Translate this page into:
A case of extranodal natural killer/T-cell lymphoma, initially misdiagnosed as erythema nodosum
Correspondence Address:
Kyung Eun Jung
Department of Dermatology, Chungnam National University Hospital, 282 Munhwa-Ro, Jung-Gu, Daejeon 301-721
South Korea
| How to cite this article: Lee JK, Hong D, Seo YJ, Jung KE. A case of extranodal natural killer/T-cell lymphoma, initially misdiagnosed as erythema nodosum. Indian J Dermatol Venereol Leprol 2020;86:715-718 |
Sir,
We present the case of a woman with cutaneous extranodal NK/T-cell lymphoma, that posed a diagnostic challenge because the early cutaneous form resembled erythema nodosum.
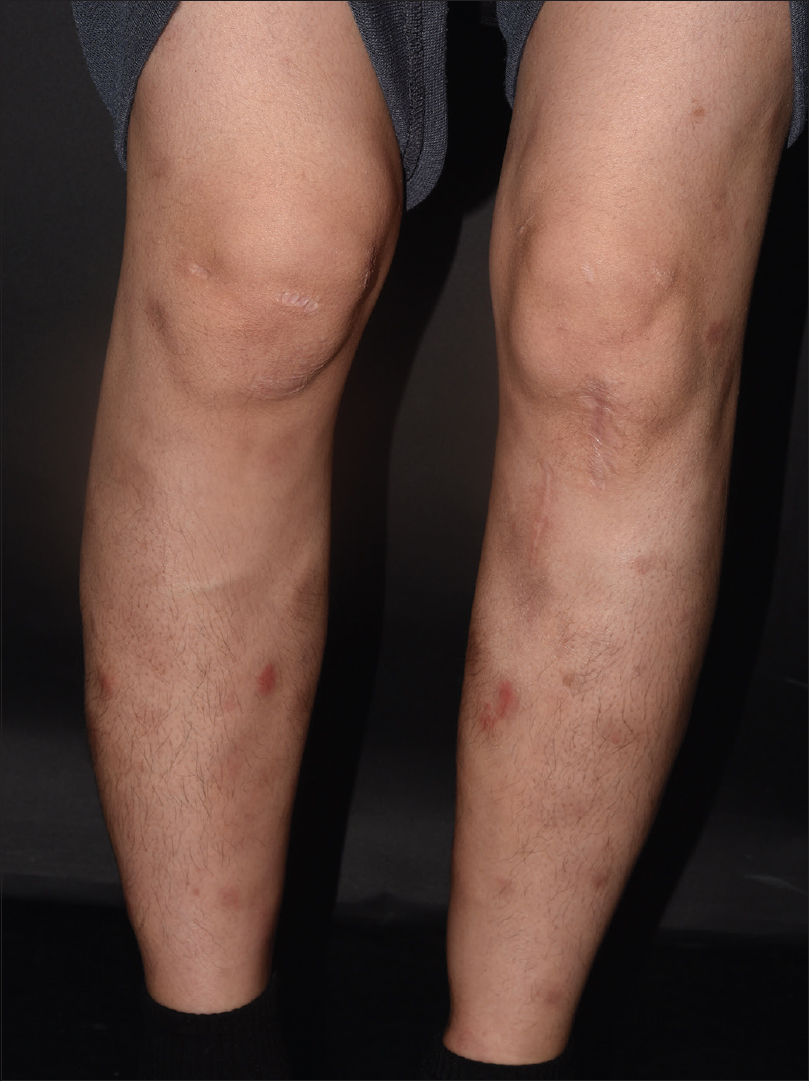
A 33-year-old woman presented with painful erythematous nodules on both pretibial areas that had developed 3 months ago[Figure - 1]. She had no prodromal symptoms and had history of neither oral nor genital ulcers. On the first visit, blood and urine examination and chest x-ray findings were normal. She had no relevant past medical history. Initial biopsy of a skin lesion revealed septal panniculitis with mixed inflammatory cells that had infiltrated the junctions of the septa and mild perivascular infiltration [Figure - 2]. Based on these clinical and histological data, we diagnosed erythema nodosum and prescribed oral methylprednisolone, colchicine and talniflumate. Despite taking these medications for 2 months (including an increase in the methylprednisolone dose), the lesions advanced to the trunk and showed aggravation with the development of ulceration on the previous leg lesions [Figure - 3]. The erythrocyte sedimentation rate was increased (54 mm/h), but all the other blood and urine tests were normal. Repeat biopsy of the lower leg lesion revealed prominent nodular infiltrations of atypical lymphocytes throughout the dermis and subcutaneous layer as well as angiocentric destruction caused by dense atypical infiltrates in the subcutis. The cells were positive for CD3, CD8, CD56 and Epstein-Barr virus by in-situ hybridization [Figure - 4]. A bone marrow biopsy revealed no evidence of malignant lymphoma, but a few atypical lymphocytes were observed in the peripheral blood smear. A computed tomographic scan of chest and pelvis showed some enlarged lymph nodes, without hepatosplenomegaly. The diagnosis of the patient was revised - to extranodal NK/T-cell lymphoma. The skin lesions exhibited remission, followed by resolution after 6 cycles of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide. However, the cancer relapsed after 6 months and the patient is currently on the waitlist for allogenic haematopoietic stem cell transplantation.
 |
| Figure 1: Erythema nodosum-like subcutaneous nodules on both anterior lower legs observed at the initial consultation |
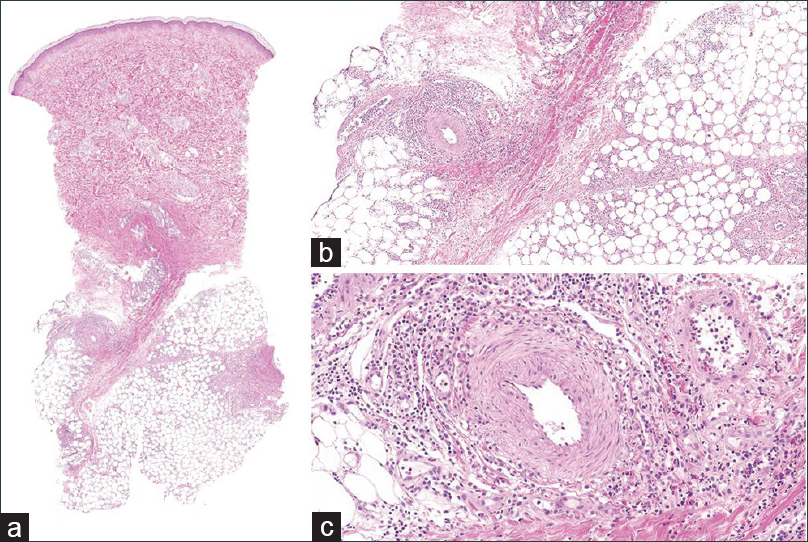 |
| Figure 2: (a)Scanner view of initial biopsy of a lower leg nodule (H and E, ×10). (b) Initial biopsy of a lower leg nodule, showing septal panniculitis with mixed inflammatory cells (H and E, ×40). (c) Initial biopsy of a lower leg nodule, showing mixed cell infiltration of a perivascular area without atypical cells (H and E, ×200) |
 |
| Figure 3: Persistent erythematous nodules and some lesions with ulceration on both anterior lower legs were observed, even after the patient received a 2 month course of oral steroids |
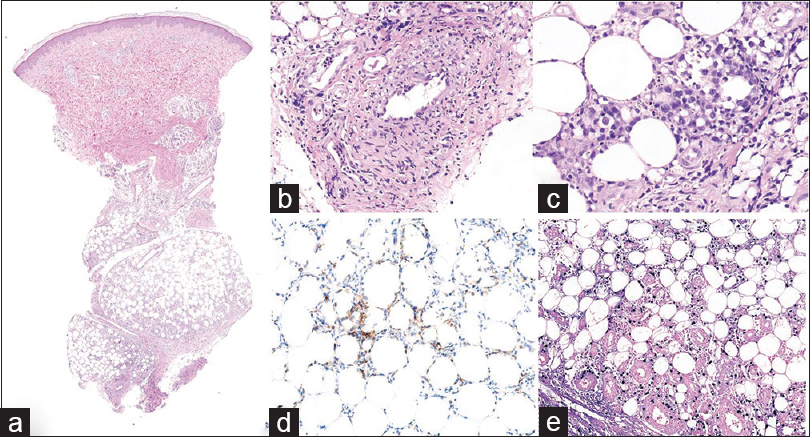 |
| Figure 4: (a)Scanner view of follow-up biopsy of a lower leg nodule (H and E, ×10). (b) Later biopsy revealed atypical lymphocytes infiltrating the vessels, destroying the endothelial structure and depositing fibrin (H and E, ×200). (c) Dense pleomorphic lymphocytes in the subcutaneous layer in the later biopsy (H and E, ×400). (d) Atypical lymphoid cells positive for CD3 in the later biopsy (CD3, ×200). (e) Atypical lymphoid cells positive for CD8 in the later biopsy (CD8, ×200) |
Immunohistochemical staining was performed, in retrospect, on the initial biopsy slides, which showed the lymphocytes in the dermis positive for CD3, CD4, CD8, and Epstein-Barr virus in-situ hybridization, but negative for CD56 [Figure - 5]. The lymphocytes expressing positivity for Epstein-Barr virus in-situ hybridization were sparse compared to that of the latter biopsy. Therefore, the result has shown that we had initially misdiagnosed our patient as erythema nodosum and that the early stage of the cutaneous extranodal NK/T-cell lymphoma may histologically present as a benign panniculitis.
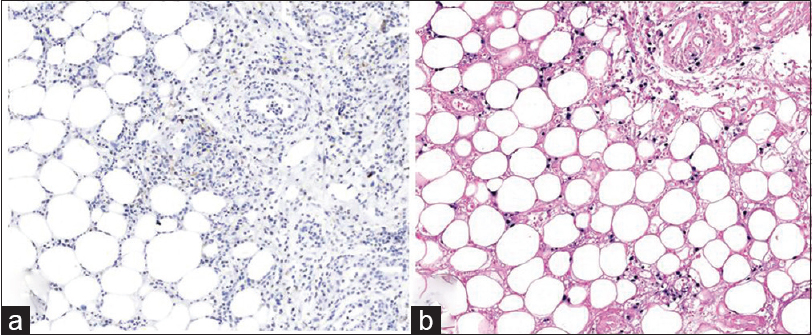 |
| Figure 5: (a) Retrospective examination of the initial biopsy showing lymphocytes positive for CD3 (CD3, ×200). (b) Retrospective examination of the initial biopsy showing lymphocytes positive for CD8 (CD8, ×200) |
Extranodal NK/T-cell lymphoma is a rare type of non-Hodgkin's lymphoma with a dismal prognosis and is most common in Asians and South Americans.[1] The tumor is poorly responsive to multi-agent chemotherapy and thus difficult to cure.[2] It is classified into 2 major groups based on the clinical features and outcomes: nasal type (the tumor originates in the nasal cavity) and extra-nasal type (the primary tumor can develop in the intestine, liver, testes, or skin).[1],[2],[3],[4] Cutaneous extranodal NK/T-cell lymphoma is the most common extra-nasal type and the lesions tend to be more aggressive, in comparison with isolated nasal lesions ;and are associated with worse prognosis.[2],[3]
Histopathology of cutaneous extranodal NK/T-cell lymphoma reveals infiltrations of pleomorphic tumor cells in a nodular or diffuse pattern throughout the dermis; the cells extend to the subcutaneous layer in an angiocentric/angiodestructive pattern.[1],[2] The cells typically express positivity for CD56 and cytoplasmic CD3, Epstein-Barr virus in-situ hybridization, and cytotoxic proteins.[1],[2],[3]
The histologic features of extranodal NK/T-cell lymphoma may be differentiated from that of other diseases such as lupus panniculitis, subcutaneous panniculitis-like T-cell lymphoma, and primary γδ cutaneous T-cell lymphoma, which all are depicted by lymphoid cells heavily infiltrating the lobular areas of the subcutis.[4],[5] The subcutaneous lymphoplasmocytic infiltrate with lymphoid follicles, hyaline necrosis of fat, deposit of mucins, and lymphocytes that are immunohistochemically positive for B lymphocytes are indicative of lupus panniculitis.[4] Furthermore, these patients may show positive serological features of lupus erythematosus.[4] Subcutaneous panniculitis-like T-cell lymphoma demonstrates an αβ T-cell phenotype, which expresses positivity for T-cell receptor βF1. It also expresses a CD3+, CD4-, CD8+ phenotype that is consistently negative for CD56 and Epstein-Barr virus in-situ hybridization.[4],[5] Primary cutaneous γδ T-cell lymphoma, which can be demonstrated by reactivity with an antibody to T-cell receptor gamma chain, show CD3+, CD2+, CD4-, CD5-, CD8- and CD56+ phenotype.[4],[5] Epstein-Barr virus in-situ hybridization reactivity is rarely positive in this case.[4],[5]
The clinical manifestations of cutaneous extranodal NK/T-cell lymphoma include cellulitis, abscess-like lesions, ulcers and subcutaneous nodules.[2],[6] Many of the patients with nodular features had involvement of more than one area on the body or isolated thigh lesions, often associated with other symptoms such as fever, during the initial presentation.[2] Furthermore, all of these patients were diagnosed with cutaneous extranodal NK/T-cell lymphoma, based on the typical histological findings.[1],[3] Our case is unique in that the patient initially presented with what seemed to be idiopathic benign subcutaneous nodules located only on both lower legs, without any other clinical manifestation. The initial histology revealed mixed cell infiltration and a lack of atypical cells within the thickened septa, panniculitis, or around the vessels, which is characteristic of erythema nodosum. When a follow-up skin biopsy was performed, both histological changes and obvious gross changes in the skin lesions were evident. Evolution of the skin lesions from erythematous subcutaneous nodules to ulcerative lesions was associated with increased vessel infiltration by atypical lymphocytes, which destroyed the endothelial structure and resulted in fibrin deposition.
Our initial clinical and pathological diagnosis of erythema nodosum can be explained on the basis that the patient may have exhibited paraneoplastic syndrome.[7] Although erythema nodosum is rarely associated with malignancy, it may be the first sign of Hodgkin's or non-Hodgkin's lymphoma and leukemia.[7] The precise pathogenesis of malignancy-related erythema nodosum remains enigmatic; however, reviews have suggested it may be related to altered immune system in response to the existing cancer cells.[7] However, our patient's evolution of the skin lesion from an erythema nodosum-like lesion to an ulcerative lesion and retrospective studies showing Epstein-Barr virus in-situ hybridization suggest we had initially misdiagnosed our patient as erythema nodosum.
The most distinguishing feature of the initial and latter biopsies was that the former biopsy showed lesser number of Epstein-Barr virus-positive cells than that of the latter biopsy. A possible explanation for the differences in the positivity for CD56 and the density of cells expressing positivity for Epstein-Barr virus in-situ hybridization, between the initial and latter biopsy may lie with the clinical characteristic of extranodal NK/T-cell lymphoma. A rapid aggressive lymphoma associated with poor prognosis, may have contributed to the alteration of the phenotype of infiltrating lymphocytes, during the period in between the initial and the latter biopsy. This may be due to the fact that Epstein-Barr virus-positive cells could have proliferated and caused the disease to advance. Furthermore, it may be possible that both CD56+ and CD56- phenotype lymphocytes were initially present, but CD56- may have been dominantly present in the early stage of the disease.[8]
Herein, our case has shown that lymphoma should be considered as a diagnostic possibilty whenever intractable and idiopathic erythematous nodules are present on the lower extremities.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lee H, Kim SH, Lee SW, Zheng Z, Bang D, Kim DY. A case of extranodal natural killer/T-cell lymphoma mimicking refractory Behçet's disease. Acta Derm Venereol 2015;95:491-2.
[Google Scholar]
|
| 2. |
Lee WJ, Jung JM, Won CH, Chang SE, Choi JH, Chan Moon K, et al. Cutaneous extranodal natural killer/T-cell lymphoma: A comparative clinicohistopathologic and survival outcome analysis of 45 cases according to the primary tumor site. J Am Acad Dermatol 2014;70:1002-9.
[Google Scholar]
|
| 3. |
Kim WS. Recent updates on extranodal NK/T-cell lymphoma. Korean J Med 2009;77:565-70.
[Google Scholar]
|
| 4. |
Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, et al. Subcutaneous panniculitis-like T-cell lymphoma: Definition, classification, and prognostic factors: An EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood 2008;111:838-45.
[Google Scholar]
|
| 5. |
Bagheri F, Cervellione KL, Delgado B, Abrante L, Cervantes J, Patel J, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma. J Skin Cancer 2011;2011:824528.
[Google Scholar]
|
| 6. |
Moon IJ, Kang HJ, Lee MW, Lee WJ. Primary cutaneous extranodal natural killer/T-cell lymphoma presenting as bilateral erythematous patches on the arms. Indian J Dermatol Venereol Leprol 2017;83:453-6.
[Google Scholar]
|
| 7. |
Chowaniec M, Starba A, Wiland P. Erythema nodosum Review of the literature. Reumatologia 2016;54:79-82.
[Google Scholar]
|
| 8. |
Seishima M, Yuge M, Kosugi H, Nagasaka T. Extranodal NK/T-cell lymphoma, nasal type, possibly arising from chronic Epstein-Barr virus infection. Acta Derm Venereol 2010;90:102-3.
[Google Scholar]
|
Fulltext Views
5,881
PDF downloads
1,459





