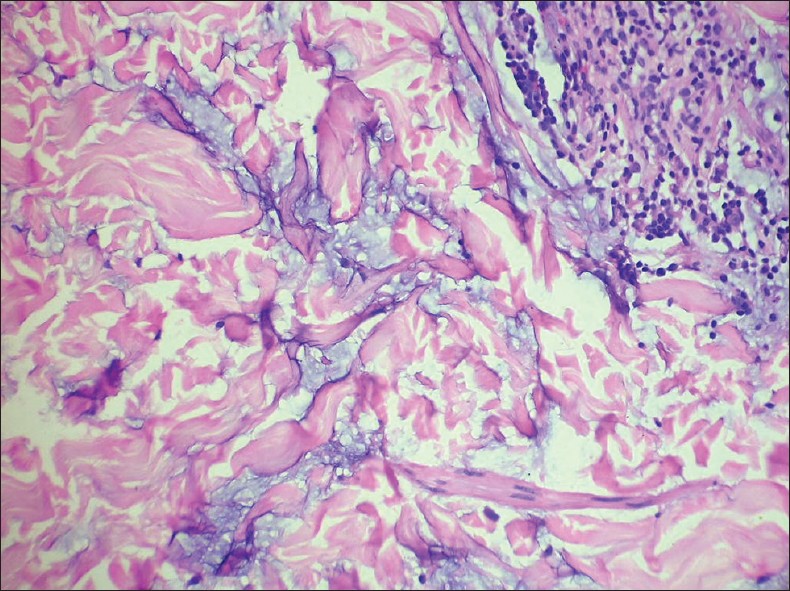 |
| Figure 7: Pools of bluish-grey mucin between bundles of collagen in the reticular dermis. Lupus erythematosus (H and E, ×400) |
The papillary dermis is usually thickened, mildly edematous in the earlier stages with scattered lymphocytes and melanophages, while long-standing lesions may become sclerotic. Dilated thin-walled vessels (telangiectasias) are common in long-standing lesions of DLE.
Involvement of the panniculus
Lobular lymphocytic panniculitis is a typical histological finding in lupus profundus. The panniculitis results in deep seated nodules which may or may not be associated with epidermal and dermal changes of cutaneous LE. Abundant lymphocytic nuclear dust is usually seen along with a dense diffuse lymphocytic infiltrate that involves the entire fat lobule. Plasma cells may be seen along with the dense lymphocytic infiltrate. A close differential diagnosis is the entity subcutaneous panniculitis like T-cell lymphoma.
Follicular involvement in LE
Cicatricial alopecia is a common end result of DLE lesions on the scalp. The follicular infundibula show interface changes similar to the epidermis, including thickening of the basement membrane. Follicular infundibular dilatation with a conical outline and thick keratin plugs is often seen in the early stages, whereas follicular loss and replacement of the destroyed follicle by vertical or concentric fibrotic tracts are seen in the end stage of cicatricial alopecia due to LE.
Differential diagnoses
- Dermatomyositis: Findings are very similar to LE, including thickening of the basement membrane; however, a sparse superficial perivascular lymphocytic infiltrate, many telangiectasias, and usually absence of dermal mucin are features in favor of dermatomyositis.
- Jessner′s lymphocytic infiltrate and reticular erythematous mucinosis: Some authors consider these conditions to be variants of tumid LE; however, there is no consensus on this issue.
- Lichenoid LE: Lichenoid lymphocytic infiltrates and epidermal hyperplasia are difficult to differentiate from LP. A deep perivascular and periadnexal lymphocytic infiltrates and a positive DIF go in favor of LE.
- EM and TEN: Confluent epidermal necrosis or numerous individually necrotic keratinocytes throughout the epidermis may be seen in LE. A thickened laminated stratum corneum and epidermal atropy along with dermal mucin deposits are findings in favor of LE.
- Drug reactions: Interface drug reactions may simulate LE. Prominent parakeratosis, findings of a mixed infiltrate containing eosinophils and neutrophils, and many necrotic keratinocytes located high in the epidermis are findings associated with a drug reaction.
- Subcutaneous panniculitis like T-cell lymphoma: This may closely resemble lupus profundus both clinically and histologically. Overlap features have been described and the lymphoma developing in the setting of pre-existing LE has also been described. [5] Fat cell rimming by CD8 + ve T cells has been reported to occur in the lymphoma.
Lichen planus
A fully developed lesion of LP shows a very typical histological picture of broad epidermal acanthosis with focal wedge-shaped hypergranulosis (often at the tips of orifices of acrosyringia and bases of follicular infundibula). The stratum corneum is thick, compact, and orthokeratotic. This epidermal pattern is repeatable and seen even in small (<2 mm micropapules) as well as in larger lesions and may considered to be the basic histological unit of LP [Figure - 8].
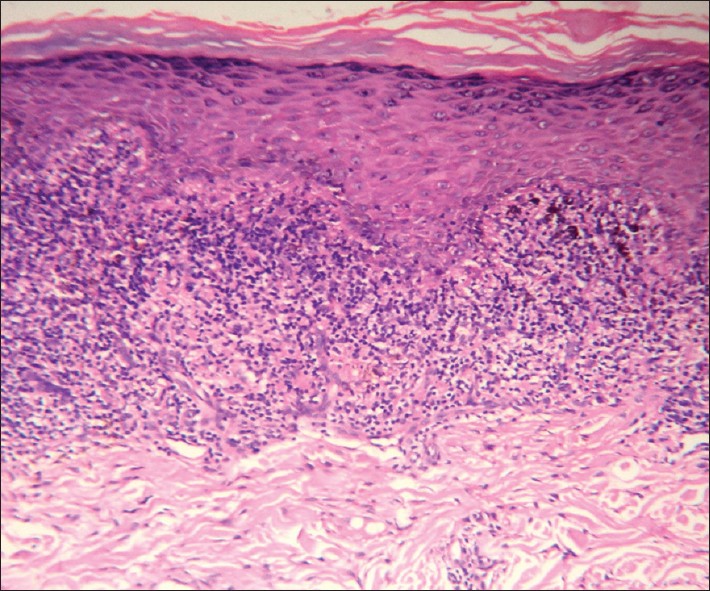 |
| Figure 8: Wedge-shaped hypergranulosis, compact orthokeratosis, irregular rete ridges and a lichenoid lymphocytic infiltrate in the widened papillary dermis. Lichen planus (H and E, ×100) |
Interface changes of basal cell vacuolization are seen early in the evolution of the lesion and may persist focally but in a well-developed papule of LP, the basal cells may not be seen and then the lichenoid infiltrate is seen to abut the spinous layers without intervening basal cells, a finding referred to as "squamatization" of basal cells. The spinous keratinocytes are large and show pale glassy cytoplasm. Colloid (Civatte) bodies that represent anucleated remnants of apoptotic basal keratinocytes may be seen in the basal layer as well in the uppermost papillary dermis, where they may be present as single units or in small clumps. Small subepidermal clefts (Max-Joseph spaces) may be seen at the base or sides of the rete which show short angulated hyperplasia resulting in the typical "sawtooth" pattern of the rete.
A dense lichenoid infiltrate of lymphocytes occupies the widened papillary dermis and may be admixed with melanophages. Melanophages in LP tend to occur near the base of the lichenoid infiltrate unlike in lesions of cutaneous LE, where the melanophages are scattered in the papillary dermis close to the basal layer of the epidermis.
Papillary dermal changes reflect the course of devolution of the lesion and may show a markedly thickened and fibrotic papillary dermis with thickened wiry bundles of collagen and a gradual decrease in the density of the inflammatory infiltrate. In lesions that clinically show atrophy, the epidermis is flat and atrophic and the papillary dermis replete with numerous melanophages without much inflammatory infiltrate [Figure - 9].
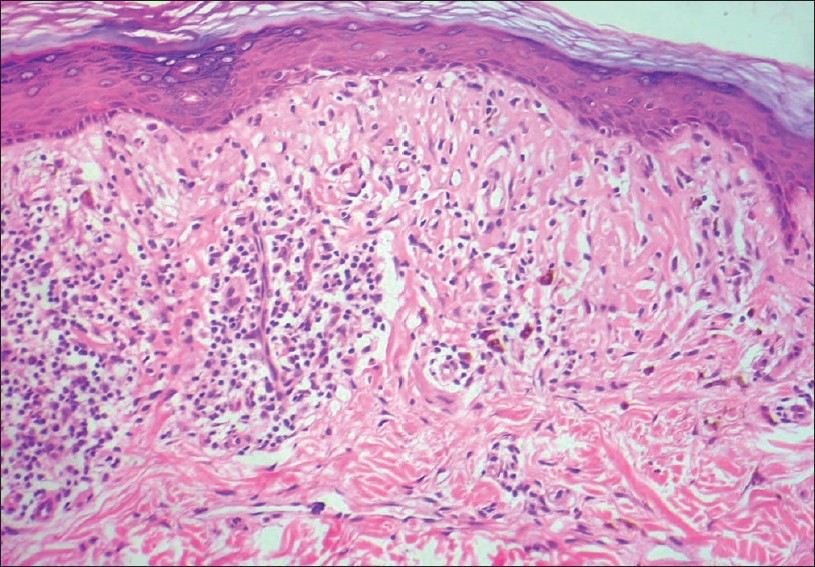 |
| Figure 9: Thick papillary dermis with wiry collagen, sparse lymphocytic infiltrate, and few melanophages. Flat epidermis with loss of the rete ridges. Lichen planus, resolving lesion (H and E, ×100) |
Hypertrophic LP shows irregular jagged hyperplasia of mainly follicular infundibular epithelium with focal hypergranulosis and a markedly thickened compact horny layer. The hyperplastic follicular infundibula show lichenoid infiltrates at their bases and sides with small subepidermal clefts and few colloid bodies. The interfollicular papillary dermis shows markedly thickened vertically oriented bundles of collagen, evidence of repeated rubbing of the lesion [Figure - 10]a and b.
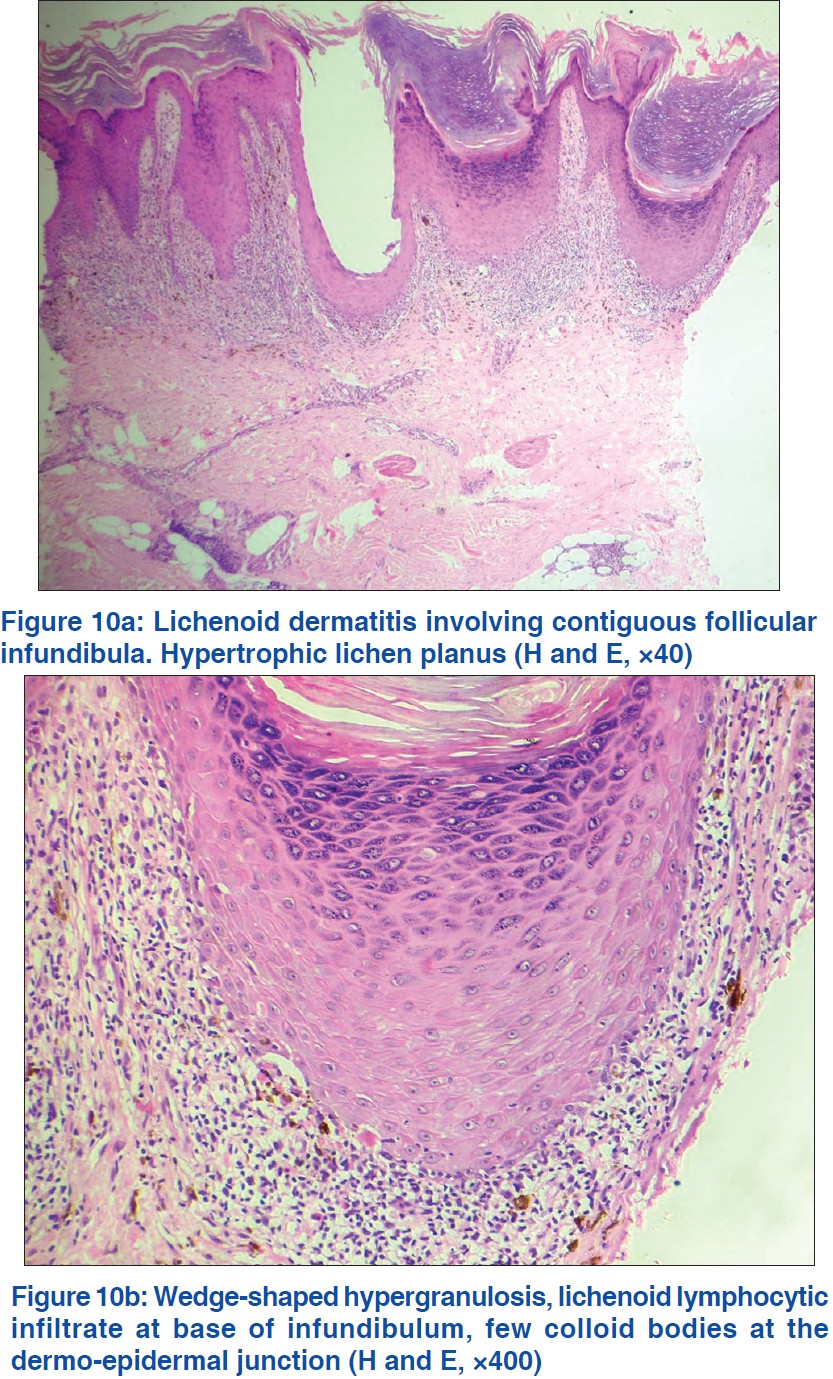 |
| Figure 10 |
Mucosal LP shows features similar to skin lesions; however, in some cases, interface changes may not be seen and the dermal infiltrate is not typically lichenoid because the papillary dermis does not exist in the oral mucosa.
Follicular LP affecting the scalp results in cicatricial alopecia and may progress to the clinical condition called pseudopelade. In early lesions, the histology is typical of LP with lichenoid lymphocytic infiltrate and flask-like dilated follicular infundibula filled with compact keratin. Late cases show loss of follicles and vertical fibrotic tracts in the dermis that represent sites of destroyed follicles.
LP pigmentosus and Ashy dermatosis are probably related or similar conditions that some consider as part of the spectrum of LP. While uncommon in the West, it is a common, cosmetically disfiguring condition in India and South Asia. Histologically, it shows interface dermatitis that is usually of the vacuolar-interface variety unlike typical LP. Numerous melanophages are present in the papillary dermis. Mild basal cell vacuolization and clumps of colloid bodies in the upper papillary dermis are sometimes seen [Figure - 11].
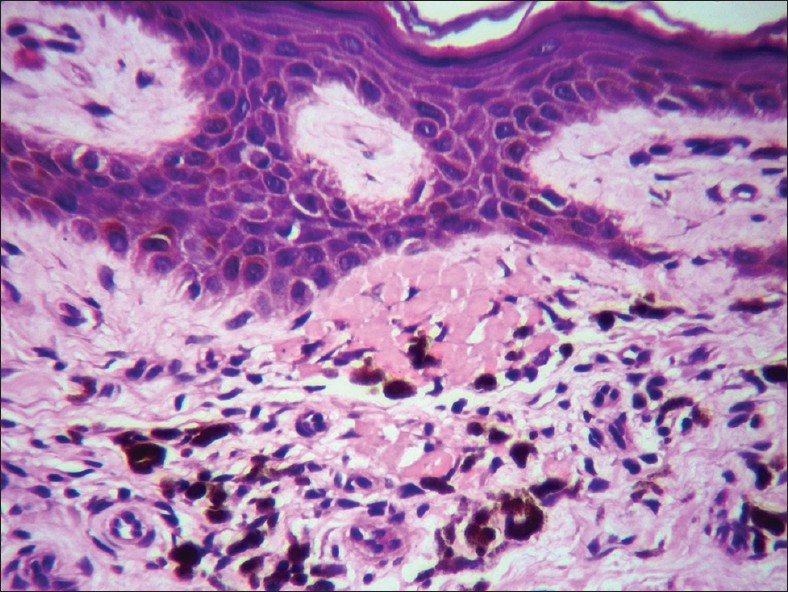 |
| Figure 11: Clumps of numerous colloid bodies in the upper papillary dermis with numerous melanophages. Lichen planus pigmentosus (H and E, ×400) |
Differential diagnoses
- Lichenoid drug eruptions: Eruptions that are both clinically and histologically similar to LP can be induced by drugs or by occupational exposure to chemicals. Lichenoid drug eruptions generally show parakeratosis, numerous individually necrotic keratinocytes scattered in the mid- and upper spinous layers, and presence of eosinophils and sometimes plasma cells in the dermal infiltrate. A deep perivascular infiltrate of lymphocytes is also usually seen unlike in true idiopathic LP.
- Lichenoid Graft vs. Host reaction: Early lesions are very similar to LP both clinically and histologically. In graft vs host reactions, while the epidermal changes mirror LP, the dermal infiltrate is generally sparse and does not align in a lichenoid pattern. In late lesions, dermal sclerosis usually supervenes and can be used to distinguish the two conditions.
- Lichenoid DLE: It can resemble LP and can only be reliably differentiated by DIF.
Erythema multiforme
This is considered as the prototype of "acute cytotoxic interface dermatitis." Findings similar to EM are seen in Stevens Johnsons syndrome (SJS) and TEN. These three conditions are believed to form a spectrum of disease with similar pathogenetic mechanisms [Figure - 12].
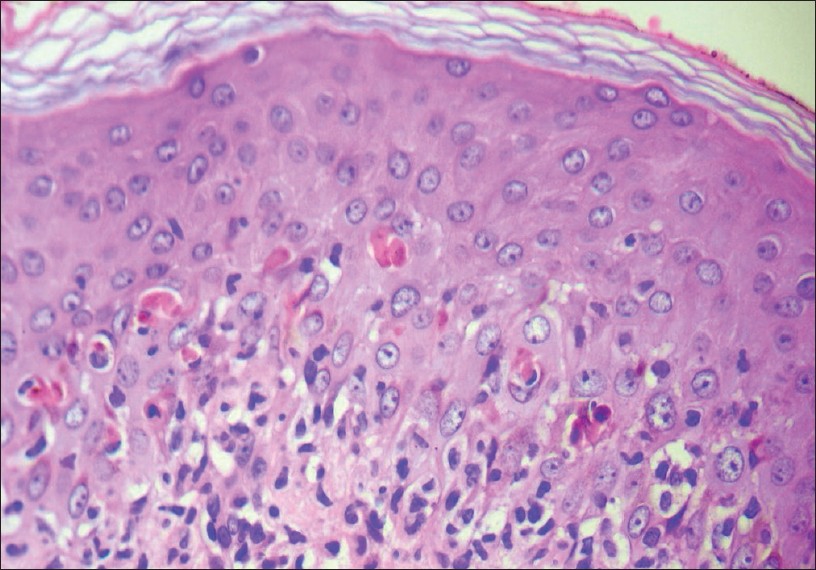 |
| Figure 12: Numerous necrotic keratinocytes scattered in the lower spinous zone with lymphocytes obscuring the dermo-epidermal junction. Note the normal basket weave stratum corneum. Erythema multiforme (H and E, ×200) |
Early lesions show a sparse to moderately dense superficial perivascular infiltrate of lymphocytes. Few lymphocytes ascend into the basal cell layer and induce basal cell vacuolization resulting in individual cell necrosis. Numerous apoptotic keratinocytes are seen scattered at various levels throughout the epidermis and may also be seen aggregated in concentric array within eccrine acrosyringia giving rise to a clump of necrotic keratinocytes in the upper spinous zone. [6]
There may be associated mild spongiosis and some ballooning of individual spinous keratinocytes. These latter changes are more often seen in lesions that develop bullae and in the more severe expressions of Stevens Johnson syndrome and TEN, where confluent epidermal necrosis is the norm along with separation of the necrotic epidermis from the papillary dermis. Changes similar to early EM are often seen at the periphery of necrotic areas in TEN and SJS.
The epidermal damage even if extensive does not affect keratinization and while there is mild to moderate epidermal hyperplasia, the stratum corneum retains its normal basket weave pattern without any parakeratosis.
Follicular involvement is not uncommon in EM and affected follicular infundibula show basal cell vacuolization and scatter of necrotic keratinocytes in the spinous layers.
Interestingly, the density of the dermal inflammatory infiltrate is inversely proportionate to the epidermal damage. Being fairly dense in typical targetoid lesions of EM, it is very sparse or even absent in TEN. Eosinophils are not seen as a rule even if the condition is drug induced and helps to differentiate from FDEs which may show very similar histological findings but always are associated with eosinophils.
Differential diagnoses
- FDE: While the epidermal changes may resemble those seen in EM, the dermal infiltrate contains numerous eosinophils and neutrophils. In recurrent FDE, numerous melanophages are seen in the moderately thickened and fibrotic papillary dermis.
- Early PLEVA: Findings that support a diagnosis of PLEVA vs EMF are small mounds of parakeratosis that may house few neutrophils and a superficial and deep wedge-shaped perivascular lymphocytic infiltrate.
- Acute graft vs. host reaction: Acute GVHD has a superficial perivascular lymphocytic infiltrate, vacuolar changes of basal cells, and individual and clustered necrotic keratinocytes in the spinous layers and can be extremely difficult to differentiate from EMF. However, acute GVHD shows compact keratin often with small mounds of parakeratosis. "Satellite cell necrosis," i.e., finding of lymphocytes closely associated with a necrotic keratinocyte in the spinous layers has in the past been described as pathognomonic of GVHD, but this is fallacious as it can be seen in other interface dermatitis.
Pityriasis lichenoides (PLEVA and Pityriasis lichenoides chronica)
Although both PLEVA and pityriasis lichenoides chronica (PLC) are considered to be variants of the same disease, namely, Mucha Haberman disease. The histological findings are different and will be considered separately.
PLEVA in early lesions shows superficial and deep perivascular wedge-shaped lymphocytic infiltrates with vacuolar changes in the dermo-epidermal junction. As lesions evolve, the epidermis becomes hyperplastic and the density of the dermal infiltrate increases to become lichenoid.
Scatter of individually necrotic keratinocytes as in EM may be seen throughout the spinous layers and the thickened horny layer shows foci of parakeratosis with few neutrophils. The upper spinous keratinocytes may show ballooning and appear pale and the granular layer is usually deficient beneath the mound of parakeratosis. The upper dermis has numerous extravasated erythrocytes.
PLC, on the other hand, usually shows only a superficial perivascular lymphocytic infiltrate with very focal mild basal cell vacuolization which may not be evident in well-developed or resolving lesions. In the absence of interface changes, stratum corneum changes may be used to make a definite diagnosis. [7] The granular layer is well formed and there is a thick laminated stratum corneum that has foci of thin flat parakeratosis without neutrophils and with flecks of melanin. The prominently thick horny layer with focal parakeratosis contributes to the "wafer-like" scale seen clinically in lesions of PLC. Extravasated erythrocytes may be seen in the upper papillary dermis and even within the lower and mid-spinous layers but are not diagnostic for this condition [Figure - 13].
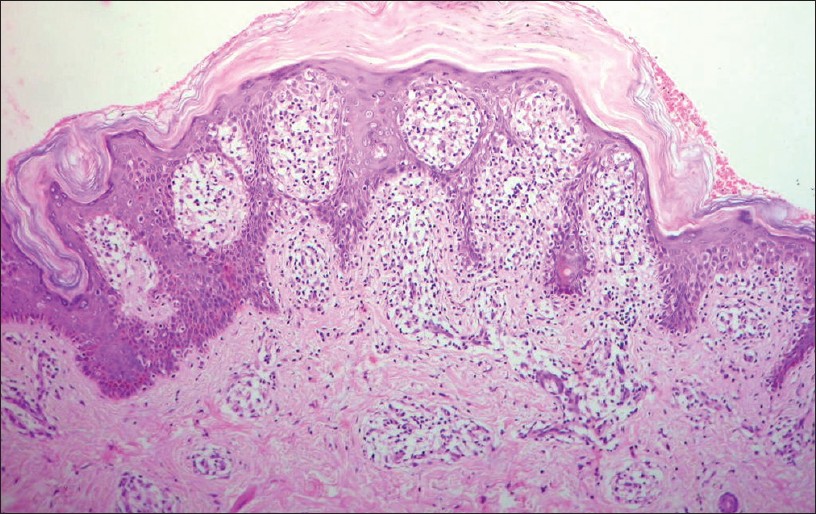 |
| Figure 13: Lichenoid-interface dermatitis. Note the thick wafer-like scale containing flat parakeratosis and flecks of melanin. Pityriasis lichenoides chronica (H and E, ×100) |
Differential diagnoses
- Guttate psoriasis: Guttate psoriasis shares with PLEVA the presence of small parakeratotic mounds with neutrophils but does not show any interface changes.
- Pityriasis rosea: It is a spongiotic dermatitis, but in developed lesions when the spongiosis is minimal or absent may be difficult to differentiate from PLC.
- Lymphomatoid papulosis: It shows denser dermal infiltrates of pleomorphic lymphoid cells along with many eosinophils as well as neutrophils. Interface changes are minimal as are necrotic keratinocytes.
Lichen striatus
Lichen striatus combines several patterns of inflammation all of which may not be seen in individual lesions. Although classified as a primary interface dermatitis, it often shows psoriasiform hyperplasia of the epidermis with foci of mild to moderate spongiosis with few scattered individually necrotic keratinocytes in the spinous layers. Small mounds of parakeratosis are often seen in the thickened stratum corneum. The interface changes in well-developed lesions may be minimal and a diagnosis of psoriasiform or spongiotic dermatitis may be rendered.
A finding that is quite characteristic of lichen striatus is the presence of a fairly dense peri-eccrine and periadnexal infiltrate of lymphocytes. [8] At times, this finding alone may serve to differentiate lichen striatus from other eczematous dermatoses.
Graft vs. host reaction
Acute GVHD is quite similar to EM with vacuolar alteration of the basal cells and a sparse to moderately dense lymphocytic infiltrate. The stratum corneum, however, is usually thickened and compact or laminated. The lichenoid variety of GVHD which may precede the sclerodermoid changes often shows a lichenoid infiltrate with a prominent granular layer in the acanthotic epidermis.
Fixed drug eruptions
The histology varies depending on the site biopsied. Early dusky red edematous plaques (of recurrent FDE) will show changes very similar to EM but in addition also show eosinophils and neutrophils in the dermal infiltrate and numerous melanophages in the papillary dermis. A biopsy of a necrotic area or of a blister shows changes similar to TEN/SJS with confluent epidermal necrosis and a sparse infiltrate of lymphocytes and eosinophils in the upper dermis. Biopsies of late lesions which clinically show dark black pigmentation show "dermal melanosis" with numerous melanophages in the papillary dermis without any diagnostic epidermal findings.
Diseases Where Interface Changes may be Seen but are not Primarily Considered as Interface Dermatitis
A big list of diseases which includes mycosis fungoides, urticarial pemphigoid, lichen sclerosus, lichenoid purpura, secondary syphilis, lichen nitidus, poikiloderma due to various causes, leprosy, and even neoplastic diseases like LPLK, SCC, BCC, regressing melanomas, etc., do show variable interface changes.
The interface changes in these conditions are always secondary and do not form part of the histological criteria used for diagnosis of that particular disease. In practice, findings of interface changes in these conditions only serve to confuse and confound the histopathologist.
Summary
Interface dermatitis refers to diseases in which the primary pathology is related to the dermo-epidermal junction and is reflected by morphological changes involving the basal cells, the dermo-epidermal junction, and the papillary dermis. Secondary changes of the epidermis are helpful for differentiating the various diseases that show interface dermatitis.
A long list of diseases shows interface changes; however, LE in its various clinical and histological presentations, LP and variants including lichenoid drug eruptions, EM, TEN/SJS and FDEs, dermatomyositis, GVHD, and pityriasis lichenoides, both PLEVA and PLC are considered major interface dermatitides.
Less commonly biopsied entities like morbilliform drug and viral eruptions and eruptions associated with lymphocyte recovery and skin damaged by chemotherapy or radiation are also primary interface dermatitis.
It is important for the clinician and the histopathologist to be aware that interface changes may be seen in many other diseases, infective, inflammatory, and neoplastic, to avoid mistakes in diagnoses of those conditions.
Algorithmic approach to diagnosis of interface dermatitis based on epidermal changes:
- Interface dermatitis with acute cytotoxic changes: [3]
- Few necrotic keratinocytes: Early EM, morbilliform drug and viral eruptions, eruption of lymphocyte recovery.
- Numerous necrotic keratinocytes: Fully developed EM, acute LE, TEN, radiation- and chemotherapy-induced skin damage, FDE (eosinophils, neutrophils, and melanophages), pityriasis lichenoides (parakeratosis).
- Interface dermatitis with premature terminal differentiation: [3]
- Dense lymphocytic infiltrates: LP, lichenoid keratosis, lichenoid drug reaction especially photolichenoid, acute GVHD, DLE, lichen striatus.
- Few lymphocytes: Dermatomyositis, lichenoid GVHD.
- Mixed infiltrates: Lichenoid drug reaction (eosinophils), keratosis lichenoides chronica (plasma cells).
- Interface dermatitis with psoriasiform hyperplasia: [3]
- Lymphocytes and siderophages: Lichenoid purpura.
- Eosinophils predominant: Urticarial pemphigoid, some drug eruptions.
- Lymphocytes mostly: Mycosis fungoides, lichen striatus, pityriasis lichenoides, early lichen sclerosus, center of porokeratosis.
- Plasma cells: Secondary syphilis, early acrodermatitis chronica atrophicans.
- Interface dermatitis with irregular epidermal hyperplasia: [3]
- Hypertrophic LP.
- Verrucous DLE.
- Some drug eruptions.
- Interface dermatitis with epidermal atrophy: [3]
- Plasma cells: Late stage of acrodermatitis chronica atrophicans.
- Band of melanophages: Regressing malignant melanoma, late pigmented patches of FDE.
- Lymphocytic infiltrate: Atrophic LP, long-standing lesions of LE, dermatomyositis, poikiloderma of any etiology, atrophic lesions of lichen sclerosus, center of lesion of porokeratosis.
| 1. |
Langley RG, Walsh N, Nevill T, Thomas L, Rowden G. Apoptosis is the mode of keratinocyte death in cutaneous graft-versus-host disease. J Am Acad Dermatol 1996;35:187-90.
[Google Scholar]
|
| 2. |
Ackerman AB, Boer A, Bennin B, Gottlieb G. Histologic Diagnosis of Inflammatory Skin Diseases: An Algorithmic Method Based on Pattern Analysis. 3 rd ed. New York: Ardor Scribendi; 2005.
[Google Scholar]
|
| 3. |
Philip Le Boit. Dermatitis involving the dermo-epidermal junction. In: Maize JC, Burgdorf WH, Hurt MA, LeBoit PE, Metcalf JS, Smith T, et al., editors. Cutaneous Pathology. Philadelphia: Churchill Livingstone; 1998. p. 87-145.
[Google Scholar]
|
| 4. |
Leboit PE. Interface dermatitis. How specific are its histopathologic features?. Arch Dermatol 1993;129:1324-8.
[Google Scholar]
|
| 5. |
Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: Redefinition of diagnostic criteria in the recent World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med 2009;133:303-8.
[Google Scholar]
|
| 6. |
Zohdi-Mofid M, Horn TD. Acrosyringeal concentration of necrotic keratinocytes in erythema multiforme: A clue to drug etiology. Clinicopathologic review of 29 cases. J Cutan Pathol 1997;24:235-40.
[Google Scholar]
|
| 7. |
Joshi R. Stratum corneum findings as clues to histological diagnosis of pityriasis lichenoides chronica. Indian J Dermatol Venereol Leprol 2008;74:156-7.
[Google Scholar]
|
| 8. |
Gianotti R, Restano L, Grimalt R, Berti E, Alessi E, Caputo R, et al. Lichen striatus-a chameleon: An histopathological and immunohistological study of forty-one cases. J Cutan Pathol 1995;22:18-22.
[Google Scholar]
|
Fulltext Views
72,011
PDF downloads
11,153





