Translate this page into:
A comparative evaluation of aluminum chloride hexahydrate gel iontophoresis versus tap water iontophoresis in people with primary palmar hyperhidrosis: A randomised clinical trial
Corresponding author: Dr. Elham Ghandali, Physiotherapy Research Center, School of Rehabilitation, Shahid Beheshti University of Medical Sciences, Tehran, Iran. eghandali@sbmu.ac.ir
-
Received: ,
Accepted: ,
How to cite this article: Hosseini SM, Ghandali E, Moghimi HR, Khademi-Kalantari K, Moghaddam ST, Baghban AA, et al. A comparative evaluation of aluminum chloride hexahydrate gel iontophoresis versus tap water iontophoresis in people with primary palmar hyperhidrosis: A randomised clinical trial. Indian J Dermatol Venereol Leprol. 2024;90:52-8. doi: 10.25259/IJDVL_975_2022
Abstract
Background
Primary palmar hyperhidrosis causes a lot of problems for patients and negatively affects their quality of life. Currently, iontophoresis with tap water and aluminum chloride hexahydrate is used for primary palmar hyperhidrosis. Yet, little evidence exists about iontophoresis with aluminum chloride hexahydrate in the form of gel. This study investigated the effect of aluminum chloride hexahydrate gel iontophoresis compared to tap water iontophoresis on primary palmar hyperhidrosis.
Methods
In this randomised controlled trial study, 32 patients with primary palmar hyperhidrosis were divided randomly into two groups (n = 16). Participants received 7 sessions of iontophoresis with aluminum chloride hexahydrate gel or tap water every other day on the dominant hand. The sweating rate was measured by gravimetry and iodine–starch tests before and after the last treatment session.
Results
Following the iontophoresis, the rate of sweating in both hands in the two groups was significantly reduced (P < 0.001). However, the sweating rate in the treated hand and the non-treated hand showed no significant difference. There was no significant difference observed in sweating rate reduction between both groups over time, but the larger effect size values observed in the aluminum chloride hexahydrate gel iontophoresis group may suggest the superiority of this gel over tap water in reducing the rate of sweating.
Limitations
Further investigations with longer follow-up are needed to confirm the hypothesis regarding the effectiveness of aluminum chloride hexahydrate gel iontophoresis over other types of iontophoresis. In addition, contraindications of iontophoresis such as pregnancy, pacemakers, and epilepsy should be considered.
Conclusion
The present study provides preliminary evidence suggesting that aluminum chloride hexahydrate gel iontophoresis is an effective alternative treatment to decrease sweating rate in extended areas with fewer side effects in patients with primary palmar hyperhidrosis.
Keywords
Aluminum chloride hexahydrate
hyperhidrosis
iontophoresis
Plain Language Summary
Palmar hyperhidrosis (excessive sweating) is a common problem that negatively affects the quality of life of sufferers. Iontophoresis is a common treatment for palmar hyperhidrosis, but this method has side effects such as skin redness and burns. On the other hand, it has been suggested that if the medium for iontophoresis is in the form of a gel, its side effects will be less and the treatment result will be better. Aluminum chloride hexahydrate is an antiperspirant used for hyperhidrosis. In the present study, aluminum chloride hexahydrate was first made into a gel, and then iontophoresis was performed with that gel to treat palmar hyperhidrosis. The present study suggests that aluminum chloride hexahydrate gel iontophoresis is an effective alternative treatment to decrease sweating rate in extended areas with fewer side effects in patients with primary palmar hyperhidrosis.
Introduction
Primary palmar hyperhidrosis is a disorder marked by excessive eccrine sweating beyond physiological need. This condition carries a psychological, social, and professional burden that has a detrimental effect on the daily activities of patients.1 Almost 0.6 to 2.8% of the general population, mostly people aged between 25 and 64 years, experience palmar hyperhidrosis.2,3 Various surgical and nonsurgical treatments (e.g., topical, iontophoresis, local, and systemic anticholinergics) are available for patients suffering from primary hyperhidrosis.4 Iontophoresis is considered FDA approved and an effective treatment for palmoplantar hyperhidrosis.5 Some authors even suggest iontophoresis as an appropriate substitution for prolonged pharmacotherapy.6 However, contraindications to iontophoresis, such as pregnancy, pacemakers, and epilepsy, should be considered.7
Iontophoresis is defined as the process of delivering an ionized substance through intact skin by means of direct electrical current.8 Iontophoresis of tap water, aluminum chloride, or glycopyrronium solutions all has been suggested with acceptable outcomes, although with some side effects.5,7 Side effects such as skin irritation, pain, blistering in tap water iontophoresis has been reported in some cases in previous studies.1 The use of anticholinergics such as glycopyrronium bromide in iontophoresis has a number of disadvantages over tap water iontophoresis. The majority of these patients showed systemic side-effects of dry mouth or throat.9 It has been proposed that gels may be preferable to liquids for iontophoresis because of fewer side effects. The simplicity of fabrication into the device, skin contour matching, and better stability could introduce gel as an appropriate delivery vehicle for iontophoresis.10 A vehicle made of agarose gel improves contact with the underlying skin and eliminates any possible “hot spots” due to air bubbles that may occur with the standard solution-based vehicle. Iontophoresis using the gel formulation as a vehicle for aluminum chloride hexahydrate delivery has the potential to lower the voltage required to overcome skin resistance, and results in reduced patient discomfort during the procedure.11,12
Aluminum chloride is an antiperspirant and an acceptable treatment for patients with hyperhidrosis. Topical solution of aluminum chloride 20% is the first line of treatment for hyperhidrosis.1 Iontophoresis with aluminum chloride hexahydrate diluted in alcohol as low as 1% has been suggested with promising results but it has difficulties in preserving the solution for a long period and considerable difficulties in application.13 Considering the advantages of gel formation as a vehicle for iontophoresis with fewer side effects, we hypothesized that iontophoresis of aluminum chloride hexahydrate in the form of gel could be more effective and have fewer side effects compared to the common tap water iontophoresis.
The purpose of the present study was to compare the effectiveness of two substances (aluminum chloride hexahydrate gel and tap water) as media for iontophoresis to decrease sweating rate and reduce density and the extent of violet areas by starch–iodine test in patients with primary palmar hyperhidrosis.
Materials and Methods
Trial design
This single-center, single-blinded, randomised controlled, parallel group trial was approved by the ethics committee of the Shahid Beheshti University of Medical Sciences (Ethic Code: IR.SBMU.RETECH.REC.1396.832).
Participants
The subjects who complained of primary palmar hyperhidrosis were recruited using posters distributed through university communities. The inclusion criteria were as follows: the presence of focal, visible excessive sweating for 6 months or more, without apparent secondary causes, accompanied by at least two of the following characteristics: bilateral and relatively symmetrical sweating, frequency of more than one time per week, impairment of daily activities of the patient, age of onset <25 years, a positive family history, and cessation of sweating during sleep.14 Patients should not be pregnant, or have a history of skin problems, and pacemaker implants.8 Patients with body mass index (BMI) > 28 were excluded from study. Data collection and treatment sessions were done in the biomechanical laboratory of the Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences.
Treatment sessions
Iontophoresis with aluminum chloride hexahydrate gel 2% was done for the first group, and with tap water for the second group. Gravimetric test was performed before and after the intervention (3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks, and 24 weeks).
Aluminum chloride hexahydrate gel iontophoresis group
In the present study, aluminum chloride hexahydrate gel was prepared at the school of pharmacy of the Shahid Beheshti University of Medical Sciences. Polymers are widely used in gel formation. The most common of these are carbomer and hydroxypropyl methylcellulose.15 A water-based hydroxypropyl methylcellulose gel was used in this study. Since this gel was supposed to be applied for iontophoresis purposes, we employed a water-based gel (not an organogel) in which the continuous phase is water. Hydroxypropyl methylcellulose, an organic polymer, is extensively used for pharmaceutical gel preparation. In order to prepare the gel, 2% w/v aluminum chloride hexahydrate aqueous solution was made and then hydroxypropyl methylcellulose 400 cps as a gelling agent was added to the previous solution gradually. This process was performed under stirring for 5 hours at room temperature.16 Iontophoresis with aluminum chloride hexahydrate gel applied to the patients’ dominant (right) hand was performed using a galvanic stimulator (ActivaDose, iontophoresis delivery unit, made in Taiwan, Serial Number 12080971) with an intensity of 2 mA for 30 minutes. The other hand was regarded as a control without any treatment. Treatments were given every other day for a total of seven sessions.
The active electrode (anode, 5 × 7 cm2) covered the palmar surface of the hand from the metacarpophalangeal crease to the distal wrist crease. The reference electrode (cathode = 5 × 7 cm2) was located on the anterior surface of the forearm.17 We built a chamber under the active electrode for applying gel. This chamber was flexible and matched the skin. The chamber was filled with 2.2 mm of aluminum chloride hexahydrate gel and was located on the patient’s palm. Straps were used to fix the chamber.
Tap water iontophoresis group
Iontophoresis with tap water was done on the patients’ dominant (right) hand using the same protocol. The only difference was the use of tap water under the active electrode. Each treatment session was performed at the same time of day (10 AM–1 PM) to eliminate any possible circadian effect.
Evaluation session
Subjects underwent one pre-treatment and 7 post-treatment evaluations at 3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks, and 24 weeks after the treatment. For the gravimetric test, filter papers were weighed before and after exposure to palmar skin for 5 minutes. The weight difference indicated the rate of sweat secretion in milligrams per 5 min.18 The room temperature was maintained constant (25°C) during the treatment and evaluation sessions.13 In addition to the gravimetric test, the size of the area with increased sweating was determined by a starch iodine test before and after iontophoresis session [Figure 1].
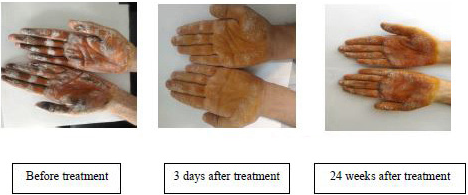
- The density and extent of dark areas before treatment, 3 days and 24 weeks after treatment in both hands of a patient in gel iontophoresis group.
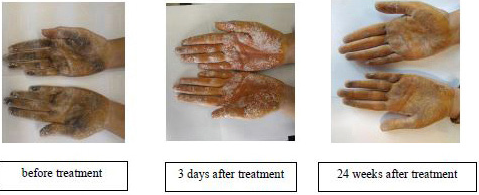
- The density and extent of dark areas before treatment, 3 days and 24 weeks after treatment in both hands of a patient in tap water iontophoresis group.
The iodine-starch test was used to show the extent of the sweating of the hands. The iodine-starch test consisted of applying a solution made up of 2 g of iodine and 4 g of potassium iodine in 100 mL of alcohol over the skin of the hand and fingers. After a few seconds, a starch powder was sprinkled over this area and after 10 minutes digital photos were taken. Sweat in contact with both substances causes the colour of the powder to turn violet.17
At the end of each treatment session, side effects such as burning, itching, and irritation were evaluated by self- reported assessment.
Sample size and randomisation
Due to the lack of a previous similar study, the sample size was determined after a pilot study. The following formula was used to calculate the sample size. The sample size in each group was estimated at 16 to achieve 90% power for the present study. Based on the hyperhidrosis disease severity scale and baseline sweating rate, the participants were randomly allocated to the aluminum chloride hexahydrate gel iontophoresis and tap water iontophoresis groups by a physiotherapist. Randomization sequence was stratified by disease severity with a 1:1 allocation using random block sizes of 2.
The physical therapist who randomised the subjects, and was responsible for treating the patients was aware of the type of treatment, whereas the assessor who measured the sweating rate was blinded to the type of intervention.
Statistical analysis
All data were analysed using SPSS version 18.0 (SPSS Inc. Chicago). A Shapiro-Wilk test was done to determine the normal distribution of variables. The independent samples t-test was used to determine if there were any significant differences between the two groups regarding the baseline sweating rate. Multifactorial ANOVA with 2 within subject factors including time (before treatment vs. 3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks and 24 weeks after treatment) and treated status (treated hand and non-treated hand) and 1 between subject including group (gel iontophoresis group and tap water iontophoresis group) was used to determine the effectiveness of treatment over time and the effect of groups and dominancy on sweating rate. P-values < 0.05 were considered statistically significant. The effect size was interpreted using Cohen’s d. The values of effect size were classified as initially suggested by Cohen and expanded by Sawilowsky.19,20
Results
The study flowchart is shown in Figure 2. Recruitment started in January 2018 and was stopped in March 2019 because the required sample size was attained. Participants attended clinic visits at the time of randomization (baseline) and at 3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks, and 24 weeks follow-up. Of the 37 participants who were referred for the study, one was excluded because of a BMI > 28, and two were excluded due to their receiving treatment for hyperhidrosis. After randomization, 17 subjects were allocated to each group. However, two were not interested in enrolling for the study. Thus, finally, data from 32 subjects (16 subjects in each group) were analysed (17 females and 15 males, age 23.8 ± 4.6 years). The demographic characteristics of participants are shown in Table 1. Shapiro-Wilk tests showed a normal distribution of all variables in both the groups. Therefore, the independent samples test was used to compare the groups for demographic characteristics and sweating rate at baseline, which showed no significant differences (P-values > 0.05). Post treatment statistical analyses for both groups (3 days, 1 week, 2 weeks, 3 weeks, 4 weeks, 8 weeks, and 24 weeks after treatment) showed a significant decrease in sweating rate versus baseline (P-values < 0.05) [Tables 2 and 3]. However, there were no significant differences in sweating rate reduction between both groups over time. Also, comparison of the sweating rate in treated hand and non-treated hand showed no significant difference. There was a noticeable reduction in the density and the extent of violet areas over time, indicating reduced sweating in both hands after the treatment [Figure 1]. This reduction was more evident in aluminum chloride hexahydrate gel iontophoresis group. Interestingly, this reduction in the darkness of the skin was not limited to the palms and was extended to the fingers. No side effects such as skin redness and burns were noticed following the iontophoresis with aluminum chloride hexahydrate gel, while in tap water iontophoresis group, irritation was seen in two patients and one patient complained of temporary burning.
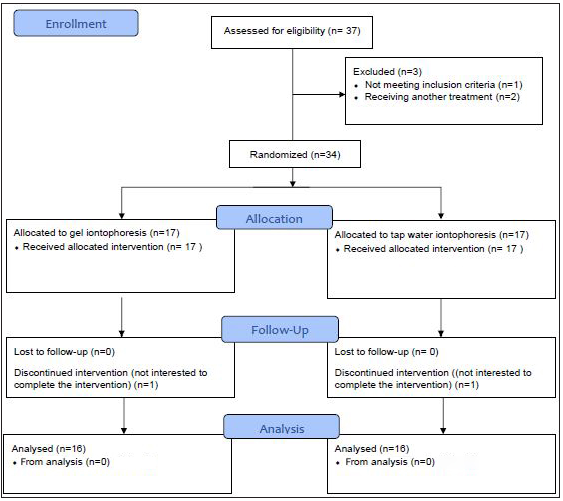
- Study flow chart.
| Variable | Tap water iontophoresis group (n = 16) | Gel iontophoresis group (n = 16) |
|---|---|---|
| Age (years) | 22.8 ± 3.9 | 24.6 ± 5.2 |
| Weight (kg) | 70 ± 16.8 | 68.8 ± 17.2 |
| Height (cm) | 171 ± 11 | 168 ± 10 |
| BMI (kg/m2) | 23.6 ± 3.2 | 23.8 ± 3.8 |
| Gel iontophoresis group | Tap water iontophoresis group | Differences in sweating rate between the two methods | P-value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Cohen’s d | Mean ± SD | Cohen’s d | Mean ± SD | ||
| Before treatment | 147.68 ± 114.68 | 134.56 ± 113.52 | 13.12 ± 179.96 | 0.74 | ||
| 3 days after treatment | 99.18 ± 98.72 | 0.47 ** | 67.81 ± 72.24 | 0.72 ** | 31.37 ± 134.04 | 0.31 |
| 1 week after treatment | 85.68 ± 79.72 | 0.65 ** | 66.87 ± 65 | 0.76 *** | 18.81 ± 113.28 | 0.47 |
| 2 weeks after treatment | 64.87 ± 46.76 | 0.98 *** | 61.75 ± 42.84 | 0.88 *** | 3.12 ± 68.48 | 0.84 |
| 3 weeks after treatment | 65.87 ± 63.8 | 0.91 *** | 76.81 ± 65.28 | 0.62** | −10.93 ± 97.2 | 0.63 |
| 4 weeks after treatment | 54.43 ± 39.08 | 1 *** | 75.50 ± 69.12 | 0.65 ** | −21.06 ± 90.4 | 0.29 |
| 8 weeks after treatment | 73.06 ± 52.56 | 0.86 *** | 88.37 ± 80.56 | 0.48** | −15.31 ± 93.52 | 0.52 |
| 24 weeks after treatment | 104.12 ± 112.12 | 0.4 * | 102.75 ± 106.4 | 0.3* | 1.37 ± 168.84 | 0.97 |
*Small effect size; **medium effect size; ***large effect size
| Gel iontophoresis group | Tap water iontophoresis group | Differences in sweating rate between the two methods | P-value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Cohen’s d | Mean ± SD | Cohen’s d | Mean ± SD | ||
| Before treatment | 163.75 ± 136.12 | 147.93 ± 112.76 | 15.48 ± 327.52 | 0.72 | ||
| 3 days after treatment | 109 ± 108.44 | 0.46** | 90.25 ± 64.08 | 0.65** | 18.75 ± 135.72 | 0.55 |
| 1 week after treatment | 102.37 ± 98.56 | 0.53** | 93.87 ± 80.92 | 0.57** | 8.5 ± 118.4 | 0.79 |
| 2 weeks after treatment | 74.37 ± 64.96 | 0.87*** | 86.50 ± 65.56 | 0.69** | −12.12 ± 93.04 | 0.6 |
| 3 weeks after treatment | 71.93 ± 74 | 0.87*** | 78.62 ± 54.96 | 0.81*** | −6.68 ± 95.52 | 0.77 |
| 4 weeks after treatment | 60.81 ± 40.8 | 1.06*** | 86.62 ± 64.04 | 0.76*** | −25.81 ± 88.08 | 0.18 |
| 8 weeks after treatment | 75.37 ± 61.64 | 0.86*** | 88.25 ± 73.56 | 0.65** | −12.87 ± 94.8 | 0.59 |
| 24 weeks after treatment | 98.50 ± 82.68 | 0.6** | 98.50 ± 73.04 | 0.54** | 0 ± 120.24 | 1 |
Discussion
Palmar hyperhidrosis negatively affects patients’ lifestyle, limits their activities, and causes emotional problems.21 Its evaluation and treatment are of great importance. In this study, the effects of two different iontophoresis treatments, with aluminum chloride hexahydrate gel 2% and tap water, were compared in patients with primary palmar hyperhidrosis over 24 weeks. The results indicated that the use of aluminum chloride hexahydrate in the form of gel is an effective intervention for people suffering from primary palmar hyperhidrosis. In addition, no side effects such as skin redness and burns were noticed following the iontophoresis with aluminum chloride hexahydrate gel, while some patients in the tap water iontophoresis group reported side effects such as irritation and burning. The extent and severity of palmar hyperhidrosis were examined through iodine–starch and gravimetry tests, respectively.22 It is suggested that gel may be more convenient than fluid for the iontophoresis; therefore, in the present study, we used aluminum chloride hexahydrate in the form of gel as an iontophoresis media for the treatment of hyperhidrosis.10 As far as we know, aluminum chloride hexahydrate in the form of gel has not been used as a media substance for iontophoresis in people with primary palmar hyperhidrosis. Although finding a way to use the skin for drug delivery is interesting, the entry of drugs through the skin could be difficult since the skin can act as a cover against therapeutic agents. The outer layer of the skin which is in contact with the external environment protects the body and limits the entrance of drugs.23 However, physiotherapy modalities, such as iontophoresis, facilitate the process of therapeutic agent delivery.6 In general, there are almost 250 sweat ducts in every square centimeter of the skin, which during iontophoresis can be used by ionic compounds to enter the skin.23 Iontophoresis is the passing of an ionized substance through intact skin by the application of an electrical current.7 Different theories, such as electrical gradient theory and plug theory, are suggested to explain the mechanism of iontophoresis for hyperhidrosis. According to the electric gradient theory, the movement of sweat in the sweat ducts is due to the electrical gradient. When iontophoresis disturbs this electrical gradient, sweat does not flow in the sweat ducts, and the person sweats less. Meanwhile, plug theory refers to the formation of diastase-resistant material in the lumens of eccrine sweat glands. Therefore, the three mechanisms suggested for the effects of iontophoresis are: (1) ion-electric field that drives ions through the skin, (2) the flow of electric current that increases the permeability of the skin, and (3) electro-osmosis.24 This study showed that the sweating rate decreased in both groups after iontophoresis treatment. Although no significant differences were seen between treatment groups, but the larger effect size values observed in aluminum chloride hexahydrate gel iontophoresis group may suggest the superior role of this gel over tap water in reducing the rate of sweating. Furthermore, sweating rate reduction was seen in a larger area on the hands with no side effects, in patients treated with aluminum chloride hexahydrate gel iontophoresis. Other studies also support the superiority of iontophoresis with aluminum chloride hexahydrate over its topical application13 or when aluminum chloride iontophoresis was compared to tap water iontophoresis.25 The present study also found the effects of iontophoresis on the other hand, such that, following the application of iontophoresis to one hand, hyperhidrosis improves in another hand. This effect was reported in other studies as well. For example, in a study investigating the effect of water iontophoresis on two hands in 11 patients with palmar hyperhidrosis similar findings were reported.26 In one study, after eight palmar hyperhidrosis treatment sessions with direct current, sweating on soles resolved simultaneously in 65 responders. It is suggested that the biofeedback mechanism could be involved in this interesting result.27 This finding was consistent with our study, which showed that treating palmar hyperhidrosis in one hand positively improves hyperhidrosis in the other hand or even in the sole of the foot. The mechanism of the iontophoresis effect on another area is not known, but it can be due to the psychological effects of the treatment program. It should also be noted that studies have not confirmed the possibility of a placebo effect in iontophoresis.28 However, all studies did not report this effect of iontophoresis. For example, Stolman showed significant differences between treated and untreated hands following 3 weeks of tap water iontophoresis in patients with palmar hyperhidrosis.6 A possible explanation for this inconsistency is that Stolman applied iontophoresis for 20 minutes at 12–20 mA, whereas in the present study iontophoresis was used for 30 minutes at 2 mA.17 The present study suggests that aluminum chloride hexahydrate gel iontophoresis is an effective alternative treatment to decrease sweating rate in an extended area with fewer side effects in patients with primary palmar hyperhidrosis, Meanwhile, further investigations with longer follow-up are needed to confirm our hypothesis regarding the effectiveness of aluminum chloride hexahydrate gel iontophoresis over tap water iontophoresis. However, contraindications to iontophoresis such as pregnancy, pacemakers, and epilepsy should be kept in mind.
Acknowledgements
This project was supported by Department of Physiotherapy, School of Rehabilitation, Shahid Beheshti University of Medical Sciences.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hyperhidrosis: review of recent advances and new therapeutic options for primary hyperhidrosis. Curr Opin Pediatr. 2014;26:460-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of botulinum toxin versus iontophoresis with topical aluminium chloride hexahydrate in treatment of palmar hyperhidrosis. Med J Armed Forces India. 2014;70:247-52.
- [CrossRef] [PubMed] [Google Scholar]
- Primary focal hyperhidrosis: current treatment options and a step‐by‐step approach. J Eur Acad Dermatol Venereol. 2012;26:1-8.
- [Google Scholar]
- Hyperhidrosis: a review and treatment options. Advances in Cosmetic Surgery. 2020;3:155-63.
- [Google Scholar]
- Gravimetry in sweating assessment in primary hyperhidrosis and healthy individuals. Clin Auton Res. 2013;23:197-200.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Iontophoresis for palmar and plantar hyperhidrosis. Dermatol Clin. 2014;32:491-4.
- [CrossRef] [PubMed] [Google Scholar]
- Focal hyperhidrosis: diagnosis and management. CMAJ. 2005;172:69-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy of iontophoresis with glycopyrronium bromide for treatment of primary palmar hyperhidrosis. J Eur Acad Dermatol Venereol. 2012;26:1167-70.
- [CrossRef] [PubMed] [Google Scholar]
- Enhancement of iontophoretic transport of diphenhydramine hydrochloride thermosensitive gel by optimization of pH, polymer concentration, electrode design, and pulse rate. AAPS PharmSciTech. 2007;8:320-5.
- [Google Scholar]
- A novel gel based vehicle for the delivery of acetylcholine in quantitative sudomotor axon reflex testing. Auton Neurosci. 2009;150:127-30.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of a gel versus solution-based vehicle for the delivery of acetylcholine in QSART. Auton Neurosci. 2010;158:123-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect and persistency of 1% aluminum chloride hexahydrate iontophoresis in the treatment of primary palmar hyperhidrosis. Iran J Pharm Res. 2011;10:641.
- [PubMed] [PubMed Central] [Google Scholar]
- Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51:274-86.
- [CrossRef] [PubMed] [Google Scholar]
- Gelation behavior of in situ forming gels based on HPMC and biphasic calcium phosphate nanoparticles. Carbohydr Polym. 2014;99:257-63.
- [CrossRef] [PubMed] [Google Scholar]
- Effect and persistency of botulinum toxin iontophoresis in the treatment of palmar hyperhidrosis. Australas J Dermatol. 2008;49:75-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperhidrosis: evolving concepts and a comprehensive review. Surgeon. 2010;8:287-92.
- [CrossRef] [PubMed] [Google Scholar]
- Statistical power analysis Jbr the behavioral. Sciences Hillsdale (NJ): Lawrence Erlbaum Associates. 1988;18:74.
- [Google Scholar]
- New effect size rules of thumb. Journal of Modern Applied Statistical Methods. 2009;8:26.
- [Google Scholar]
- Palmar hyperhidrosis: long‐term follow‐up of nine children and adolescents treated with botulinum toxin type A. Pediatr Dermatol. 2009;26:439-44.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of excess sweating of the palms by iontophoresis. Arch Dermatol. 1987;123:893-6.
- [PubMed] [Google Scholar]
- Transdermal iontophoresis: combination strategies to improve transdermal iontophoretic drug delivery. Eur J Pharm Biopharm. 2005;60:179-91.
- [CrossRef] [PubMed] [Google Scholar]
- Iontophoresis in dermatology. Indian J Dermatol Venereol Leprol. 2005;71:236.
- [CrossRef] [PubMed] [Google Scholar]
- A new strategy of iontophoresis for hyperhidrosis. J Am Acad Dermatol. 1990;22:239-41.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of hyperhidrosis manuum by tap water iontophoresis. Acta Derm Venereol. 1988;69:346-8.
- [Google Scholar]
- Safe control of palmoplantar hyperhidrosis with direct electrical current. Int J Dermatol. 2002;41:602-5.
- [CrossRef] [PubMed] [Google Scholar]
- Placebo‐controlled evaluation of direct electrical current administration for palmoplantar hyperhidrosis. Int J Dermatol. 2004;43:503-5.
- [CrossRef] [PubMed] [Google Scholar]







