Translate this page into:
A case of drug-induced hypersensitivity syndrome induced by icotinib managed by intravenous immunoglobulin and systemic corticosteroids
Correspondence Address:
Li Li
Department of Dermatology, West China Hospital of Sichuan University, No. 37 Guoxue Lane, Chengdu
People's Republic of China
| How to cite this article: Chen X, Wang S, Li L. A case of drug-induced hypersensitivity syndrome induced by icotinib managed by intravenous immunoglobulin and systemic corticosteroids. Indian J Dermatol Venereol Leprol 2018;84:350-352 |
Sir,
Drug-induced hypersensitivity syndrome is a rare and life-threatening skin disease. There is a paucity of literature about epidermal growth factor receptor inhibitors associated drug-induced hypersensitivity syndrome. Here we report a case of drug-induced hypersensitivity syndrome induced by icotinib, a tyrosine kinase inhibitor of epidermal growth factor receptor inhibitors, successfully treated with intravenous immunoglobulin (IVIg) and systemic corticosteroids.
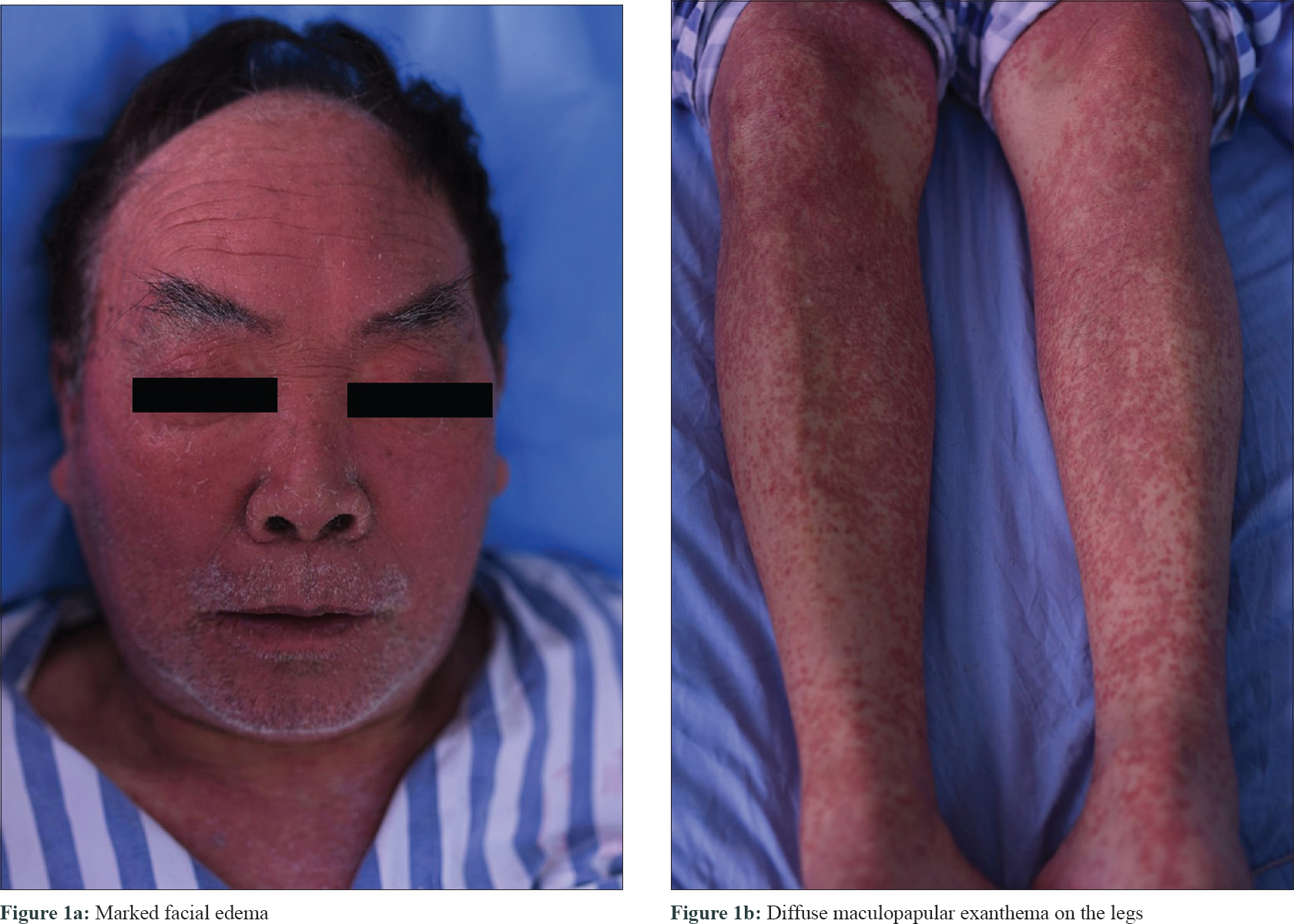
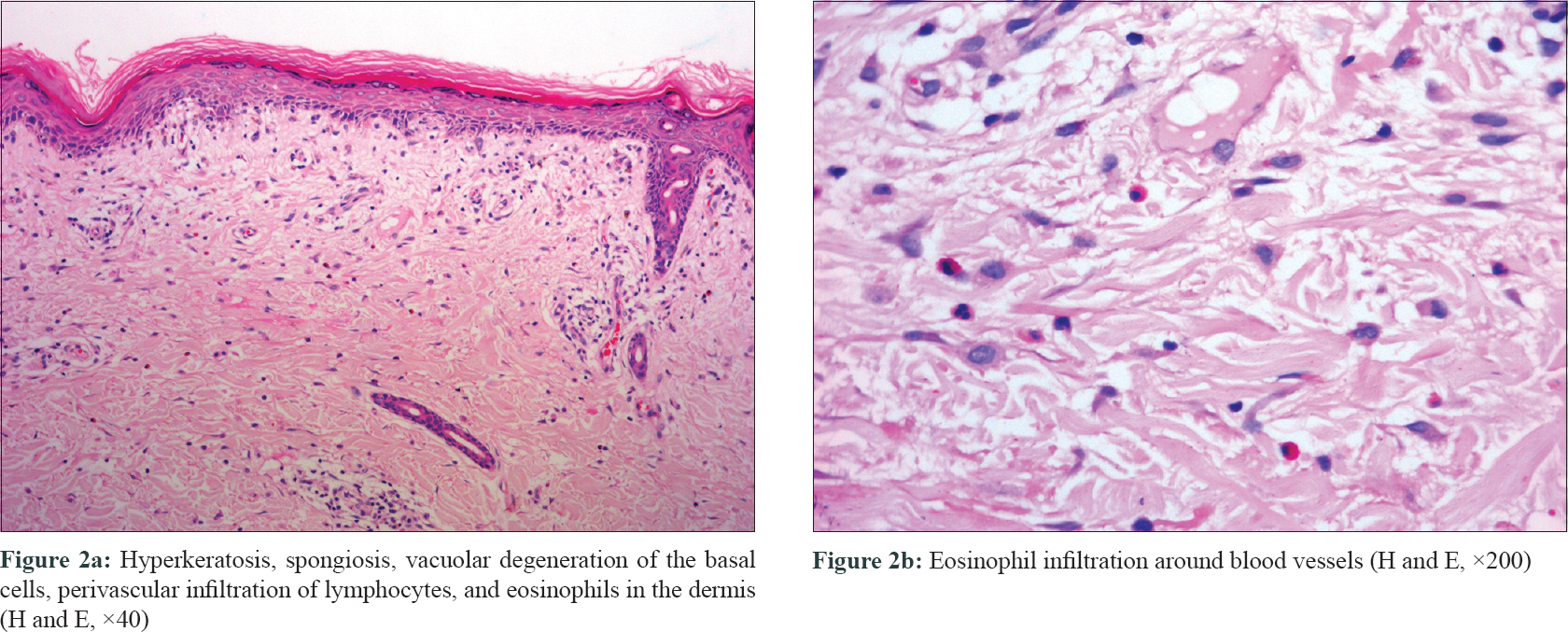
A 63-year-old Chinese man was diagnosed with nonsmall cell lung cancer and treated with icotinib (6.25 mg/kg/day) 1 month ago. Twenty-two days after starting icotinib, the patient presented with high fever (up to 40°C) and generalized maculopapular exanthema. High fever was relieved after use of methylprednisolone (1.5 mg/kg/day) for 5 days, but skin rash aggravated. Physical examination revealed marked facial edema [Figure - 1]a, generalized maculopapular exanthema on the trunk and limbs [Figure - 1]b, without lymphadenopathy or oral mucosal involvement. Laboratory examination showed leukocytosis (26.43 × 109/L) and eosinophilia (11.60 × 109/L). Liver function tests were also abnormal, with elevated aspartate aminotransferase (103 U/L, Normal value <40 U/L) and alanine aminotransferase (324 U/L, Normal value <50 U/L). No abnormal lymphocyte in peripheral blood smear, immunologic abnormality (Normal serum immunoglobulins IgG, IgA, IgM, IgE, and B lymphocyte count), or renal dysfunction were detected. Blood culture, viral serologies for hepatitis B and C, Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus were negative. Histopathology showed hyperkeratosis, spongiosis, vacuolar degeneration of the basal cells in the epidermis, and perivascular infiltration of lymphocytes and eosinophils in the dermis [Figure - 2].
 |
| Figure 1 |
 |
| Figure 2 |
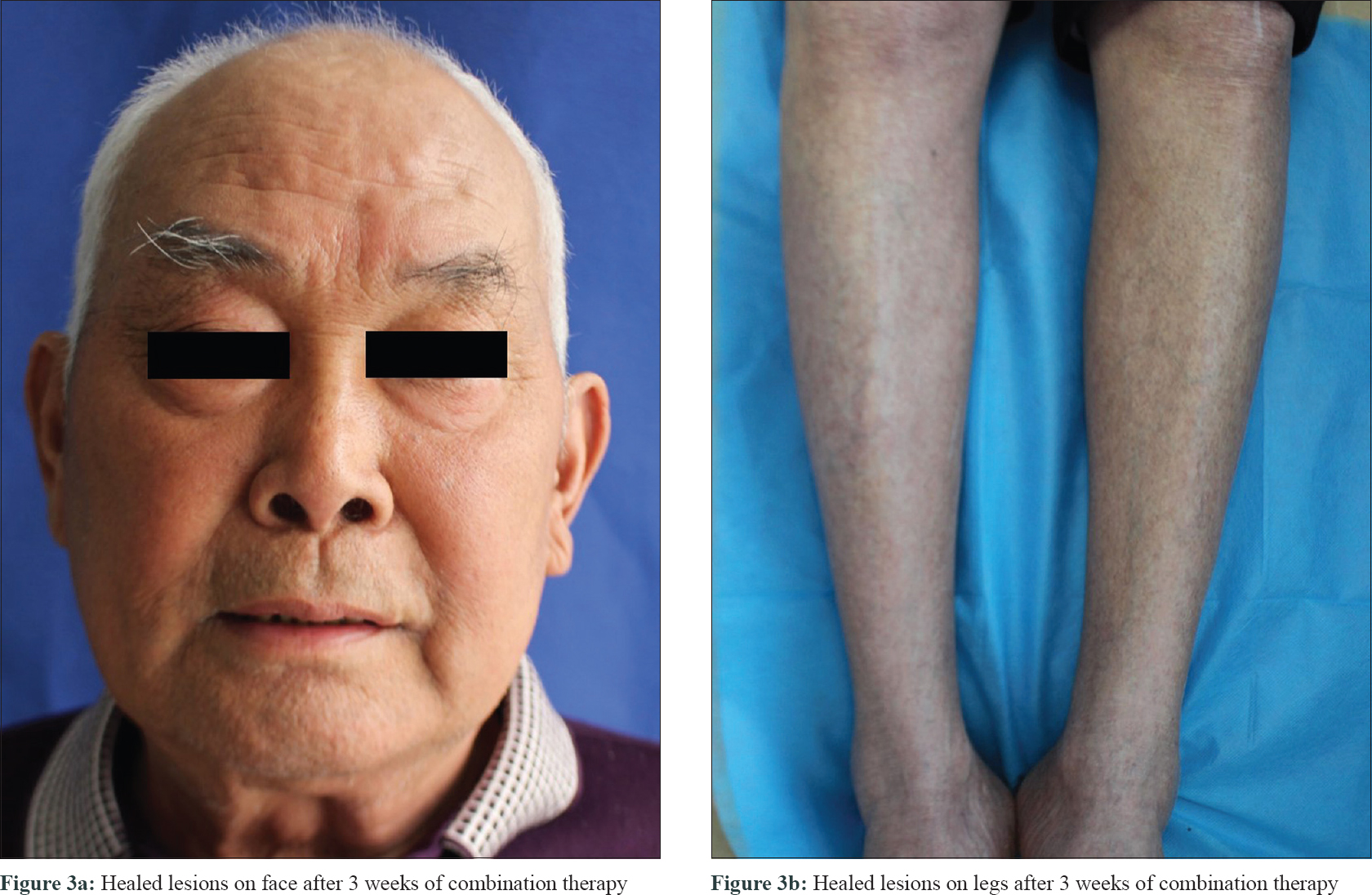
The RegiSCAR [1] score was estimated to be 5, which supported the diagnosis of drug-induced hypersensitivity syndrome. It was probably induced by icotinib according to the criteria of the Naranjo adverse drug reaction probability scale. Icotinib was immediately discontinued. Given the patient's advanced age, history of malignancy, and poor response to high-dose methylprednisolone, treatment was augmented with IVIg (0.5g/kg/d, for 5 days). The results of leukocytosis, eosinophilia, aspartate aminotransferase, and alanine aminotransferase returned to the normal range in 1 week. The methylprednisolone dose was tapered every 3–5 days. Most of the skin lesions disappeared after 3 weeks [Figure - 3], and methylprednisolone was discontinued after 6 weeks. There has been no clinical evidence of recurrence during the 3-month follow-up period.
 |
| Figure 3 |
Drug-induced hypersensitivity syndrome is a rare example of drug hypersensitivity reaction. Clinical features include extensive rash, high fever, lymphadenopathy, hepatitis, hematologic abnormalities with eosinophilia and atypical lymphocytes, and may involve damage in several other systems, especially kidney, heart, lungs, and pancreas. Marked swelling of the face, scaling and crusting in seborrheic distribution and involvement of palms/soles, are clues to the diagnosis.[2]
There is still no international consensus on the diagnostic criteria for drug-induced hypersensitivity syndrome. At present, criteria adopted by the European RegiSCAR is regarded as the best one to meet the needs in the diagnosis of drug-induced hypersensitivity syndrome.[2] The presentation of our case meets the criteria of RegiSCAR with a score of 5, including fever ≥38.5°C, rash on more than 50% of body, eosinophilia and liver involvement. The detections of human herpes virus-6 and human herpes virus-7 could not be carried out due to resource constraints. Lymphadenopathy, which is common (70–75% of cases) in drug-induced hypersensitivity syndrome,[2] was not found in our patient. It could be due to the initiation of treatment in the early stage of drug-induced hypersensitivity syndrome.
The exact pathogenesis of drug-induced hypersensitivity syndrome remains unclear. It may be a multifactorial model related to specific drugs, altered immune response, sequential reactivation of herpes viruses, and some human leukocyte antigen alleles.[2]
The main drugs associated with drug-induced hypersensitivity syndrome include anticonvulsants, antidepressants, sulfonamides and sulfones, antiinflammatory drugs, angiotensin-converting enzyme inhibitors and beta-blockers.[2]
Icotinib is an oral epidermal growth factor receptor-tyrosine kinase inhibitor agent, which is approved by China Food and Drug Administration to treat nonsmall cell lung cancer and has been marketed in 2011. A few studies [3] demonstrated that rash, pruritus, and hand-foot syndrome could appear in patients treated with icotinib. But no severe cutaneous adverse effect has been reported before.
Little has been known about the mechanisms that underlie epidermal growth factor receptor inhibitors-associated cutaneous toxicities, although it is presumed to be caused by the inhibition of the follicular and interfollicular epidermal growth signalling pathways in the skin.[4]
Although systemic glucocorticoids are the mainstay treatment for drug-induced hypersensitivity syndrome, in serious conditions, other approaches, such as IVIg, cyclosporine and plasmapheresis, should be taken into consideration especially when patients are not responding to systemic steroids.[2] Studies conducted in 2012[5] suggested that IVIg must not be used as a single treatment in drug reaction with eosinophilia and systemic symptoms due to poor benefit and increased risk of harms. Combination therapy of IVIg and systemic glucocorticoids was applied in our case, which turned out to be effective. It is unclear whether it was the IVIg, or combination of systemic steroid and IVIg that was responsible for the improvement in our case.
In conclusion, we reported herein a case of drug-induced hypersensitivity syndrome syndrome induced by epidermal growth factor receptor inhibitors to alert the potential rare serious adverse effects of icotinib. We also demonstrated that combination of IVIg and systemic glucocorticoids may be an effective method to treat this entity.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br J Dermatol 2007;156:609-11.
[Google Scholar]
|
| 2. |
Criado PR, Criado RF, Avancini JM, Santi CG. Drug reaction with Eosinophilia and Systemic Symptoms (DRESS)/Drug-induced Hypersensitivity Syndrome (DIHS): A review of current concepts. An Bras Dermatol 2012;87:435-49.
[Google Scholar]
|
| 3. |
Chen J, Zheng X, Liu DY, Zhao Q, Wu YW, Tan FL, et al. Therapeutic effects and adverse drug reactions are affected by icotinib exposure and CYP2C19 and EGFR genotypes in Chinese non-small cell lung cancer patients. Asian Pac J Cancer Prev 2014;15:7195-200.
[Google Scholar]
|
| 4. |
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006;55:657-70.
[Google Scholar]
|
| 5. |
Joly P, Janela B, Tetart F, Rogez S, Picard D, D'Incan M, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol 2012;148:543-4.
[Google Scholar]
|
Fulltext Views
2,710
PDF downloads
2,468





