Translate this page into:
A clinical rating scale for the assessment of facial aging in Indian population
2 Department of Pharmacology, College of Medicine and Sagore Dutta Hospital, Kolkata, West Bengal, India
Correspondence Address:
Supriyo Choudhury
A/111, Survey Park, Santoshpur, Kolkata - 700 075, West Bengal
India
| How to cite this article: Sen S, Choudhury S, Gangopadhyay A, Halder C, Biswas P, Jain A. A clinical rating scale for the assessment of facial aging in Indian population. Indian J Dermatol Venereol Leprol 2016;82:151-161 |
Abstract
Background: Estimation of facial aging has assumed growing importance due to the advent of several antiaging therapies. Evidence-based estimation of global facial aging is often necessary, especially for validation of these treatment modalities. Most available methods are expensive and have been used in fair skinned individuals. Aim: We attempted to develop a clinical rating scale for the estimation of global facial aging applied on an Indian population which has brown to black skin. We have also measured the association of this rating scale score with the chronological age. Methods: Initially, a 14- item summated rating scale was developed with inputs from five dermatologists and a clinical pharmacologist. The rating scale was applied to 105 consenting subjects with healthy facial skin between 30 to 90 years of age. Intra- and inter-rater reliability was assessed. Results: The summated rating score showed a significant positive correlation with the chronological age (Pearson's correlation coefficient 0.834, P < 0.001). We omitted one item from the scale due to a low inter-rater agreement. The resulting 13-item rating scale was internally consistent (Cronbach's alpha: 0.905), with substantial inter- and intra-rater reliability (intraclass correlation coefficient: 0.973 and 0.788, respectively). Principal components and predictive equation for perceptible age were identified on further computation. Limitations: Participants of this study were limited to a particular ethnic group from West Bengal and other neighboring states of Eastern India. Conclusions: We have developed and validated a 13-item rating scale for the quantification of global facial aging suitable for Indian (brown to black) skin type. This scale can be utilized effectively for clinical estimation of global facial aging.INTRODUCTION
Aging is an inevitable process of accumulation of physical, psychological and social changes over time and is practically irreversible. Facial aging creates special concern due to its esthetic and social impact. Various intrinsic and extrinsic factors affect the appearance of the human face over time including genetic makeup, loss of teeth, smoking, sun exposure, other environmental influences and gravity. [1] The human face may look older than it actually is due to accelerated skeletal, soft tissue and dermatological changes. [2] Loss of facial skin elasticity and hydration and loss of facial volume results in the development of wrinkles. [1] Signs of facial aging may show considerable ethno-racial variation due to a diversity in the genetic blueprint of different geographical populations. [3] Perceptible aging changes in the face are a serious area of concern for many individuals and since time immemorial human beings have tried to arrest and mask these changes to try and regain a youthful appearance. [4],[5] Technological developments in cosmetic dermatology have led to the introduction of various anti-aging strategies for the face such as fillers, botulinum toxin injection, lasers, chemical peeling and surgical interventions. [6],[7],[8],[9],[10],[11]
Since beauty and perception of age are largely subjective concepts, there have been various efforts to quantify facial aging so as to be able to assess the need and impact of esthetic interventions. These approaches included summated rating scales using face photographs, estimation of biomarkers of aging, confocal microscopy of facial skin, etc. [12],[13],[14] However, these approaches are largely resource and technology driven. Researchers have also attempted to estimate facial aging according to a specific facial area (such as clinical score for lateral canthal lines) since surgical intervention focuses on a particular area of the face at a time. However, in real life this piecemeal approach does not have much clinical relevance when the anti-aging treatment is applied to the whole face. [15] There is a scarcity of validated and reliable global facial aging scales. Rzany et al. have developed one such scale validated for European skin types using photographs. [12] Bernois et al. developed a similar rating scale for the quantification of facial aging among Indian women from the Western part of India. [16] In these scales, quality of the photograph and its display system (screen quality and size, image luminosity, etc.) becomes critical in estimating facial age. [17] Moreover, some aging manifestations such as skin texture, laxity of skin and wrinkle intensity are best estimated by simultaneous visual as well as tactile assessment which is not possible while estimating facial age from a photograph. This limitation underlies the need for the development of a facial aging scale for Indian skin types that is not dependent on photography.
Through the present study, we attempted to develop and validate a simple summated clinical rating scale (Facial Aging Rating and Evaluation scale) for quantification of facial age in Indian subjects, to be used by dermatologists by means of live clinical assessment. We also assessed the correlation between facial aging as quantified by this score, and chronological age of the subjects.
METHODS
This analytical observational study was conducted in the dermatology outpatient department of a tertiary care hospital in eastern India (Institute of Postgraduate Medical Education and Research, Kolkata, West Bengal) after obtaining approval from the Institutional Ethics Committee. Subjects from 30 to 90 years of age of both genders, with Fitzpatrick skin types IV through VI (common in the Indian population) were selected. [18] Subjects having abundant facial hair, loss of more than 3 teeth from either the upper or the lower set, moderate to heavy smokers (greater than 10 cigarettes per day), predominantly outdoor workers with more than 3 hours of sun exposure per day, scarring, human immunodeficiency virus (HIV)-related lipoatrophy, facial skin disease or any uncontrolled systemic or dermatological diseases that may influence facial appearance were excluded. Only anti-aging therapy-naive subjects were selected.
We adopted a convenience sampling strategy. All study related activities were conducted after obtaining written informed consent from the selected subjects. The correlation coefficient of aging score and chronological age using the rating scale by Rzany et al. was reported as 0.77. [12] We assumed a sample correlation coefficient of 0.60 between the summated score of our aging scale and chronological age and a population correlation coefficient of 0.77. Considering 90% power and 5% alpha error, we intended to obtain complete data from 101 evaluable subjects and recruited 115 consenting subjects, anticipating a 15% dropout rate. The sample size was calculated using nMaster version 2.0 (Copyright: Biostatistics Resource and Training Centre, CMC, Vellore).
Construct definition was established at the outset of rating scale development by the authors who selected commonly perceived facial aging signs as items for the proposed rating scale. The morphological effects of facial aging are conventionally assessed across three main domains of the face, upper third (forehead and brows), middle third (mid-face and nose) and lower third (chin, jaw line and neck). The group selected 2 items from the upper third, 4 from middle third, 5 from lower third and 2 additional items as general attributes of facial aging. The items selected are shown in [Table - 1]. All items were scored on a scale of 0-3 according to increasing order of severity, as indicated. The initial rating scale was modified after taking the opinion of a peer group of five dermatologists and one clinical pharmacologist, in order to achieve construct validity. The rating scale was independently applied to study subjects by two dermatologists separately on the same day. The severity scales for the majority of the items were objectively quantifiable as reflected in [Table - 1] and [Table - 2]. Observers were trained by showing prototypes of each category, or by showing characteristic images prior to rating for relatively subjective items (e.g., item number 11 and 12). One dermatologist re-applied the scale on the same subjects at an interval of 14 ± 2 days. Duplicate and repeat assessments were conducted to assess inter- and intra-rater reliability, respectively.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 20 statistical software (IBM Corporation) was used for analysis. Summary statistics is presented for demographic variables. Differences between subgroups were assessed by Student′s independent samples t-test and Mann-Whitney U-test for numerical parametric and non-parametric datasets respectively; two-tailed P < 0.05 was considered statistically significant. Intra- and inter-rater reliability was computed for each skin aging item using intra-class correlation coefficient. An intra-class correlation coefficient of 0.00-0.20 was considered slight, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 substantial and 0.81 or greater as almost perfect agreement. [19] Items with substantial intra- and inter-rater agreement (lower bound of 95% confidence interval of intra-class correlation coefficient >0.6) were retained while those not fulfilling this criterion were modified. Internal consistency of the scale was tested using Cronbach′s alpha with an all item value >0.8 considered necessary for declaring the scale as an internally consistent scale. [20] Corrected inter-item correlation coefficient was calculated to check the validity of individual items with a value ≥0.4 being considered acceptable. The association between chronological age and rating scale total score was quantified using Pearson′s correlation coefficient.
As a secondary objective, factor analysis and principal component analysis was performed to identify if there were any common dimensions to the scale. Exploratory factor analysis was carried out for all three rating cycles to establish the consistency of rating (factorial structure) across raters. This was followed by confirmatory factor analysis. The summated item score of three rating cycles was considered to confirm the number of dimensions of our rating scale. The Kaiser rule of eigenvalue >1 and eigenvalue scree plots were the main criteria used to determine the number of dimensions. [21],[22] In interpreting the rotated factor pattern, an item was said to load on a given dimension if a 2 /h 2 (a 2 = square of the factor loading for that dimension, h 2 = variable communality) was 0.5 or greater (Furntratt criterion). [12]
RESULTS
Of 115 subjects recruited, three complete sets of observations were available for 105 subjects. Ten subjects did not turn up for repeat assessment after 2 weeks and their data has been excluded.
Mean (standard deviation) age of the 105 subjects was 52.3 (12.9) years with the proportion of subjects in decadal age bands from third to eighth decade of life being 17.1%, 25.7%, 26.7%, 15.2%, 13.3% and 1.9%, respectively. Of 105 subjects 50.5% were men. There was no statistically significant difference in the mean ages of male and female subjects, as seen by Student′s independent samples t-test (P = 0.410).
The inter- and intra-rater reliability validation and internal consistency assessment have been summarized in [Table - 3]. The scoring of all three ratings was consistent. Cronbach′s alpha for the scale comprising all 14 items was 0.897. However, among 14 items, solar keratosis was found to have a low index of inter-rater agreement compared to other items. It also showed a low corrected item total correlation coefficient (0.023) signifying a lack of importance of this item in the rating scale. Hence, we decided to delete the item from the rating scale for further analysis. The summary of the actual score for all three ratings (individual item score and total score) are presented in [Table - 4] and the mean scores of decadal age bands are presented in [Table - 5].
[Figure - 1] shows the scatter plot associating average (three rating) summated score of the 13-item rating scale and chronological age of the 105 evaluable subjects. Pearson′s correlation coefficient between chronological age and the total score was 0.834 (P < 0.001).
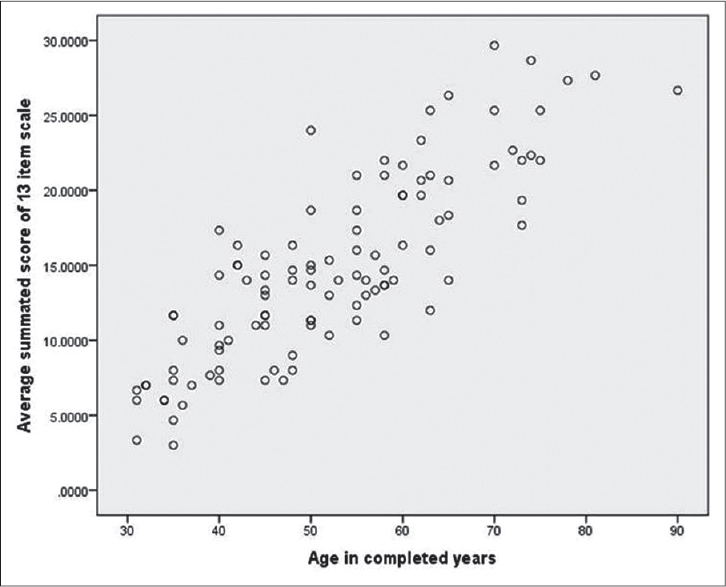 |
| Figure 1: Scatter plot associating average (three rating) summated score of 13-item rating scale and chronological age |
The Mann-Whitney U-test did not reveal any significant difference in the facial aging scores of males and females (P = 0.239). When individual item scores were compared between genders, the difference in scores for perioral and lip wrinkles at rest was statistically significant (P value 0.012, 0.049, respectively), with the mean rank greater for women (females 58.38 vs. males 47.72 for lip wrinkles and females 60.07 vs. males 46.07 for perioral wrinkles).
We also performed a simple linear regression for prediction of perceptible age from the score and found a linear relationship between the summated score of the 13-item rating scale and the perceptible age in years. The predictive equation is as follows:
Age in years = 27.4455 + 1.6252 × summated score of 13-item scale.
[Figure - 2] depicts the relationship of chronological age and predicted age from the regression equation with total summated score of the 13-item rating scale.
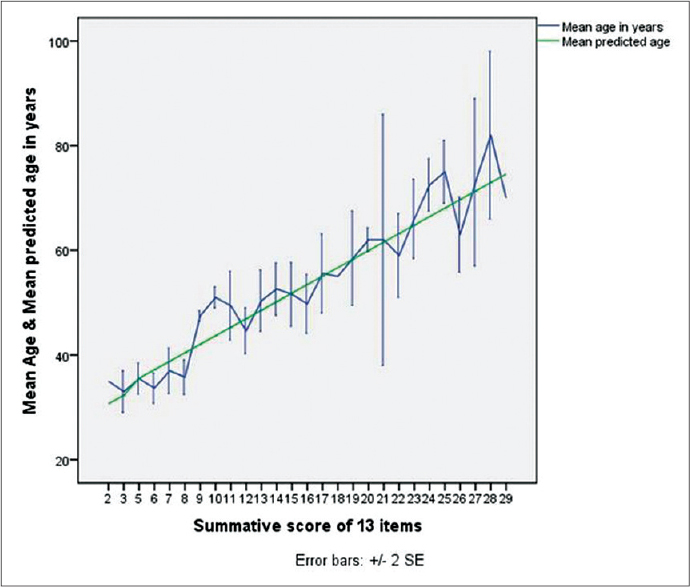 |
| Figure 2: Relationship of chronological age and predicted age from the regression equation with total summated score of 13-item rating scale |
Factor analysis and principal component analysis identified two common dimensions to the 13-item facial aging scale after deleting the solar keratosis item. An acceptable solution in terms of goodness-of-fit (Kaiser-Meyer-Olkin measure of sampling adequacy >0.8) was obtained with two factors for all three rating cycles by exploratory factor analysis. The principal axis method was used to extract the dimensions and this was followed by a varimax rotation. A pattern of loadings >0.5 emerged which is consistent across raters. Therefore, the items across all three sets of ratings were merged by summing. On confirmatory factor analysis, two dimensions or factors were retained accounting for 33.7% and 30.2% of the total variance of facial aging score, respectively. [Table - 6] denotes the loading of individual items on these two dimensions. A substantial correlation was observed between factors 1 and 2 (r = 0.673).
Photographs depicting the facial aging signs are presented as [Figure - 3], [Figure - 4], [Figure - 5], [Figure - 6], [Figure - 7], [Figure - 8], [Figure - 9], [Figure - 10], [Figure - 11], [Figure - 12], [Figure - 13], [Figure - 14], [Figure - 15].
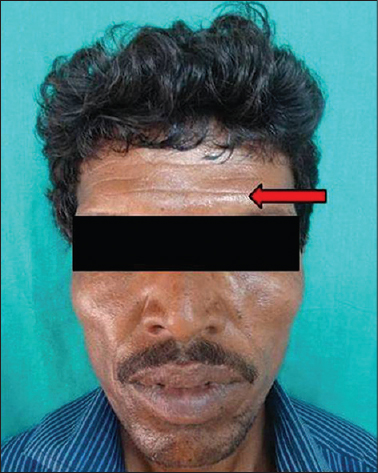 |
| Figure 3: Forehead lines |
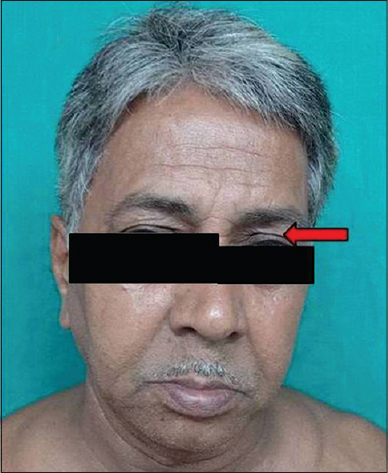 |
| Figure 4: Upper eyelid laxity |
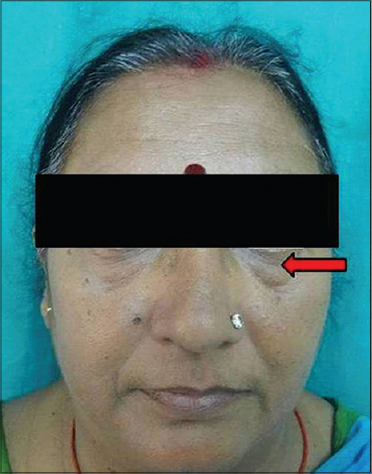 |
| Figure 5: Infraorbital laxity |
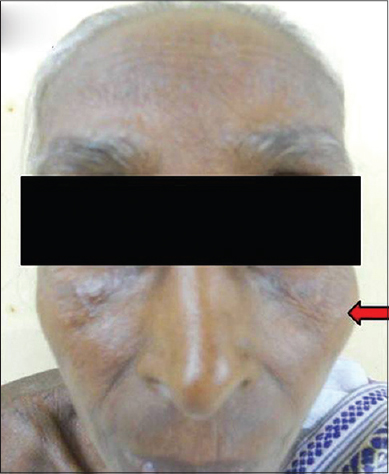 |
| Figure 6: Visible malar prominence |
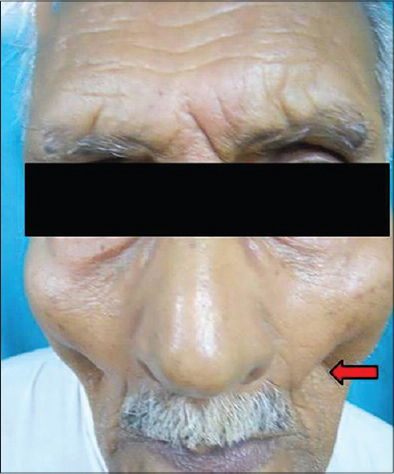 |
| Figure 7: Fullness of buccal fat pad |
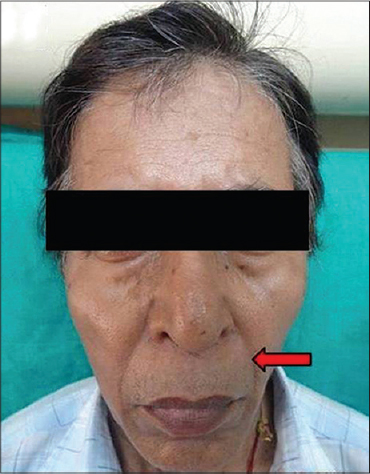 |
| Figure 8: Nasolabial fold |
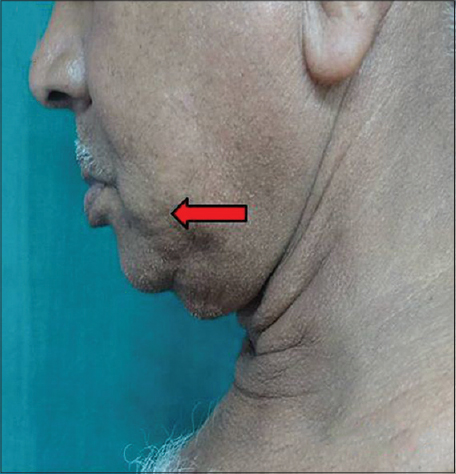 |
| Figure 9: Cheek skin elasticity |
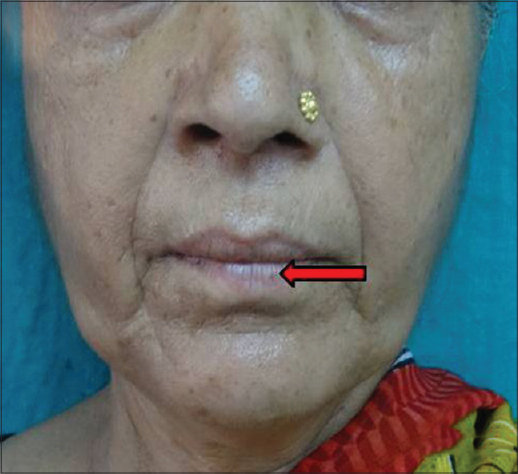 |
| Figure 10: Lip wrinkles at rest |
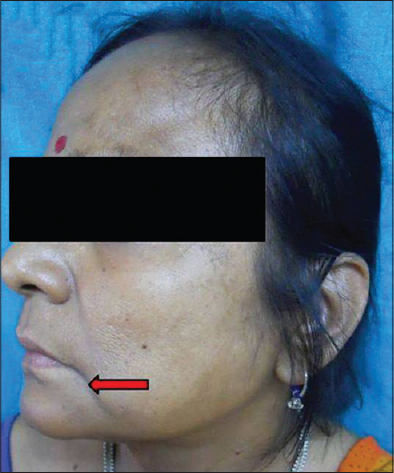 |
| Figure 11: Descent of corner of mouth |
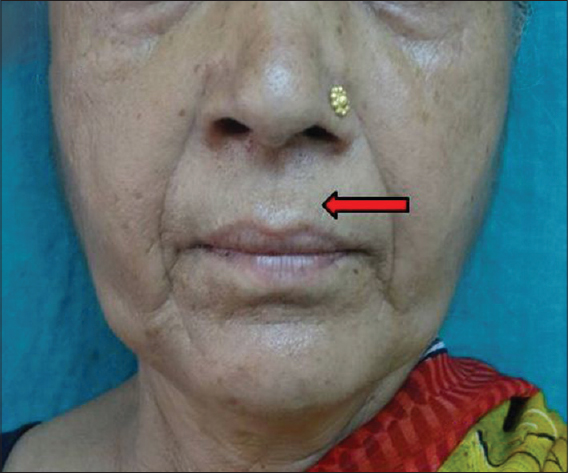 |
| Figure 12: Perioral wrinkles |
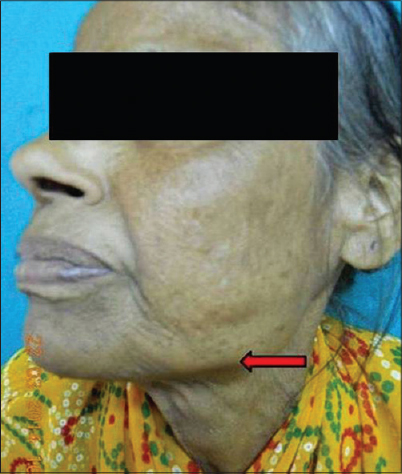 |
| Figure 13: Jawline at rest |
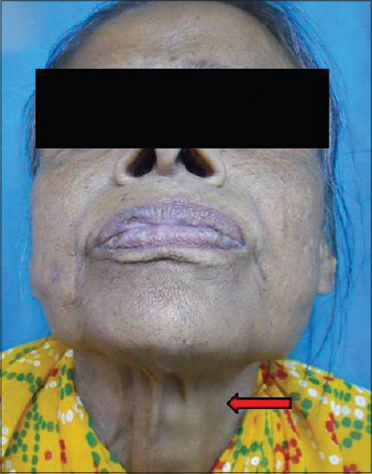 |
| Figure 14: Neck volume at rest |
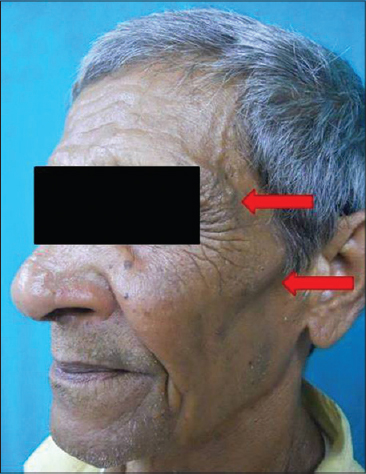 |
| Figure 15: Pigmented spot |
DISCUSSION
The development of a validated rating scale for the clinical estimation of facial aging is required for objectively evaluating anti-aging therapy. In this study, we have developed an easy-to-use clinical rating scale for facial aging suitable for application in brown to dark skin population. The age distribution of study subjects was homogenous over a range of 30-90 years. On literature review, we were unable to find any previous reports on the development of facial aging rating scale by clinical assessment. The avoidance of photography has inherent advantages as already stated.
Our study group felt a need for constructing facial aging scale due to perceived limitations of the existing tools. The items representing aging signs of lower face and neck were inadequately selected in the validated geriatric facial aging scale developed by Allerhand et al. [23] The rational mechanism of the statistically derived dimensions from facial aging scale (MERZ scale) is inadequately addressed by Rzany et al. [12] These scales were validated on subjects with either an age greater than 80 years or less than 65 years, respectively. To overcome limitations of a narrow age cohort, we included subjects over a wide age range.
Our study revealed substantial intra- and inter-rater agreement of overall scores on repetition of rating. The scale, therefore, appears to be a reliable instrument for estimation of facial aging. All items in the scale individually showed an acceptable degree of reliability, except solar keratosis, which showed moderate intra-rater and poor inter-rater reliability. Although, this disagreement may have been avoidable if the raters had used a dermoscope during evaluation, we discarded this item from the final scale since we wanted a scale independent of any sort of instrument use. A moderate inter-rater reliability of cheek skin elasticity (intra-class correlation coefficient = 0.52, 95% confidence interval: 0.30-0.68) suggests that this item may not be as objective as the others. However, this item was retained in the scale as the intra-class correlation coefficient was >0.5.
A review of literature shows that the most important determinant of perceptible age is the chronological age; hence, the latter was was chosen as the external criterion for the purpose of validation of our summated rating scale. [4] A satisfactory positive linear correlation between total score and chronological age, and increasing scores through the decadal age bands, supports the validity and usefulness of this scale. From the regression equation, dermatologists will be able to predict the perceptible age of their patients and hence, the requirement of anti-aging therapy can be determined and evaluated.
Overall facial aging score and chronological age were comparable between genders. However, a gender difference in the severity of perioral and lip wrinkles was observed with women showing more lip and perioral wrinkles than men of a comparable age. The reason for this difference is still obscure and a potential area for further research. On literature review, it was found that lip and perioral wrinkles develop due to degenerative changes of skin and orbicularis oris muscle rather than from loss of lip volume. Genetic factors may explain the localized accelerated degeneration in woman subjects. [24] Rzany et al. also found a greater propensity towards lip wrinkles among women. [12]
Principal component analysis was performed to extract common dimensions of our scale. The factor loading pattern of individual items suggests two main dimensions, both of which can explain the variability of more than 30% of this scale. The first dimension consists of the items forehead lines at rest, upper eyelid laxity, visible bony prominences of upper cheek, fullness of cheeks, nasolabial fold at rest, cheek skin elasticity, lip wrinkles at rest, perioral wrinkles at rest and pigmented spots. The other dimension consists of upper eyelid laxity, infraorbital laxity, nasolabial fold at rest, lip wrinkles at rest, descent of corner of the mouth, jaw line at rest and neck volume at rest. It is noteworthy that items from the first dimension mostly result from degeneration of skin and subcutaneous tissue. On the other hand, items from the second dimension result from gravitational effect These were named as wrinkles and sagging, respectively, by Allerhand et al., who found a similar component composition in their facial aging scale. [23] Interestingly, we did not find pigmentation to be an important factor, in contrast to the study by Guinot et al., in which the study population had fair skin. [25] Pigmented spots do not appear to be an important determinant of the score, possibly due to darker skin hue of Indians. A similar factorial structure was reported by Bernois et al., where the study population consisted of woman subjects from western India. [16] Although wrinkling and sagging have been considered as distinct factors, the two are actually related. The effect of gravity can only work when skin and subcutaneous tissue degenerates.
Limitations of the study need to be mentioned. There is no scope for post-rating re-evaluation of the scores in clinical assessment. We took photographs of hallmark aging signs which might be utilized for raters′ training purpose subsequently. Besides, this scale was not tested on subjects below 30 years and above 90 years of age since aging signs are not prominent below 30 and subjects above 90 rarely visit the outpatient department. The majority of subjects were from a particular ethnic group, individuals born and brought up in West Bengal or neighboring states of Eastern India and this may influence the generalizability of the scale. There is a need for validating the scale at extremes of age and in various ethnicities. However, our findings are broadly in agreement with those reported from the western part of India suggesting that our scale may be valid in other parts of India. [16] Due to resource constraints, we could not evaluate the cost and convenience of using a photographic scale compared to our clinical scale. Cross-validation of this scale in a different country with similar skin types may be an interesting area of future work. Another area of further research could be a comparative assessment of facial aging scores for various age groups, especially focusing on the target age groups among whom this scale is more likely to be applied in clinical practice. In this study, the summary statistics of facial aging scores for age groups represented the pattern of aging and normal aging scores among the participants, over ranges of chronological age.
CONCLUSION
We have developed a 13-item rating scale for the evaluation of facial aging in Indian subjects through clinical examination of skin changes. This should prove useful in anti-aging research and in esthetic practice in the Indian population. Dermatologists can use this scale to screen patients who are seeking treatment for facial aging.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgment
The authors would like to thank Prof. Dr. Avijit Hazra, department of pharmacology, Institute of Post Graduate Medical Education and Research, for his valuable comments and suggestions regarding the methodology and statistical aspect of the research.
The authors would also like to thank Prof. Dr. Gobinda Chatterjee, head of the department of dermatology, IPGMER and Kolkata for helping in editing the article before submission.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sveikata K, Balciuniene I, Tutkuviene J. Factors influencing face aging. Literature review. Stomatologija 2011;13:113-6.
[Google Scholar]
|
| 2. |
Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg 1996;15:134-8.
[Google Scholar]
|
| 3. |
Querleux B, Baldeweck T, Diridollou S, de Rigal J, Huguet E, Leroy F, et al. Skin from various ethnic origins and aging: An in vivo cross-sectional multimodality imaging study. Skin Res Technol 2009;15:306-13.
[Google Scholar]
|
| 4. |
Gunn DA, Rexbye H, Griffiths CE, Murray PG, Fereday A, Catt SD, et al. Why some women look young for their age. PLoS One 2009;4:e8021.
[Google Scholar]
|
| 5. |
Kreyden OP. Antiaging - A scientific topic or just a social trend? J Cosmet Dermatol 2005;4:228-9.
[Google Scholar]
|
| 6. |
Weinkle S, Lupo M. Attitudes, awareness, and usage of medical antiaging treatments: Results of a patient survey. J Clin Aesthet Dermatol 2010;3:30-3.
[Google Scholar]
|
| 7. |
Brandt FS, Cazzaniga A. Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging 2008;3:153-9.
[Google Scholar]
|
| 8. |
Lipozencic J, Bukvic Mokos Z. Dermatologic lasers in the treatment of aging skin. Acta Dermatovenerol Croat 2010;18:176-80.
[Google Scholar]
|
| 9. |
Raspaldo H, Niforos FR, Gassia V, Dallara JM, Bellity P, Baspeyras M, et al. Lower-face and neck antiaging treatment and prevention using onabotulinumtoxin A: The 2010 multidisciplinary French consensus - Part 2. J Cosmet Dermatol 2011;10:131-49.
[Google Scholar]
|
| 10. |
Effron C, Briden ME, Green BA. Enhancing cosmetic outcomes by combining superficial glycolic acid (alpha-hydroxy acid) peels with nonablative lasers, intense pulsed light, and trichloroacetic acid peels. Cutis 2007;79 1 Suppl Combining:4-8.
[Google Scholar]
|
| 11. |
Beer KR. Combined treatment for skin rejuvenation and soft-tissue augmentation of the aging face. J Drugs Dermatol 2011;10:125-32.
[Google Scholar]
|
| 12. |
Rzany B, Carruthers A, Carruthers J, Flynn TC, Geister TL, Görtelmeyer R, et al. Validated composite assessment scales for the global face. Dermatol Surg 2012;38:294-308.
[Google Scholar]
|
| 13. |
Chang AL, Lingala B, Chang TC, Kern DG, Wood SM, Toyoda H, et al. An exploratory study to determine the association between assessed facial skin aging and plasma isoprostane levels in middle-aged Japanese women. Dermatol Surg 2012;38:462-70.
[Google Scholar]
|
| 14. |
Longo C, Casari A, De Pace B, Simonazzi S, Mazzaglia G, Pellacani G. Proposal for an in vivo histopathologic scoring system for skin aging by means of confocal microscopy. Skin Res Technol 2013;19:e167-73.
[Google Scholar]
|
| 15. |
Hund T, Ascher B, Rzany B, SMILE STUDY GROUP. Reproducibility of two four-point clinical severity scores for lateral canthal lines (crow′s feet). Dermatol Surg 2006;32:1256-60.
[Google Scholar]
|
| 16. |
Bernois A, Huber A, Derome C, Drouault Y, de Quéral D, Schnebert S, et al. A photographic scale for the evaluation of facial skin aging in Indian women. Eur J Dermatol 2011;21:700-4.
[Google Scholar]
|
| 17. |
Zhao W, Chellappa R, Phillips PJ, Rosenfeld A. Face Recognition: A Literature Survey, ACM Computing Surveys. Vol. 35. Association for Computing Machinery, New York; 2003. p. 399-458.
[Google Scholar]
|
| 18. |
Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869-71.
[Google Scholar]
|
| 19. |
Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 1979;86:420-8.
[Google Scholar]
|
| 20. |
Tavakol M, Dennick R. Making sense of Cronbach′s alpha. Int J Med Educ 2011;2:53-5.
[Google Scholar]
|
| 21. |
Gorsuch RL. Factor Analysis. 2 nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. p. 175-206.
[Google Scholar]
|
| 22. |
Cattell RB. The scree test for the number of factors. Multivariate Behav Res 1966;1:245-76.
[Google Scholar]
|
| 23. |
Allerhand M, Ooi ET, Starr J, Alcorn M, Penke L, Drost E, et al. Skin ageing and oxidative stress in a narrow-age cohort of older adults. Eur Geriatr Med 2011;2:140-4.
[Google Scholar]
|
| 24. |
Iblher N, Stark GB, Penna V. The aging perioral region - Do we really know what is happening? J Nutr Health Aging 2012;16:581-5.
[Google Scholar]
|
| 25. |
Guinot C, Malvy DJ, Ambroisine L, Latreille J, Mauger E, Tenenhaus M, et al. Relative contribution of intrinsic vs. extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol 2002;138:1454-60.
[Google Scholar]
|
Fulltext Views
6,499
PDF downloads
2,638





