Translate this page into:
A case of cyclophosphamide-induced euprolactinemic galactorrhea
Corresponding author: Dr. Swagata Arvind Tambe, Department of Dermatology Venereology, Leprology, Seth V.C.Gandhi & M.A. Vora Municipal General Hospital, Mumbai, India. swagatatambe@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jangid KM, Tambe SA, Mahobia S, Siddiqui Khatoon Mohd Hanif S. A case of cyclophosphamide-induced euprolactinemic galactorrhea. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_535_2024
Dear Editor,
Galactorrhea is a nonpurulent milky discharge from the breast that occurs spontaneously or with manual expression, in the absence of pregnancy or the postpartum state1. It is the most common symptom of hyperprolactinemia, occurring in more than 90% of women with hyperprolactinemia1,2. Common causes of hyperprolactinemia include drugs like dopamine receptor antagonists, opioids, and verapamil, as well as tumours such as sellar and/or pituitary adenomas, hypothyroidism, renal insufficiency, and nipple stimulation2. Euprolactinemic galactorrhea refers to galactorrhea not accompanied by hyperprolactinemia. Here, we describe cyclophosphamide-induced galactorrhea with normal prolactin levels in a 55-year-old woman with pemphigus vulgaris3.
A 55-year-old married woman came to the hospital with multiple vesicles, bullae, and erosions all over her body that had developed over the past one and a half months. Due to these extensive skin erosions and oral cavity involvement, she was unable to eat and became generally weak. She was admitted to the intensive care unit for sepsis and fluid electrolyte imbalance. A diagnosis of pemphigus vulgaris was confirmed through a skin biopsy, direct immunofluorescence, and desmoglein antibody tests.
Once her sepsis resolved, she received two doses of rituximab (1 g, administered 15 days apart), which led to significant improvement in her skin condition. She was also started on prednisolone at a dose of 1 mg/kg, with a gradual tapering schedule, along with 40 mg of pantoprazole daily. One month after beginning oral steroids, cyclophosphamide 50 mg daily was added as a steroid-sparing agent. Four weeks later, she experienced fullness in both breasts and bilateral milky nipple discharge [Figure 1]. She underwent a thorough evaluation following these symptoms.
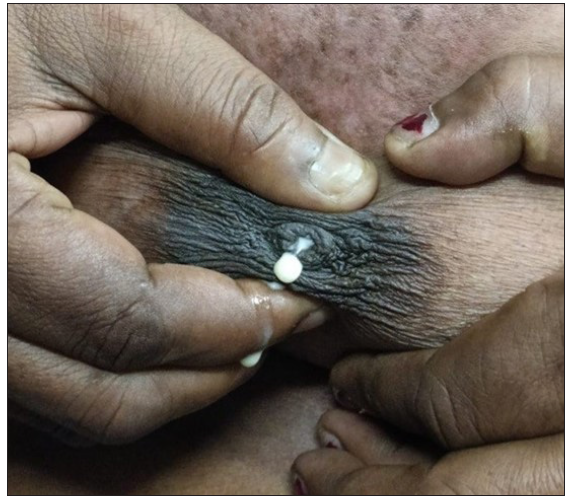
- Right breast showing milky nipple discharge.
Physical examination revealed no abnormalities. All her routine laboratory investigations, including renal function tests, were within normal limits. She had normal levels of prolactin (15.27 ng/mL, reference range: 2.8–29.2 ng/mL), cortisol (4.08 mcg/dL, reference range: 3.1–16.7 mcg/dL), luteinizing hormone (6.46 mIU/mL, reference range: 1.9–12.5 mIU/mL), and follicle-stimulating hormone (2.6 mIU/mL, reference range: 2.5–10.2 mIU/mL). Her thyroid profile was also normal. Magnetic resonance imaging of the brain was normal, as was an ultrasonography of the pelvis. She was referred to the gynaecology and endocrinology departments, but no issues were found. After ruling out all possible causes of galactorrhea with normal prolactin levels, she was diagnosed with cyclophosphamide-induced euprolactinemic galactorrhea. She was advised to stop taking cyclophosphamide, and mycophenolate sodium 360 mg was started. Prednisolone in tapering doses and pantoprazole 40 mg daily were continued until the resolution of all lesions. Withdrawal of cyclophosphamide led to a complete resolution of her symptoms without recurrence. Based on the Naranjo adverse drug reaction (ADR) probability scale, the adverse event appeared after the drug was administered, improved when the drug was discontinued, and no other possible causes were identified; the causality score was 5, indicating that the reaction was probably due to cyclophosphamide. Furthermore, the galactorrhea resolved within a few days of discontinuing cyclophosphamide.
Euprolactinemic galactorrhea is defined as galactorrhea occurring with normal prolactin levels. This condition has been reported with various drugs, including selective serotonin reuptake inhibitors, tricyclic antidepressants, and domperidone. There is also a single case report of azathioprine-induced euprolactinemic galactorrhea4.
The mechanism behind euprolactinemic galactorrhea remains unclear. It is hypothesised that increased tissue sensitivity to the metabolic and lactogenic effects of prolactin, as well as hyperresponsive 5-hydroxytryptamine type 1A receptors, could be potential explanations. In our case, serum prolactin levels were normal, and no other systemic cause for the galactorrhea was identified.
Cyclophosphamide is an immunosuppressive drug used alone or in combination with corticosteroids to treat pemphigus vulgaris and other autoimmune blistering disorders. It is also employed in the management of autoimmune connective tissue disorders such as lupus erythematosus, systemic sclerosis, dermatomyositis, vasculitis, and various systemic conditions. However, due to its adverse effect profile, cyclophosphamide is generally reserved for patients with severe dermatological conditions. Known adverse effects of cyclophosphamide include microscopic haematuria, haemorrhagic cystitis, myelosuppression (leukopenia, anaemia, and thrombocytopenia), amenorrhea, azoospermia, and ovarian failure5.
A literature search revealed only one case report of azathioprine-induced euprolactinemic galactorrhea4. To our knowledge, no cases of cyclophosphamide-induced euprolactinemic galactorrhea have been reported to date.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Prolactin secretion and biological activity in females with galactorrhoea and normal circulating prolactin concentrations at rest. Clin Endocrinol (Oxf). 1985;22:661-78.
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressant-induced hyperprolactinaemia: Incidence, mechanisms and management. CNS Drugs. 2010;24:563-74.
- [CrossRef] [PubMed] [Google Scholar]
- The aetiology of galactorrhoea in women with regular menstruation and normal prolactin levels. Hum Reprod. 1992;7:912-4.
- [CrossRef] [PubMed] [Google Scholar]
- A case report on azathioprine-induced euprolactinemic galactorrhea. Indian J Pharmacol. 2021;53:234-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclophosphamide in dermatology. Australas J Dermatol. 2017;58:5-17.
- [CrossRef] [PubMed] [Google Scholar]





