Translate this page into:
Paclitaxel induced systemic lupus erythematosus in a case of cervical carcinoma
Correspondence Address:
Swapnil A Sanghavi
Department. of Skin and VD, Seth G S Medical College and KEM Hospital, Parel, Mumbai-12, Maharashtra
India
| How to cite this article: Sanghavi SA, Chikhalkar S, Khopkar US. Paclitaxel induced systemic lupus erythematosus in a case of cervical carcinoma. Indian J Dermatol Venereol Leprol 2013;79:537-539 |
Sir,
Paclitaxel (taxol) is a drug derived from the bark of the Pacific yew tree and is widely used in cancer treatment, especially for breast, ovarian, and lung cancers. [1] Paclitaxel induced lupus erythematosus is a rare adverse effect, with only a few cases described in literature. We report a case of systemic lupus erythematosus developing secondary to paclitaxel use for cervical carcinoma.
A fifty five year old female presented with red itchy lesions over face, dorsal aspect of both hands and feet since 5 days. On inquiry, she also had photosensitivity and pain in both knees. She gave no history of chest pain, palpitations, difficulty in breathing, neurological complaints or hematuria. Patient was diagnosed as a case of cervical carcinoma and had undergone hysterectomy with bilateral salpingo-oophorectomy 2 months back. She had recieved 2 cycles of chemotherapy with paclitaxel and carboplatin, the 1st cycle starting 1 and ½ months back.
Physical examination revealed the presence of erythematous to dusky patches over both cheeks and the nose [Figure - 1], dorsal aspect of both hands and feet [Figure - 2]. Oral cavity revealed the presence of erosions over the palate, gingivae and the buccal mucosa. Both knee joints showed swelling with tenderness on movement. An X-ray examination detected nonerosive arthritis in both knee joints.
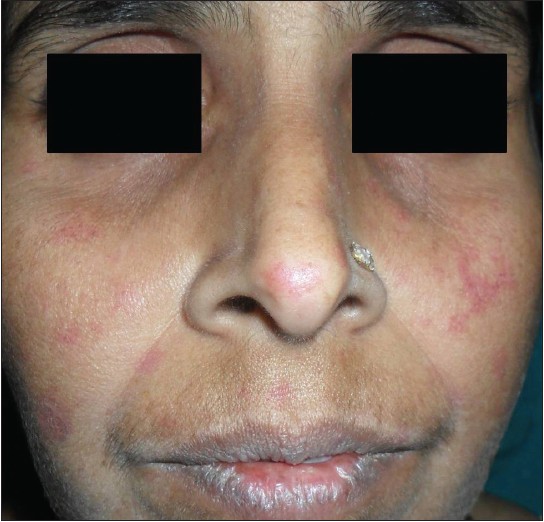 |
| Figure 1: Erythematous patches over both cheeks and nose |
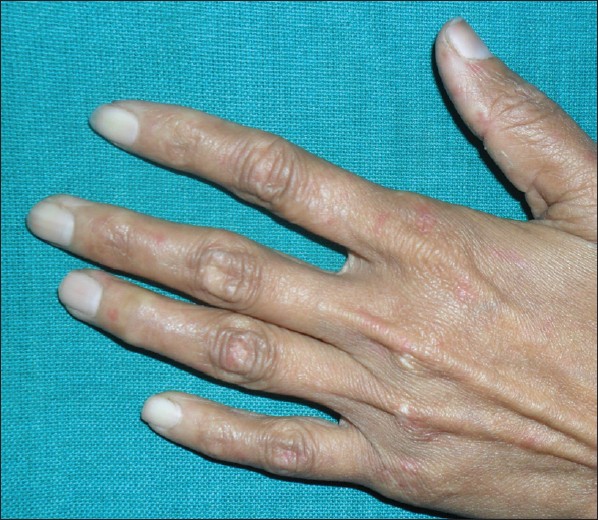 |
| Figure 2: Erythematous patches over dorsal aspect of hands |
Complete blood count examination was normal except for the presence of normocytic normochromic anemia (Hb 8.3 gm/dl). ESR was 70 mm/min. C3 and C4 levels were within normal limits. High titres of antinuclear (ANA titres of 1:320) and anti-histone antibodies were present. Anti double stranded DNA antibodies were negative. Urine analysis was normal.Biopsy from the lesion on the face showed epidermal flattening, vacuolar interface changes in the basal layer of the epidermis along with melanin incontinence. The superficial and mid dermis showed the presence of perivascular and periappendageal lymphocytic infiltrate [Figure - 3]. DIF could not be performed due to non affordability.
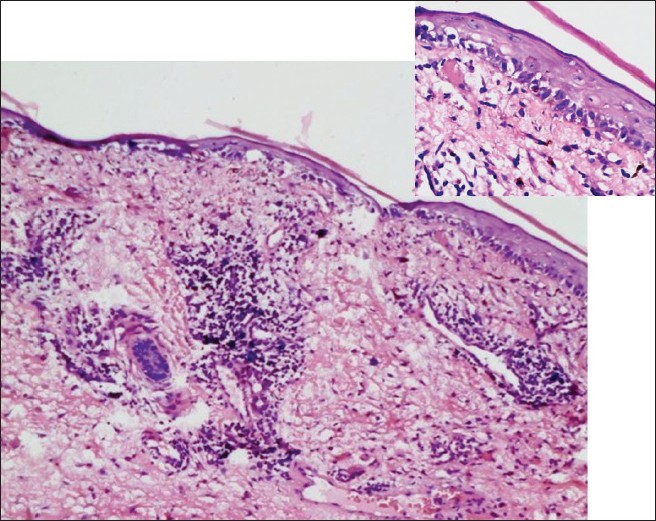 |
| Figure 3: Biopsy showing atrophic epidermis, vacuolar interface changes at DE junction (inset) and perivascular and periappendageal lymphocytic infiltrate in the superficial and mid dermis (H and E, ×100) |
Paclitaxel was discontinued and her cutaneous symptoms disappeared within 3 weeks.
Paclitaxel is a recent antineoplastic agent belonging to the taxane family that acts by promoting the polymerisation of mitotic tubulin and stabilisation of the resulting microtubules. Its activity has been demonstrated in advanced and refractory genital, breast, lung, and head and neck cancers. It has been implicated in various cutaneous adverse effects like onycholysis, acral erythema, erythema multiforme, SJS, bullous fixed drug eruption, and pustular eruption. [1]
Both paclitaxel and docetaxel have been implicated in the causation of auto immune disorders. In 2003, Kupfer et al. [2] first reported scleroderma-like changes in a patient ten days after paclitaxel administration. In 2007, Adachi and Horikawa, [3] presented the first two cases of cutaneous lupus erythematosus due to paclitaxel use in breast cancer patients. Both patients were cases of Sjogren′s syndrome and had high titres of Anti SSA/ Ro antibodies. Lortholary et al. [4] , also in 2007, reported a case of paclitaxel induced cutaneous lupus erythematosus in a woman with ovarian cancer. Till date, only one case of paclitaxel induced systemic lupus erythematosus has been reported in literature. In 2008, C A Dasanu et al. [1] reported a case of SLE developing in a woman with ovarian cancer following treatment with paclitaxel and carboplatin. ANA, anti ds DNA and anti-histone antibodies were all positive.
For the diagnosis of drug induced LE, the following factors should be preliminarily confirmed. [5]
- The patient has 1 or more clinical symptoms of SLE
- Antinuclear antibodies are present
- The patient had no history of SLE before using the culprit drug
- The drug was taken anytime from 3 weeks to 2 years prior to the appearance of symptoms
- Clinical improvement is rapid when the drug is discontinued, whereas antinuclear antibodies and other serologic markers slowly decrease toward more normal levels.
Our case had malar rash, oral ulcers, arthritis and positive ANA, thus fulfilling four of the SLICC classification criteria for diagnosis of SLE. [6] Also the presence of anti-histone antibodies along with the fact that the symptoms cleared rapidly with the withdrawal of the drug suggest the diagnosis of drug induced lupus erythematosus.
The differential diagnosis includes drug induced photosensitivity reaction. However absence of systemic features, autoantibodies and suggestive histopathology can easily differentiate it from lupus erythematosus.
Till date, no case of lupus, either cutaneous or systemic, has been attributed to or associated with carboplatin, an agent that has been used extensively for a few decades, thus excluding it as a potential contributing agent in this case. To the best of our knowledge this is the 1st reported case of paclitaxel induced SLE in Indian literature.
| 1. |
Dasanu CA, Alexandrescu DT. Systemic lupus erythematosus associated with paclitaxel use in the treatment of ovarian cancer. South Med J 2008;101:1161-2
[Google Scholar]
|
| 2. |
Kupfer I, Balguerie X, Courville P, Chinet P, Joly P. Scleroderma-like cutaneous lesions induced by paclitaxel: A case study. J Am Acad Dermatol 2003;48:279-81
[Google Scholar]
|
| 3. |
Adachi A, Horikawa T. Paclitaxel-induced cutaneous lupus erythematosus in patients with serum anti-SSA/Ro antibody. J Dermatol 2007;34:473-6.
[Google Scholar]
|
| 4. |
Lortholary A, Cary-Ten Have Dallinga M, El Kouri C, Morineau N, Ramée JF. [Paclitaxel-induced lupus]. Presse Med 2007;36:1207-8.
[Google Scholar]
|
| 5. |
Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res 2009;301:99-105.
[Google Scholar]
|
| 6. |
Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677-86.
[Google Scholar]
|
Fulltext Views
2,140
PDF downloads
763





