Translate this page into:
A descriptive observational study on clinical and dermoscopic features of benign melanocytic neoplasms
Correspondence Address:
Siddhi Bhalchandra Chikhalkar
Department of Dermatology, Skin OPD 17/18, Seth GS Medical College and KEM Hospital, Acharya Donde Marg, Parel, Mumbai - 400 012, Maharashtra
India
| How to cite this article: Malladi NS, Chikhalkar SB, Khopkar U, Kharkar V. A descriptive observational study on clinical and dermoscopic features of benign melanocytic neoplasms. Indian J Dermatol Venereol Leprol 2020;86:251-261 |
Abstract
Background: Benign melanocytic neoplasms have nests of melanocytic cells and show characteristic dermoscopic features. Clinical and dermoscopic features have not been studied previously in the Indian population.
Aims: To study the clinical, epidemiological and dermoscopic patterns of benign melanocytic neoplasms.
Methods: This was a descriptive, observational, single centre study. In 107 patients with melanocytic neoplasms, 167 lesions were clinically examined and studied under the dermoscope and histopathological examination was done when indicated. The lesions were broadly divided as acquired and congenital. Five main dermoscopic patterns were seen–globular, homogenous, reticular, parallel and streaks. If there were two of these patterns in a particular lesion, it was termed 'mixed pattern'. The presence of three or more patterns was called 'multicomponent pattern'. Various other features were also observed.
Results: The majority of patients belonged to the third decade with a female preponderance. History of increased UV exposure and family history was significant in acquired nevi. The dermoscopic pattern progressed from predominantly reticular in junctional nevi to predominantly globular in compound nevi and lesser pigment in intradermal nevi, with more vascular structures. The congenital melanocytic nevi showed additional features of comedo- like lesions, milia- like cysts, perifollicular pigmentary changes and increased colour variation. Even though colour variation was observed in both acquired and congenital lesions, no signs of dysplasia were seen on histopathology.
Limitations: A larger sample size is required, with follow up of lesions. No parallel studies in brown skinned population were found for exact comparison.
Conclusion: Benign melanocytic proliferations are often neglected in our country. This study will help in understanding the course, clinical features and dermoscopic patterns of various benign melanocytic neoplasms, and will be a step forward towards research in our population. To the best of our knowledge, this is the first study of its kind in India.
Introduction
Benign melanocytic proliferative lesions consist of a spectrum of disorders. They range from an epidermal focus with simple increase in melanocyte number to massive accumulation of cells in multiple tissue elements. These lesions are divided into melanocytic neoplasias and hyperplasias.[1]
The term melanocytic neoplasia is used to describe the presence of melanocytic cells in epidermal nests or theques within the dermis or other tissues. A nest is defined as three or more melanocytic cells in direct contact with each other. The melanocytic neoplasms are referred to as nevi. The melanocytic cells forming these nevi are referred to as nevomelanocytes. The term melanocytic hyperplasia indicates increased melanocytes confined to basal layer of epidermis. It includes lentigines. In contrast, freckles or ephelides are a result of increased sun-induced melanogenesis, with normal number of melanocytes.[1]
Dermoscopy is a noninvasive andin vivo diagnostic tool. It visualizes subtle clinical patterns of skin structures invisible to the unaided eye. It has been primarily used for the diagnosis of pigmented lesions, although it is being used in a variety of skin disorders.[2]
We conducted this study to note the clinical and epidemiological features of benign melanocytic neoplasms and their dermoscopic patterns. We were unable to find any similar Indian study done previously.
Methods
The study was conducted in the outpatient department of KEM Hospital, Mumbai, India. Approval by the Ethics Committee was obtained. One hundred and seven patients with benign melanocytic neoplasms were enrolled in the study. A pre-procedure informed written consent was taken. This was followed by a detailed clinical history, examination and clinical photographs. Dermoscopic examination was done for 167 lesions. The findings were recorded. The Oitez Escope dermoscope with 20× and 200× magnification was used. The lesions were examined in white light and polarized light. The dermoscope was attached to the monitor of the computer screen. Live images were seen and recorded.
Acquired and congenital melanocytic nevi were included in the study while seborrheic keratoses, ephelides, lentigines and mucosal nevi were excluded. The definition of neoplasia does not include ephelides and lentigines, while dermoscopy of mucosal nevi was cumbersome. Seborrheic keratoses were clinically differentiated based on the age of onset, stuck on appearance, verrucous surface changes and follicular plugging. A biopsy was done for exclusion wherever needed. No cases with malignant transformation either clinically (sudden change in color, border or surface characteristics) or on histopathology were seen in the study.
In our study, five main patterns for melanocytic neoplasms – globular, homogenous, reticular, parallel and streaks – were assessed. When there were two of the above patterns in a particular lesion, it was termed “mixed pattern.” Presence of three patterns or more was called “multicomponent pattern.”[3]
Other features evaluated were dots, cobblestones, blotches, pseudonetwork, fissures and ridges, milia-like cysts, comedo-like lesions, vascular structures, structureless areas, curvilinear streaks, lattice pattern, hypopigmented areas, wobble sign, perifollicular hyperpigmentation, perifollicular hypopigmentation and leukotrichia.
Statistical analysis was done using Statistical Package for the Social Sciences, version 23. Different statistical tests were conducted, such as mean, standard deviation to describe the demographic factors and Pearson's Chi-square tests to establish any relation between the factors.
Results
Of the 107 participants in the study, there were 67 (62.6%) females and 40 (37.4%) males. There was increased incidence of benign melanocytic neoplasms in females with female to male ratio of 1.67:1.
The age of patients ranged from 5 months to 68 years, with the mean age being 28.48 years. Majority of the patients, 49 (45.8%) belonged to the age group of 21–30 years followed by 21 (19.6%) in age group 11–20 years and 13 (12.1%) in 31–40 years. Seventy nine (73.8%) of the subjects had an indoor occupation, while 28 (26.2%) had an outdoor occupation.
A total of 167 lesions were studied in 107 patients. One hundred and fifteen lesions (68.9%) were acquired, while 52 lesions (31.1%) were congenital. The halo phenomenon was seen in three cases of congenital melanocytic nevus. [Table - 1] shows the further subdivision.
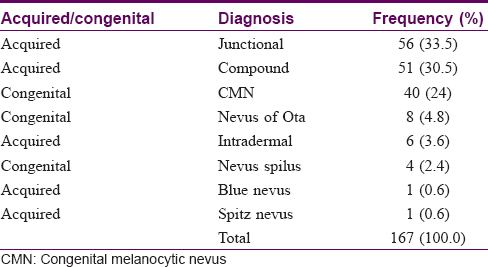
[Table - 2] depicts the age of onset of all lesions. Among 115 acquired melanocytic neoplasms, 43 (37.3%) were progressive in nature and a minority of lesions 12 (10.4%) were in the involuting stage. Four cases of eruptive melanocytic nevi were found. Three of these cases had a peripheral rim of globules, which is suggestive of progression in size of lesions.[4]

A significantly strong positive family history was seen in 90 (78%) patients with acquired melanocytic nevi (P < 0.05). History of increased UV exposure was present in 66 (57.3%) patients with acquired nevi (P < 0.05).
Ninety five (56.9%) of the total lesions were seen in Fitzpatrick skin type 5 patients, followed by 70 (41.9%) in Fitzpatrick skin type 4 persons. This is as expected in the Indian population. One case (0.6%) each was seen in patients with Fitzpatrick skin type 1 and 3 (P < 0.001). About one-third of the acquired melanocytic lesions had a size> 6 mm and about half had color variation. None showed signs of dysplasia on histopathological examination.
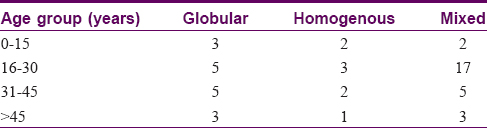
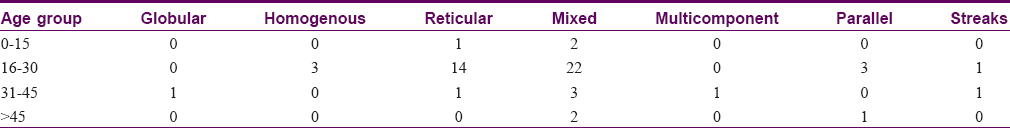
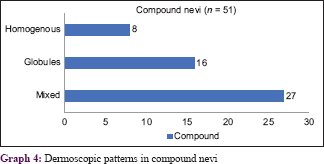
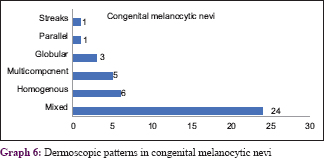
The most common dermoscopic pattern observed was the mixed pattern. This was followed by homogenous, globular, reticular, multicomponent pattern, parallel and lastly the streaks pattern [Table - 3] and [Table - 4]. [Table - 5] and [Table - 6] depicts their distribution in various age groups.

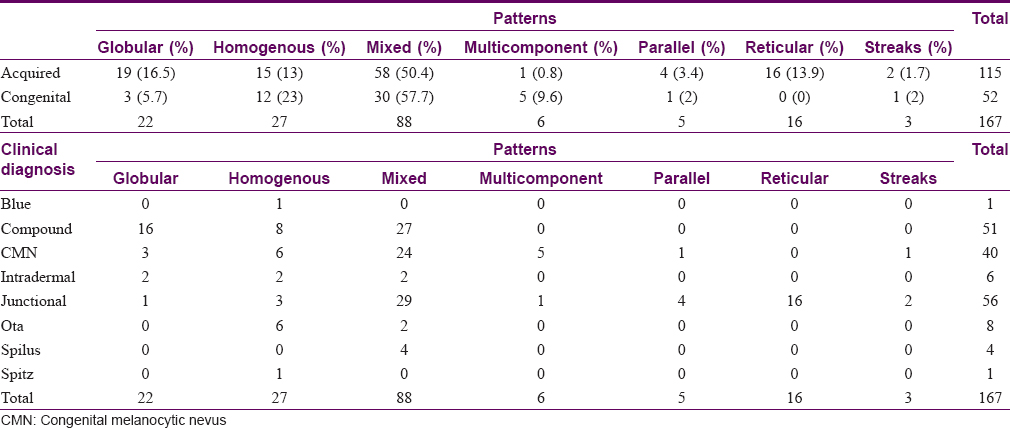
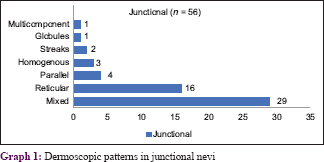
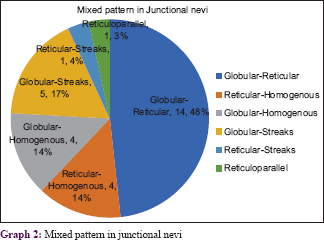
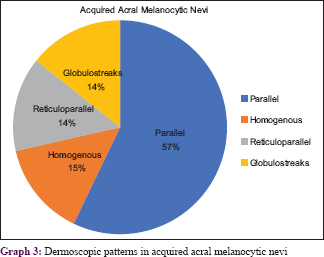
The various patterns [Figure - 1], [Figure - 2], [Figure - 3], [Figure - 4], [Figure - 5] in junctional nevi are depicted in Graphs 1 and 2. In our study, 8 cases of acral melanocytic nevi of junctional type were present. Of these, 7 were acquired melanocytic nevi [Graph 3 and [Figure - 6], while a single case was that of a congenital melanocytic nevus over the acral area.
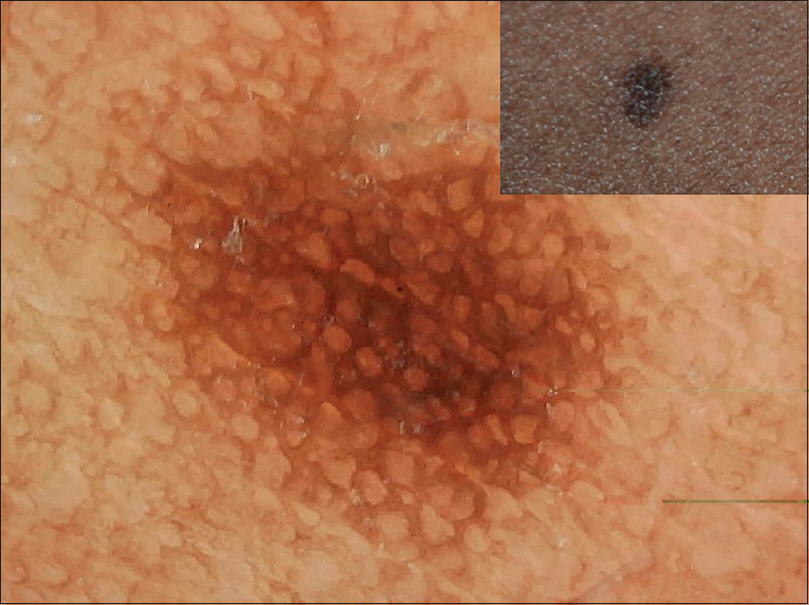 |
| Figure 1: Dermoscopy of junctional nevus showing typical reticular pattern darker in center and lighter in periphery |
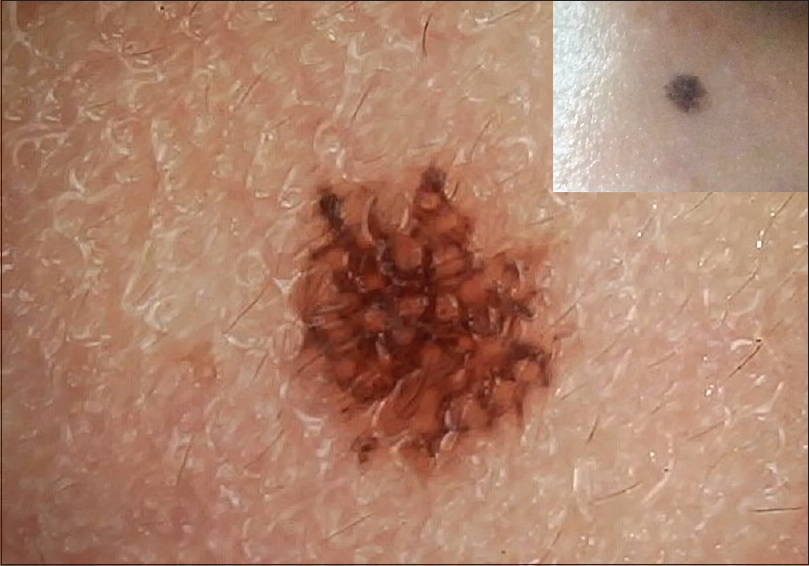 |
| Figure 2: Junctional nevus showing mixed reticular pattern with occasional interspersed globules and few dots |
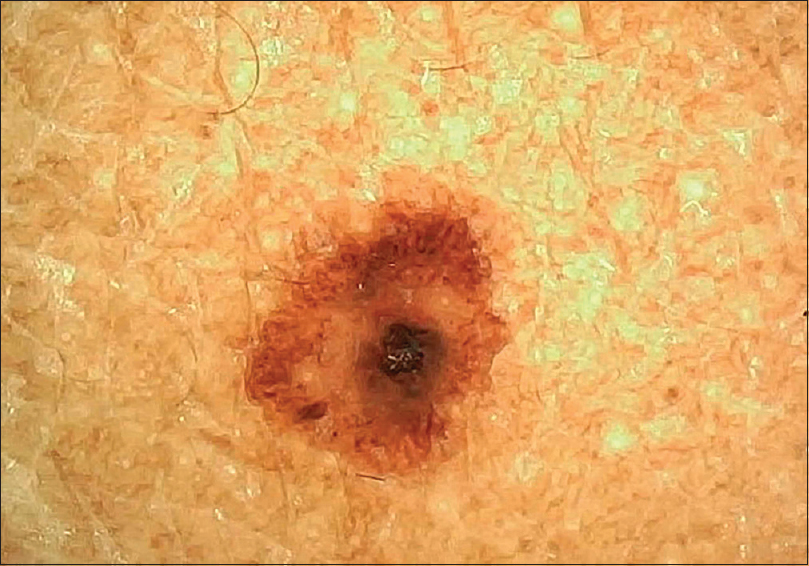 |
| Figure 3: Junctional nevus with central globule with blotches and peripheral streaks arranged in a target-like pattern |
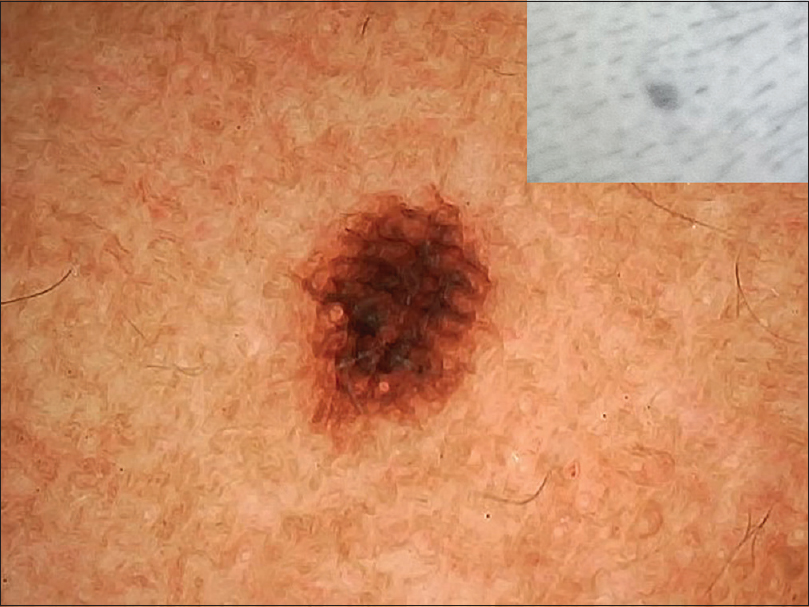 |
| Figure 4: Junctional nevus showing reticular areas with radiating streaks giving a mite-like appearance |
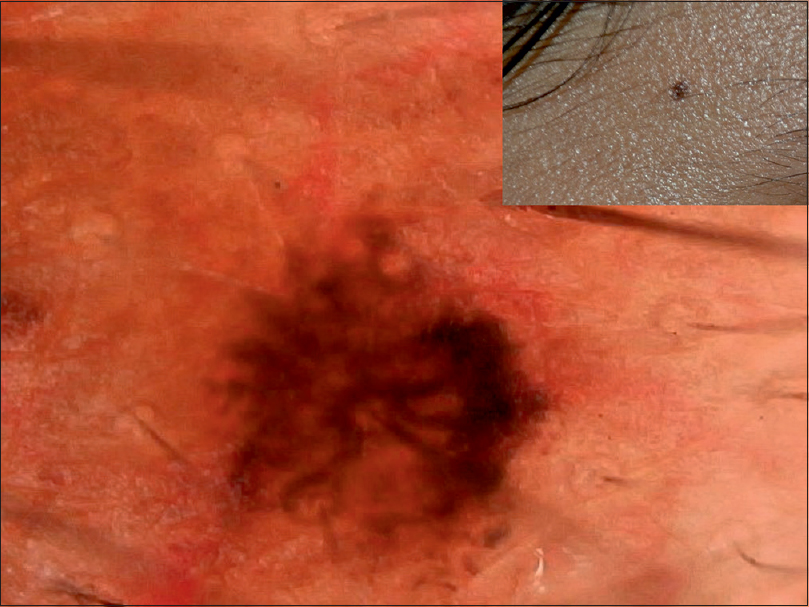 |
| Figure 5: Junctional nevus with streaks and formation of homogenous pattern at one end indicative of progression |
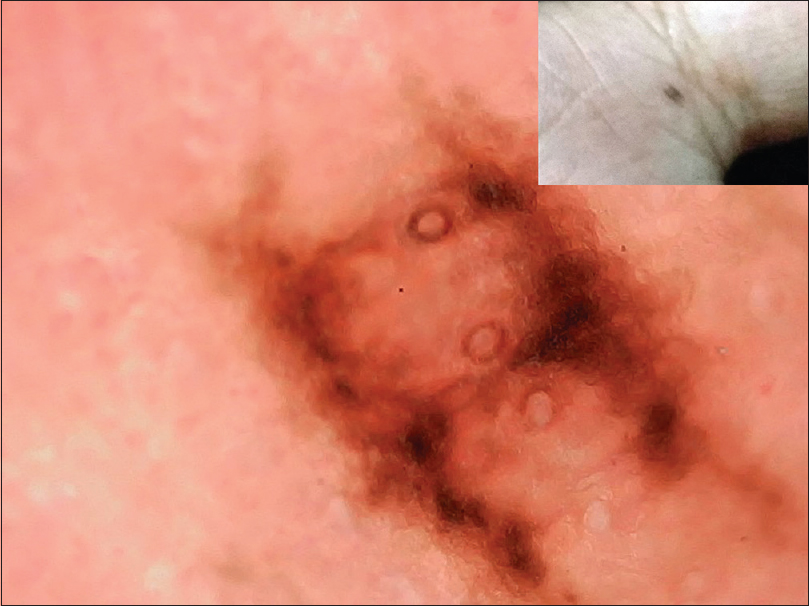 |
| Figure 6: Palmar junctional nevus in parallel lattice pattern showing pigmentation in furrows with perpendicular connecting lines. Openings of eccrine glands seen |
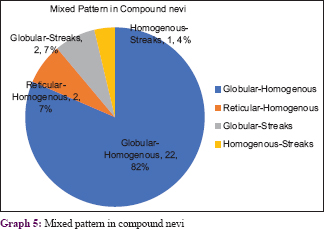
Graphs 4 and 5 show the patterns seen in compound nevi [Figure - 7], [Figure - 8], [Figure - 9].
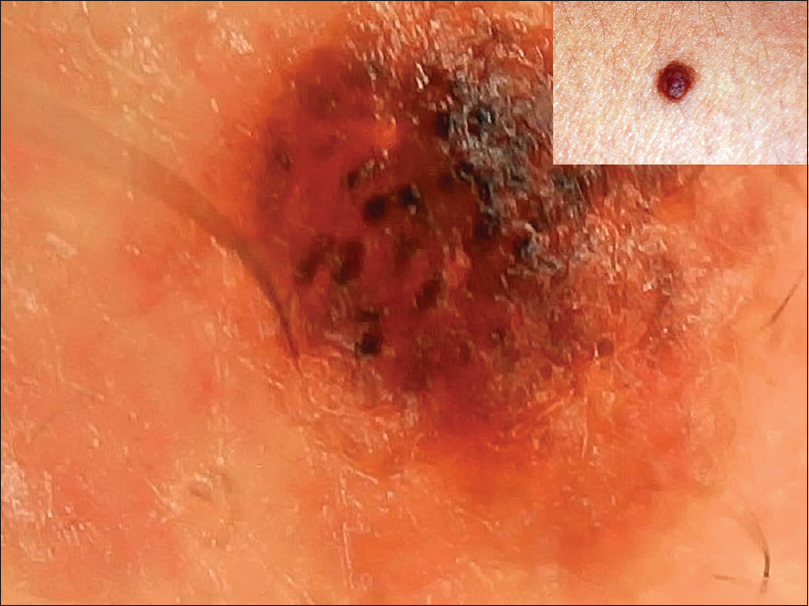 |
| Figure 7: Compound nevus showing a dark brown and black globules with background of light brown coloration |
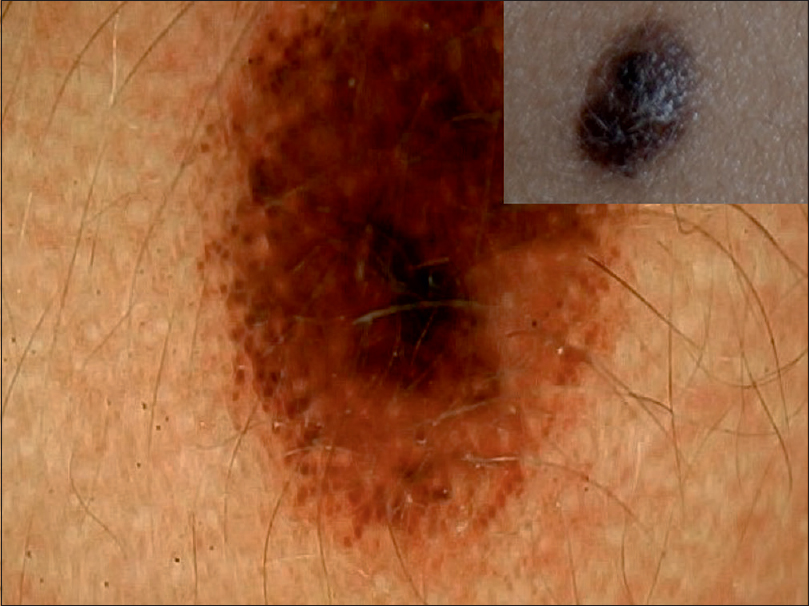 |
| Figure 8: Eruptive compound melanocytic nevus with central homogenous pattern and peripheral rim of globules indicative of progression |
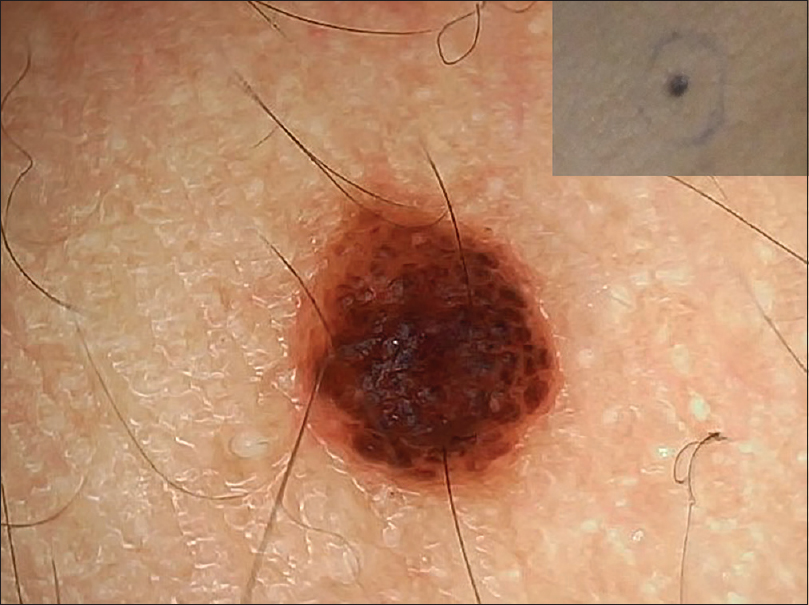 |
| Figure 9: Compound nevus showing aggregation of globules in a cobblestone pattern |
The five intradermal nevi were less pigmented and had a mixed pattern with occasional globular and homogenous patterns. Additional patterns such as vascular patterns [Figure - 10] and curvilinear streaks with mosaic pattern [Figure - 11] were present. One intradermal nevus showed homogenous pigmentation and exophytic morphology [Figure - 12]. This is suggestive of Unna nevus.
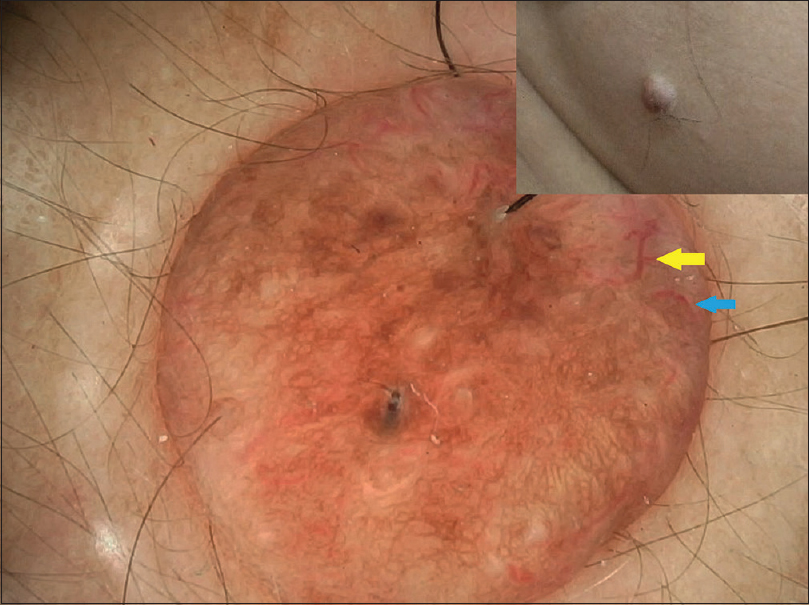 |
| Figure 10: Intradermal nevus with mixed pattern and decreased pigmentation and vascular patterns—comma vessels (blue arrow) and hairpin loops (yellow arrow) seen |
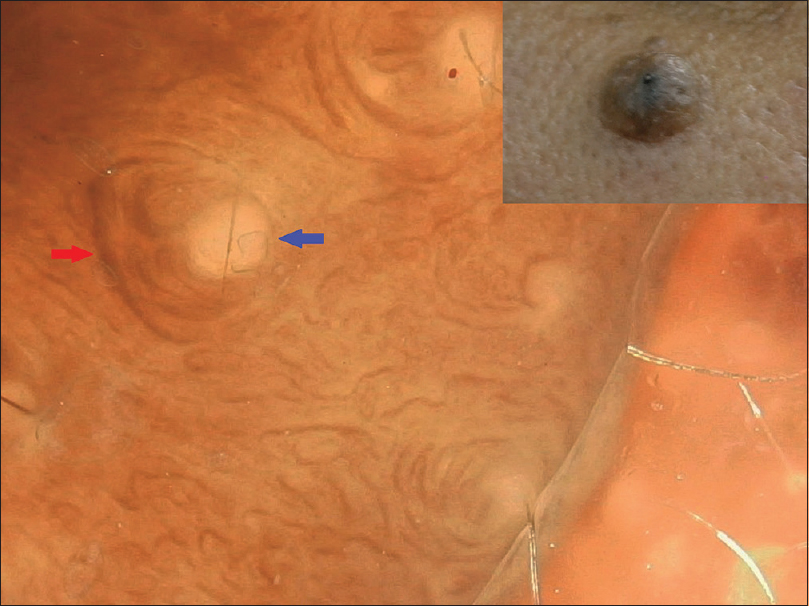 |
| Figure 11: Intradermal nevus with no pigmentation and curvilinear streaks (red arrow) around milia-like cysts (blue arrow) arranged in a mosaic pattern |
 |
| Figure 12: Unna-like intradermal nevus showing homogenous pigmentation and exophytic morphology with fissures and ridges resembling a scrotal appearance |
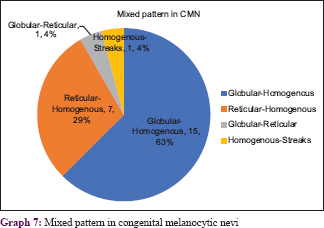
Graph 6 shows congenital melanocytic neoplasms with their various patterns [Figure - 13], [Figure - 14], [Figure - 15], [Figure - 16], [Figure - 17], [Figure - 18], [Figure - 19], [Figure - 20], [Figure - 21], [Figure - 22]. One case of congenital melanocytic nevus on the palm showed parallel furrow pattern with crista dots known as peas in pod appearance [Figure - 15] and [Figure - 16]. Congenital melanocytic nevi also showed noteworthy features such as perifollicular pigment changes [Figure - 18], comedo-like lesions, fissures and ridges [Figure - 19], increased color variation, a greenish discoloration and occasional hypopigmentation along the creases..
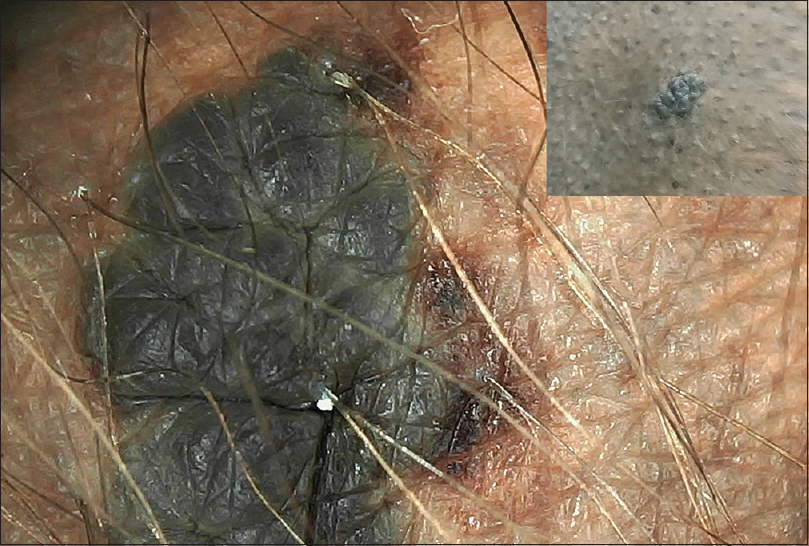 |
| Figure 13: Bathing trunk nevus with localized proliferative nodule with homogenous pattern showing color variation and greenish coloration along creases |
 |
| Figure 14: Large congenital melanocytic nevus with homogenous pattern and peripheral granularity showing greenish discoloration and perifollicular hyperpigmentation |
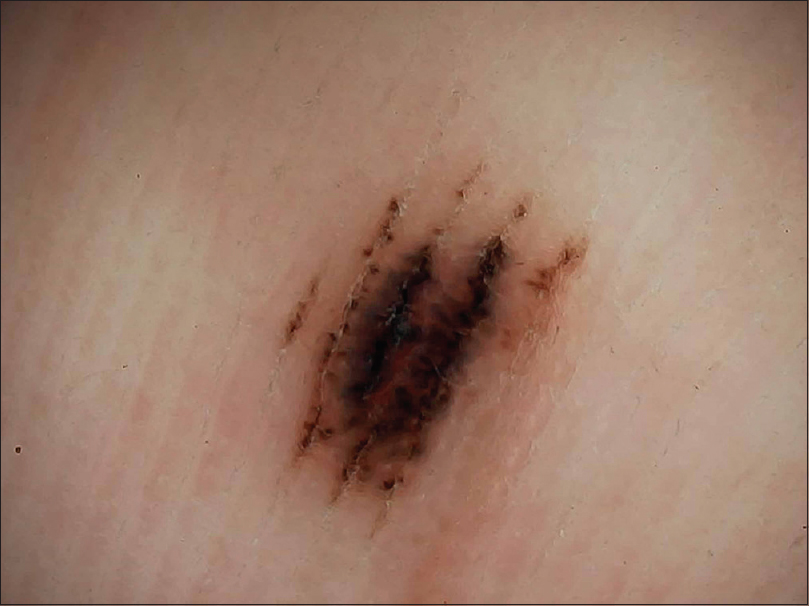 |
| Figure 15: Palmar congenital melanocytic nevus showing pigmentation along furrows and attached globules (crista dots) known as peas in pod appearance |
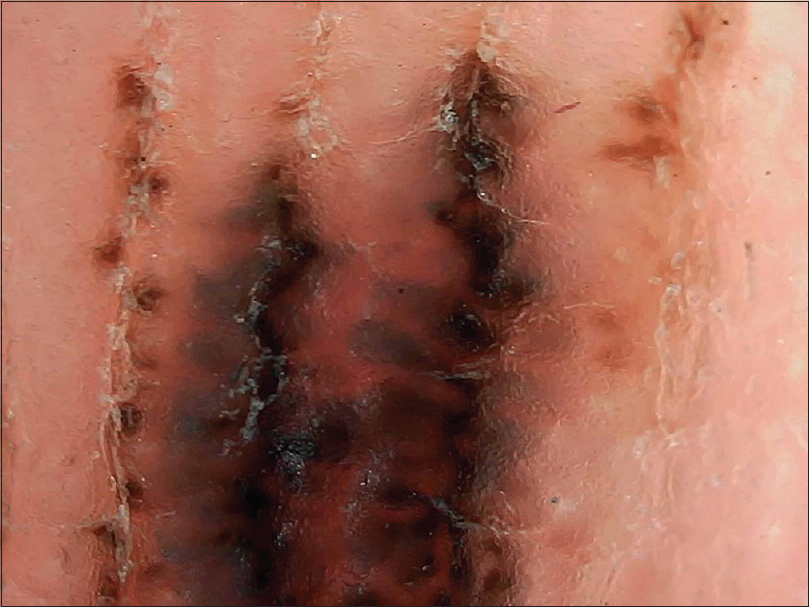 |
| Figure 16: Higher magnification (×200) of the peas in pod appearance of palmar congenital melanocytic nevus |
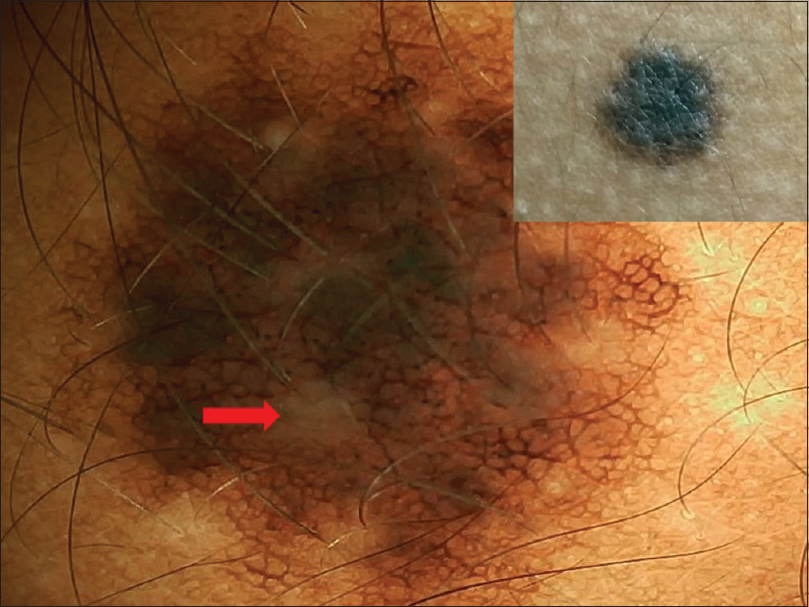 |
| Figure 17: Satellite lesion in bathing trunk nevus showing homogenous with peripheral reticular pattern with perifollicular hypopigmentation (red arrow) |
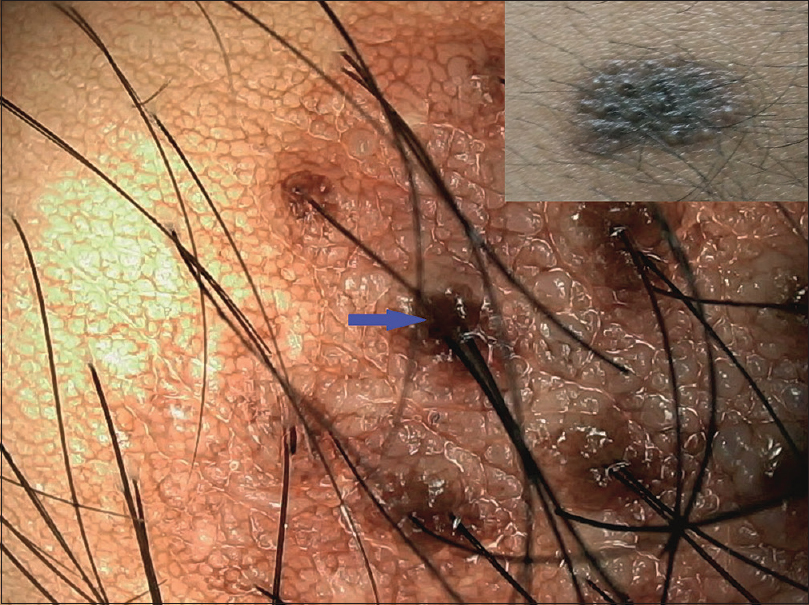 |
| Figure 18: Medium-sized congenital melanocytic nevus with central homogenous pattern and peripheral reticular pattern merging into the surrounding. Also shows perifollicular hyperpigmentation (blue arrow) and cerebriform surface |
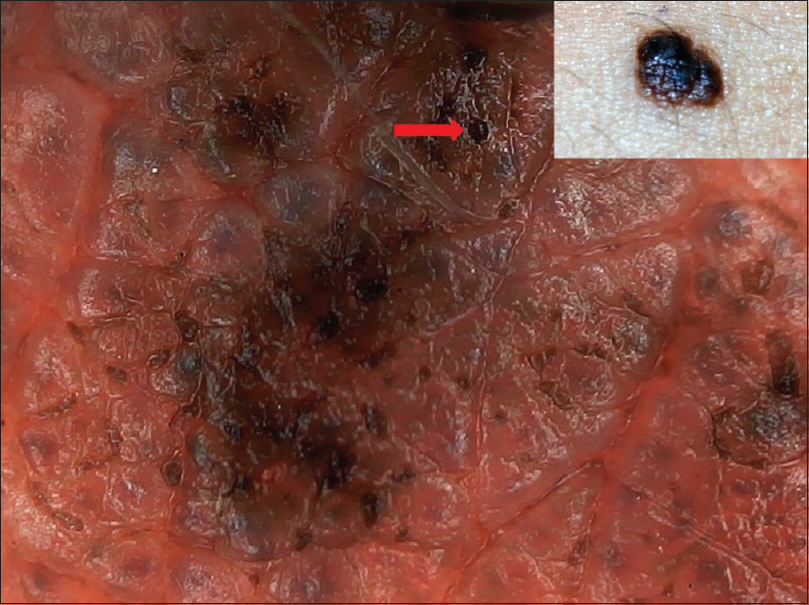 |
| Figure 19: Small congenital melanocytic nevus showing globular pattern with comedo-like lesions (red arrow) and fissures and ridges (cerebriform appearance) |
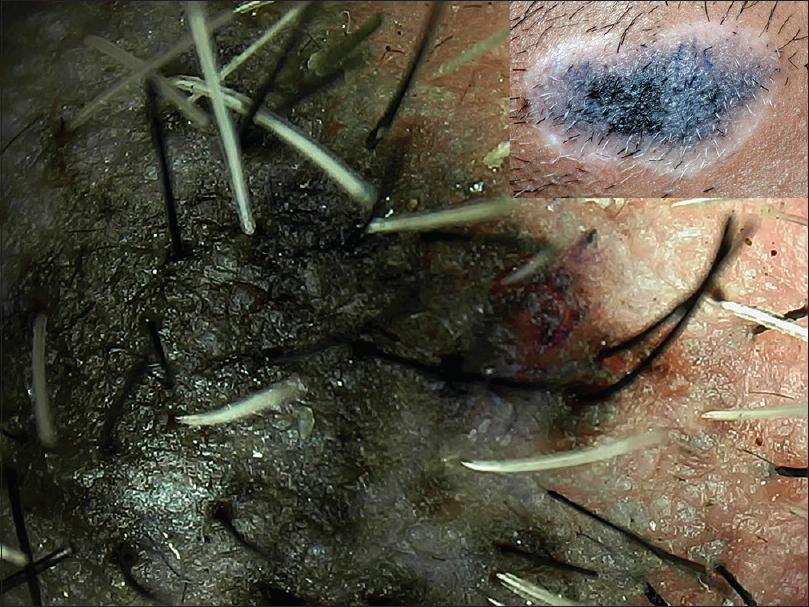 |
| Figure 20: A case of congenital melanocytic nevus with depigmented halo surrounding it (Halo nevus) showing progressive whitish coloration and leukotrichia |
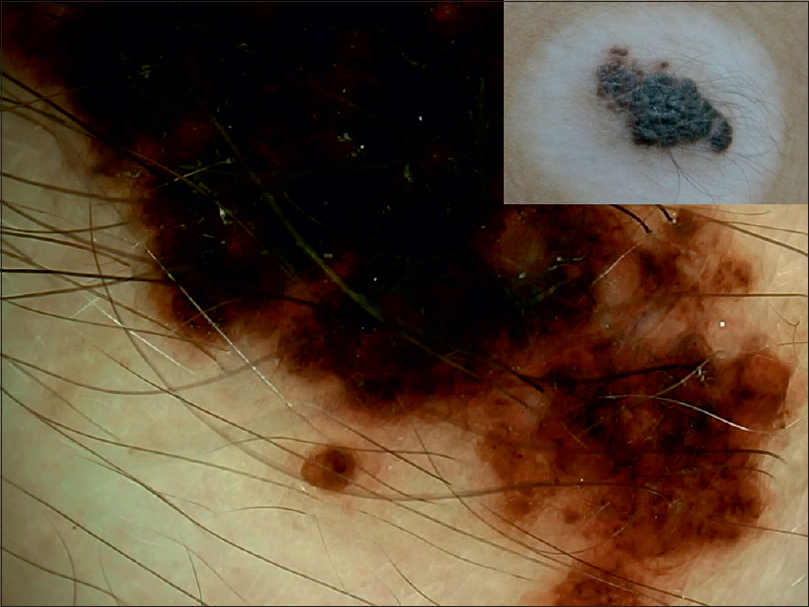 |
| Figure 21: Halo nevus in congenital melanocytic nevus showing increased granularity at the periphery (lower aspect) |
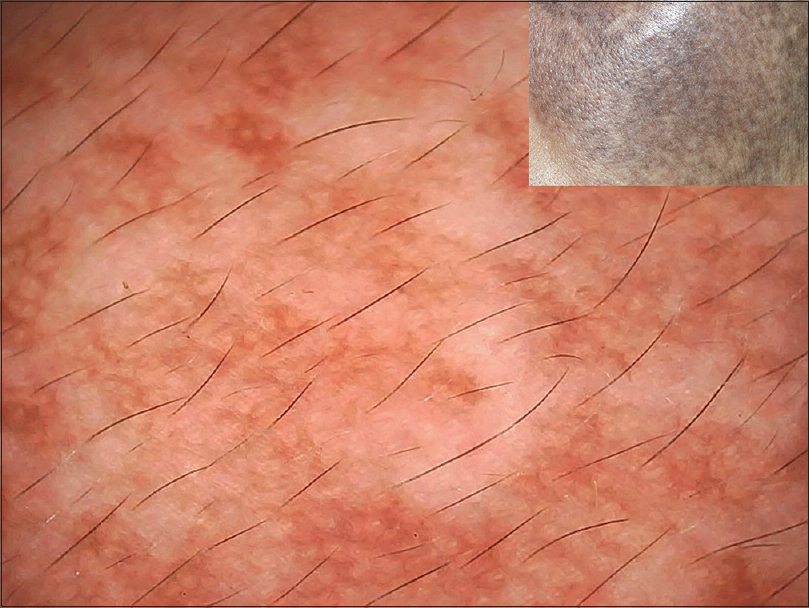 |
| Figure 22: Nevus of Ota showing unspecified brownish pigment with pseudoreticular pattern |
In our study, halo phenomenon was seen in three cases of congenital melanocytic nevi with features of leukotrichia and depigmentation around the lesions [Figure - 20]. Increased granularity at the periphery was seen in one lesion [Figure - 21].
All eight cases of Nevus of Ota had a predominantly homogenous unspecified pattern with pseudonetwork or pseudoreticular pattern [Figure - 22]. A few areas with reticular or globular pattern were present.
All four cases of Nevus spilus demonstrated a mixed pattern. They had dermoscopic features similar to either a compound nevus or junctional nevus with a mixed pattern (globulohomogenous or reticulohomogenous). Blue nevus showed homogenous pattern with blue grey discoloration and hypopigmented areas [Figure - 23].
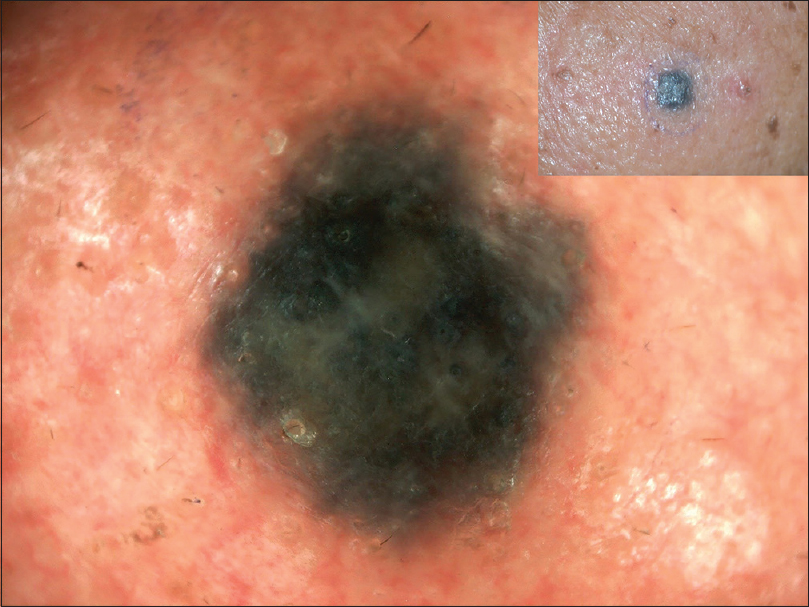 |
| Figure 23: Blue nevus showing homogenous pattern with dark blue coloration and hypopigmented areas |
A single case of Spitz nevus in a case of oculocutaneous albinism was observed. Dermoscopy revealed a homogenously pinkish nodule with papillomatous surface and evidence of vascular structures (dotted and comma-shaped) [Figure - 24]. Occasional pigment was present.
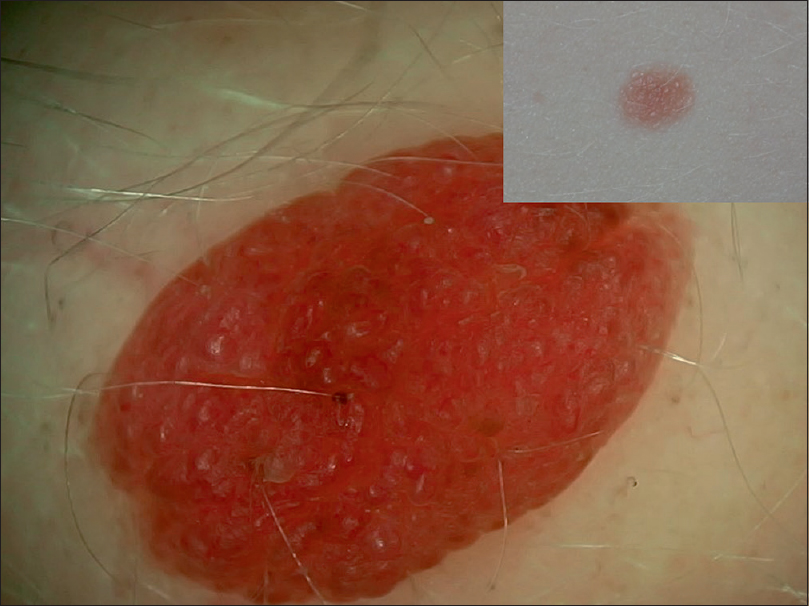 |
| Figure 24: A case of oculocutaneous albinism showing pink papillomatous surface with vascular structures such as comma vessels and dotted vessels |
Discussion
Benign melanocytic neoplasms have been divided into acquired and congenital. In acquired nevi we have included the common melanocytic nevi-like junctional nevi, compound nevi and intradermal nevi. Some rare varieties such as Spitz nevi and blue nevi are also included in this category. In the group of Congenital melanocytic nevi, rarer varieties such as Nevus of Ota and nevus spilus were also included. A difference in frequency distribution of nevi according to gender is not clear, although most series show equal prevalence in males and females.[1] In our study, there was increased incidence of benign melanocytic neoplasms in females. A recent study in Turkey by İyidal et al. on 412 patients had 57.3% female patients and 42.7% male patients.[5] This was similar to our study. The increased incidence in the female population in our study could be a result of greater cosmetic concerns in women.
Cooke et al. in a study of 872 New Zealand adults found the highest prevalence of nevi in adults aged 20 to 39.[6],[7] This was similar to our study where the majority of the subjects were in the third decade followed by second decade and then fourth decade of life. The occupational profile of the patients in our study consisted of more indoor workers than outdoor. Augustsson et al. in Sweden conducted a study among 310 adult subjects aged 30–50 years.[8] They also found that indoor workers had higher counts of nevi than outdoor workers. This suggests that exposure of unacclimatized skin to strong sunlight may be important in causation of nevi. These results support the hypothesis that intermittent exposure to ultraviolet light has a nevogenic effect, while chronic exposure might be protective.
Genetic factors directly determine an individual's potential for the development of melanocytic nevi. The final expression of this potential is influenced by solar exposure. The notion is that oncogenic BRAF could also be an early acquired somatic mutation induced by intermittent ultraviolet exposure. This oncogenic BRAF is common among acquired nevi. Other types of melanocytic nevi including intermediate to large congenital nevi, Spitz nevi and Blue nevi demonstrate mutations in other genes. These include NRAS, HRAS or GNAQ, respectively.
In our study nevi were either stable, progressive or involuting. The life of a melanocytic nevus can conceptually be divided into inception, promotion, senescence and involution.[10],[11] Most of the acquired melanocytic nevi go through a phase of growth and promotion during puberty under the influence of hormones. In adulthood or late adulthood, they involute. This results in clinical and dermoscopic evidence of vanishing pigmentation.[9]
The cases of eruptive acquired melanocytic nevi noted in the adolescent age group in our study could be due to the hormonal flux during puberty. The rare phenomenon of sudden onset or increase in the number or size of nevi has been linked with dermatological diseases and immune suppression. The most plausible theory for eruptive nevi postulates that an intact immunological state normally prevents the proliferation of melanocytic lesions.[12],[13] Immune suppression, or even an alteration in T cells, induces melanocyte-stimulating hormone or melanoma growth stimulatory activity.[13]
No parallel studies with the same parameters as our study was found for comparison. Hence comparisons have been made from the descriptions given in the literature.
Pigmented lesions may be melanocytic lesions or nonmelanocytic lesions. Dermoscopic features such as pigment network, pseudonetwork, aggregated globules, branched streaks and parallel pattern are suggestive of melanocytic lesions. Nonmelanocytic lesions could be seborrheic keratoses, basal cell carcinoma, angioma or angiokeratoma. Seborrheic keratoses are characterized by milia-like cysts, comedo-like openings, fissures and ridges, hair pin blood vessels, sharp demarcation, moth eaten border and light brown fingerprint-like structures. Basal cell carcinoma shows features of arborizing vessels, maple leaf-like areas, large blue gray ovoid cysts, spoke wheal areas and ulceration. Angiomas and angiokeratomas show red, blue black lacunae or red bluish to red black homogenous areas.[14]
The dermoscopic features of junctional nevus have a honeycomb-like network pattern in a uniform distribution. The lines of the network are light to dark brown and have regular length and width. The network often is darker in the center and fades gradually toward the edges of the lesion (i.e., thins out and blurs at the periphery). Heavy pigmentation is often present in the center and obscures the pattern. Small black dots and globules may be present either overlying the network or in between the grid.[15]
The basis of this pigment network is either melanin pigment in keratinocytes or in melanocytes along the dermo-epidermal junction. The reticulation (network) denotes the rete ridge pattern of the epidermis. The relatively hypomelanotic holes in the network represent the tips of the dermal papillae and the overlying suprapapillary plates of the epidermis.[14] The pigment network merges with the surrounding skin.
In our study, predominance of the reticular pattern and globulo-reticular pattern was observed. The increased globular pattern and homogenous pattern may be an indication of progression of the junctional nevus to a compound nevus. Some nevi exhibit a peripheral rim of small brown globules on dermoscopy; this has been shown to be a sign of nevus growth.[4] Pseudonetwork or pseudoreticular pattern was seen in lesions over the face. Structureless areas without regression or scar-like areas were seen in involuting nevi in our study. Dermoscopy has given us an insight into the three pathways for involution: with halo phenomenon, with regression structures or with neither of these features.[10] The involuting junctional nevi in our study belonged to the third category. Acquired acral nevi have a parallel pattern or mixed pattern corresponding to dermatoglyphics.
The globular pattern is a pattern commonly seen in compound nevi. It is characterized by homogeneous globules distributed uniformly throughout the lesion. Globules have a diameter larger than 0.1 mm and correspond to nests of pigmented melanocytes. When large regular globules are closely aggregated, it is known as cobblestone pattern.[15]
Blotches correspond to a large concentration of melanin pigment, while dots correspond to fine melanin particles or melanin “dust” in melanophages. In our study, we have observed that the pattern changes from a predominant reticular pattern in junctional nevi to globular and homogenous pattern in compound nevi. As per literature, intradermal nevi have fewer dermoscopic structures with smooth or papillomatous surface. Pigment network or black dots are absent. In case of presence of pigment, it is usually homogenous light brown in color or occasional globules can be seen. They are characterized by presence of vascular structures. This is an important finding. Dilated blood vessels could be visualized mostly as comma vessels or hairpin vessels, and occasionally as dotted vessels.[15]
In our study, intradermal nevi showed predominance of vascular structures. The novel features seen were curvilinear streaks in mosaic arrangement around milia-like cysts. The pattern of a blue nevus is described as homogenous structureless blue or steel blue pigmentation distributed diffusely throughout the entire lesion.[16] The border of a blue nevus is generally well defined. Pseudopod and radial streaming are absent.
As per literature, congenital melanocytic nevi show discrete light to dark brown or black globules of varying shapes, sizes and numbers.[15] Milia-like cysts, comedo-like lesions, hypertrichosis, skin furrow hypopigmentation and perifollicular pigment changes are seen. Similar features were observed in our study.
Milia-like cysts are round whitish or yellowish structures which correspond to intraepidermal keratin filled cysts. Hypertrichosis could be due to changes in local cytokine environment caused by accumulation of nevomelanocytes. Perifollicular hypopigmentation and hyperpigmentation was markedly prominent in our study in congenital melanocytic nevi. Our hypothesis is that the hyperpigmentation could be due to the accumulation of the pigmented nevus cells in perifollicular location. The nevus cells have tendency to invade the appendageal structures.[17]
Both large congenital melanocytic nevi and Beckers nevus show dermoscopic features such as focal thickening of network pattern, skin furrow hypopigmentation and perifollicular hypopigmentation. But features such as target globules, aggregated globules, homogenous diffuse pigmentation and target vessels are more common in congenital melanocytic nevi.[18]
The dermoscopic features are helpful for diagnosis of the benign melanocytic lesions. These help us to understand the course of the lesions.
Limitations
- These findings need to be explored further by studies with a larger sample size.
- Follow-up of dermoscopy of these lesions will help us to further understand the nature and course of nevi. This can provide insights to nevogenesis.
- Similar comparative studies in brown-skinned individuals could not be found for direct comparison.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Grichnik JM, Rhodes AR, Sober AJ. Benign neoplasias and hyperplasias of melanocytes. In: Goldsmith AL, editor. Fitzpatrick's Dermatology in General Medicine. 8th ed. USA: McGraw-Hill; 2012. p. 1377-96.
[Google Scholar]
|
| 2. |
Dogra S, Mittal A. Role of dermoscopy in the diagnosis of pigmentary dermatoses in skin of color. Pigment Int 2014;1:41.
[Google Scholar]
|
| 3. |
Tanaka M. Dermoscopy basics and melanocytic lesions (Part 2 of 2). Hong Kong J Dermatology Venereol 2013;21:181-7.
[Google Scholar]
|
| 4. |
Zalaudek I, Schmid K, Marghoob AA, Scope A, Manzo M, Moscarella E, et al. Frequency of dermoscopic nevus subtypes by age and body site: A cross-sectional study. Arch Dermatol 2011;147:663-70.
[Google Scholar]
|
| 5. |
İyidal AY, Gül Ü, Kılıç A. Number and size of acquired melanocytic nevi and affecting risk factors in cases admitted to the dermatology clinic. Postepy Dermatol Alergol 2016;33:375-80.
[Google Scholar]
|
| 6. |
Gallagher RP, McLean DI. The epidemiology of acquired melanocytic nevi. A brief review. Dermatol Clin 1995;13:595-603.
[Google Scholar]
|
| 7. |
Cooke KR, Spears GF, Skegg DC. Frequency of moles in a defined population. J Epidemiol Community Health 1985;39:48-52.
[Google Scholar]
|
| 8. |
Augustsson A, Stierner U, Rosdahl I, Suurküla M. Regional distribution of melanocytic naevi in relation to sun exposure, and site-specific counts predicting total number of naevi. Acta Derm Venereol 1992;72:123-7.
[Google Scholar]
|
| 9. |
Zalaudek I, Catricalà C, Moscarella E, Argenziano G. What dermoscopy tells us about nevogenesis. J Dermatol 2011;38:16-24.
[Google Scholar]
|
| 10. |
Terushkin V, Scope A, Halpern AC, Marghoob AA. Pathways to involution of nevi: Insights from dermoscopic follow-up. Arch Dermatol 2010;146:459-60.
[Google Scholar]
|
| 11. |
Stefanaki I, Antoniou C, Stratigos A. Benign melanocytic proliferations and melanocytic neavi. In: Griffiths C, editor. Rooks Textbook of Dermatology. 9th ed. UK: Wiley Blackwell; 2016. p. 132.1-132.47.
[Google Scholar]
|
| 12. |
Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol 2005;52:S96-100.
[Google Scholar]
|
| 13. |
Richert S, Bloom EJ, Flynn K, Seraly MP. Widespread eruptive dermal and atypical melanocytic nevi in association with chronic myelocytic leukemia: Case report and review of the literature. J Am Acad Dermatol 1996;35:326-9.
[Google Scholar]
|
| 14. |
Braun RP, Rabinovitz HS, Oliviero M, Kopf AW, Saurat JH. Dermoscopy of pigmented skin lesions. J Am Acad Dermatol 2005;52:109-21.
[Google Scholar]
|
| 15. |
Rao BK, Wang SQ, Murphy FP. Typical dermoscopic patterns of benign melanocytic nevi. Dermatol Clin 2001;19:269-84.
[Google Scholar]
|
| 16. |
Zalaudek I, Manzo M, Savarese I, Docimo G, Ferrara G, Argenziano G, et al. The morphologic universe of melanocytic nevi. Semin Cutan Med Surg 2009;28:149-56.
[Google Scholar]
|
| 17. |
Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol 2013;88:863-78.
[Google Scholar]
|
| 18. |
Ingordo V, Iannazzone SS, Cusano F, Naldi L. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: An analysis with a 10-fold magnification. Dermatology 2006;212:354-60.
[Google Scholar]
|
Fulltext Views
13,610
PDF downloads
3,180





