Translate this page into:
A descriptive study to characterize segmental vitiligo
Correspondence Address:
Binod K Khaitan
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi-110029
India
| How to cite this article: Khaitan BK, Kathuria S, Ramam M. A descriptive study to characterize segmental vitiligo. Indian J Dermatol Venereol Leprol 2012;78:715-721 |
Abstract
Background: Segmental vitiligo is a small but unique subset of vitiligo requiring due importance due to its lack of response to medical treatment but excellent response to surgical treatment. Characterization of the pattern of segmental vitiligo will also help to understand the pathogenesis of the disease. Aim: To characterize clinically the features of segmental vitiligo, a cross-sectional clinical study at dermatology outpatient department at AIIMS was carried out. Methods: Consecutive 188 patients were evaluated to characterize the clinical features of segmental vitiligo by detailed history, clinical examination, and photography. Frequency of each clinical feature was calculated. Results: Certain features such as early onset, initial progression of disease followed by stability, blaschkoid pattern, irregular margins, leucotrichia within and beyond the vitiligo lesion, and islands of pigmented macules within the vitiligo lesion were found to be characteristic of the disease. Conclusions: A combination of various features such as early onset of disease, blaschkoid pattern, irregular margins, leucotrichia, and islands of pigmented macules within the vitiligo lesion are helpful in diagnosis of the disease.Introduction
Vitiligo is an acquired pigmentary disorder of the skin presenting as depigmented or hypopigmented macules. It affects 0.1-2% population worldwide, [1],[2] and its prevalence in India is about 0.5-2.5%. [3],[4] Vitiligo can be classified into segmental and non-segmental vitiligo. Segmental vitiligo has depigmented macules arranged in a dermatomal or quasi-dermatomal distribution, which does not cross the midline. It differs from non-segmental vitiligo in terms of clinical features, natural history, and also treatment response. [5] Segmental vitiligo usually has an early onset in childhood in contrast to non-segmental vitiligo, which predominantly affects adults. In segmental vitiligo, the lesions develop rapidly over short span of time in a localized area and then remain stable, whereas non-segmental vitiligo has a highly variable course with periods of progression, remission, and stability. [5] Segmental vitiligo responds poorly to medical treatment, [6],[7] and surgical methods are the treatment of choice. [6] The characteristic feature of segmental vitiligo is the distribution pattern of the lesions. Many authors have considered the pattern to be dermatomal or quasidermatomal, blaschkoid or following acupuncture lines. [6]
In most cases, it is fairly easy to recognize segmental vitiligo due to its unilateral and patterned distribution; however, in some localized or early cases, where the diagnosis is uncertain, clinical criteria to differentiate segmental from non-segmental vitiligo would be helpful. Previously, we have observed certain features like irregular margins, leucotrichia, and early evolution of islands of pigmented skin within depigmented macules in segmental vitiligo. Hence, this study was conducted to characterize the clinical features of segmental vitiligo.
Methods
A cross-sectional study was conducted where we recorded the clinical features of all patients of segmental vitiligo presenting to the outpatient department of Dermatology at All India Institute of Medical Sciences, New Delhi, from May 2005 to January 2008.
Diagnosis of segmental vitiligo was made by at least two of the three authors by clinical parameters. The clinical criteria used for inclusion of patients were the presence of (i) depigmented macules, (ii) arrangement in a recognizable pattern such as dermatomal, narrow blaschkoid, broad blaschkoid, phylloid, checkerboard, or any other unknown but specific pattern, (iii) localization to an area of the body. In few cases, where there was disagreement between the authors, the cases were excluded.
A detailed history regarding age at onset, duration of disease, character of the lesion, progression, site of involvement, family history, other associated diseases, and prior treatment was taken at the time of presentation. Clinical examination included general physical, systemic, and cutaneous examination with special focus on morphology and distribution of lesions. Body surface area involvement was calculated according to the ′rule of nine.′ The pattern in which the depigmented macules were arranged was assessed by comparing with templates of dermatomes, Blaschko′s lines, checkerboard pattern, phylloid pattern, and Voigt′s lines. When depigmented macules were arranged such that they followed a particular recognizable dermatome, then it was classified as dermatomal pattern; if macules were present linearly on limbs or in curvilinear fashion with swirling on the back, then it was classified as blaschkoid pattern. Phylloid pattern referred to macules having a leaf-like pattern, and checkerboard pattern referred to arrangement of macules with sharp horizontal and longitudinal margin. Those not fitting into any of these patterns were classified as ′others.′ Islands of pigmented macules within or outside the segmental vitiligo were not recorded in first 12 patients. Baseline clinical photograph was recorded.
Statistical analyzes
The frequency of each clinical feature recorded on history and examination were calculated.
Results
There were 188 patients, 76 female and 112 male [Table - 1]. The mean age at presentation was 16.23 years. The age at onset ranged from 1 month to 63 years (mean 12.02 years) and in 132 (70.2%) patients, it was less than 15 years.

Disease progression
At the time of presentation, 96 (51.0%) patients had progressive disease, 91 (48.4%) had non-progressive disease, and in 1, there was no record of disease progression [Table - 2]. Progression was defined as extension of the pre-existing lesions or increased depigmentation of lesions and/or development of new lesions in the same segment in the last 3 months. The patients who were categorized as non-progressive (n = 91) were asked about the initial evolution of the disease. There was an initial period of progression within the segment of duration 1 year or less in 65/91 and more than 1 year in 14/91, after which the disease stabilized. Remaining 12 patients could not recall the initial period of progression.

Of the 96, who had progressive disease, the disease was unremittingly progressive from onset to presentation in 49, progressive at places within the segment and regressive at other places in 6, and progressive with intermittent periods of stability and/or regression in 41. On an average, patients developed 2-3 new lesions per segment in the 3 months before they presented to us.
Mixed vitiligo
Thirty-six patients (19.1%) had segmental vitiligo in combination with other types of vitiligo. Nineteen (10.1%) had progressed from segmental to non-segmental vitiligo away from the site of primary segment. Of these, 11 developed focal, 5 developed segmental vitiligo at another site, 2 developed acrofacial, and 1 developed lip vitiligo. Segmental vitiligo followed non-segmental vitiligo at another site in 11 patients (5.8%), of which 10 had preceding acrofacial and 1 had focal vitiligo. Interestingly, 6 patients had concurrent onset of segmental and non-segmental vitiligo, of which 2 had acrofacial type and 4 had focal vitiligo.
Number and site of involvement
Most patients (170, 90.4%) had only 1 segment involved [Figure - 1], while 17 (9%) had 2 segments [Figure - 2] and [Figure - 3] and only 1 (0.5%) had 3 segments involved. The head and neck was the initial site of onset in 98 patients (52.6%). Trunk was the second commonest site of onset in 41 (21.8%) followed by lower limb in 24 (12.7%), upper limb in 23 (12.2%), and genitals in 2 (1%). The right and left half of the body were affected in 103 (54.7%) and 95 (50.5%) patients, respectively.
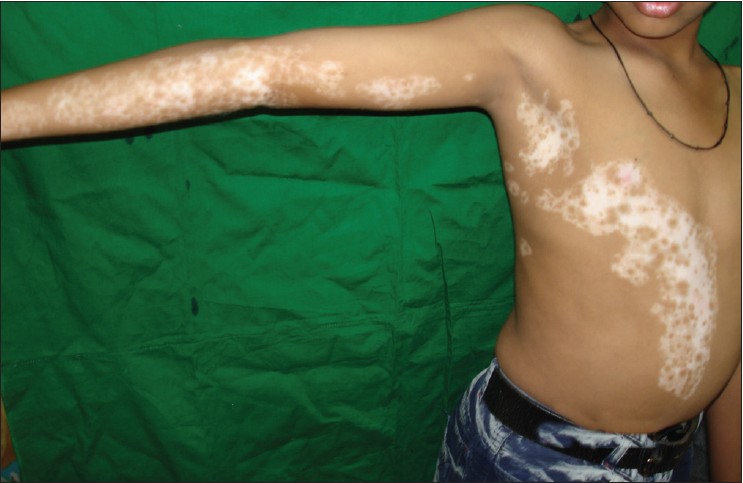 |
| Figure 1: Segmental vitiligo following Blaschko's lines, affecting only 1 segment. Single segment may extend on 2 different anatomical sites |
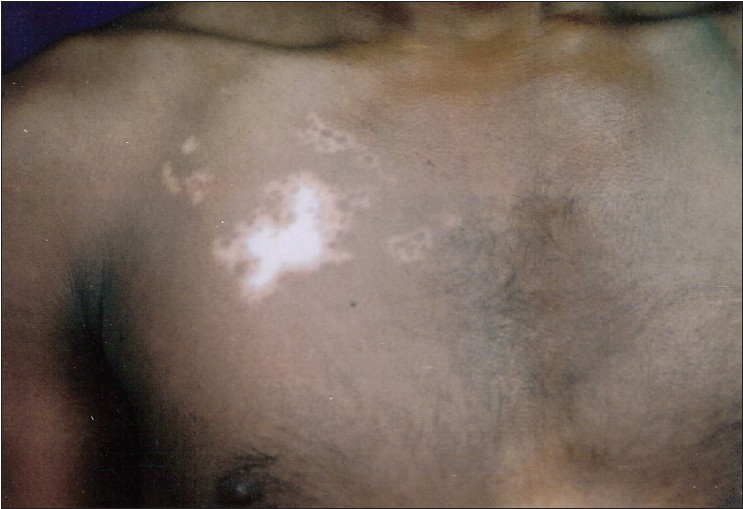 |
| Figure 2: Segmental vitiligo affecting two different segments - chest in this figure |
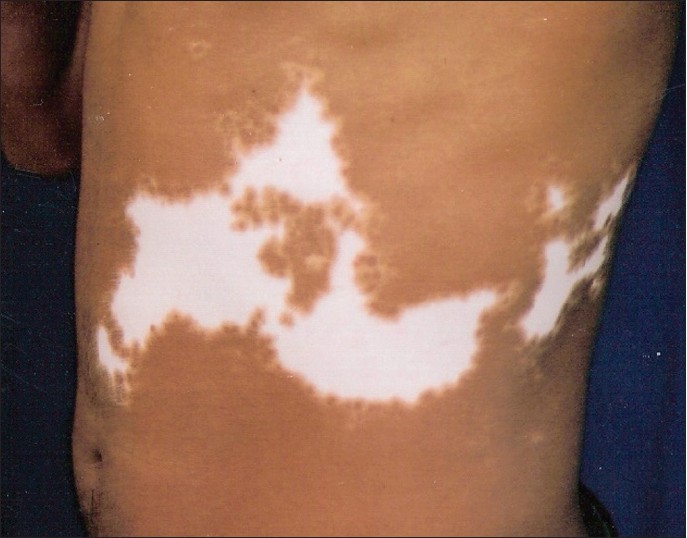 |
| Figure 3: Segmental vitiligo affecting two different segments - abdomen in this figure |
Pattern
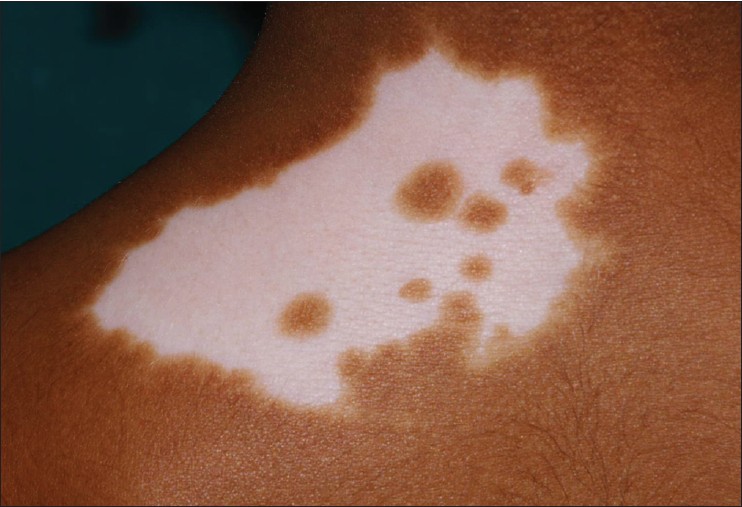
Blaschkoid pattern was observed in 93 (49.4%) [Figure - 1], dermatomal in 6 (3.1%) [Figure - 3], phylloid in 2 (1%) [Figure - 4], checkerboard in 1 (0.5%), [Figure - 5] and Voigt′s lines in 1 (0.5%). In 12, there was doubt between blaschkoid and dermatomal pattern, and in 10, it was probably but not definitively blaschkoid. No specific pattern could be ascertained in 66 patients. In 3 patients with 2 segments, the segments had different pattern of lesions.
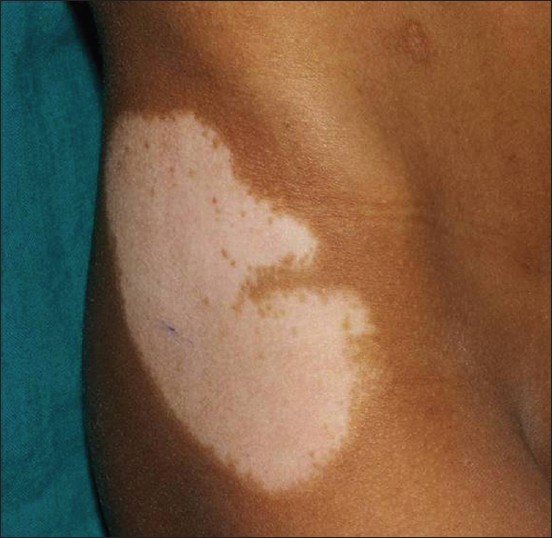 |
| Figure 4: Segmental vitiligo in phylloid pattern on thigh |
 |
| Figure 5: Segmental vitiligo in checkerboard pattern on abdomen |
Clinical characteristics
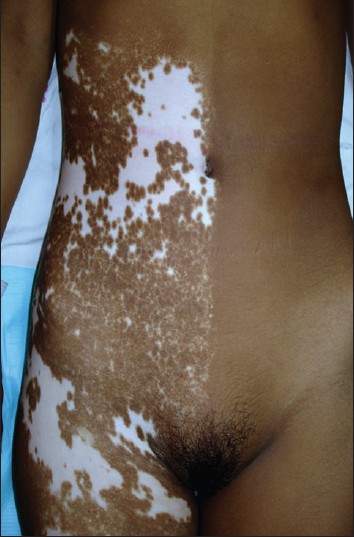
The body surface area involvement varied from less than 1% to 8%. In 121 (64.3%) patients, it was 1% or less. The remaining 67 patients had more than 1% body surface area involvement, the maximum being 8%. Leucotrichia [Figure - 6] was present in 162 (86.1%) patients. There was no leucotrichia in 12 and no hair in the affected area in 14 patients. All the hair in the lesion were white in 70 patients while there were both white and black hair in the lesion in 92 patients. Normally pigmented skin beyond the margin of segmental vitiligo also showed leucotrichia in 122 (64.8%) patients [Figure - 6].
 |
| Figure 6: Segmental vitiligo on the shoulder showing irregular margins and leucotrichia both with in and outside depigmented macules |
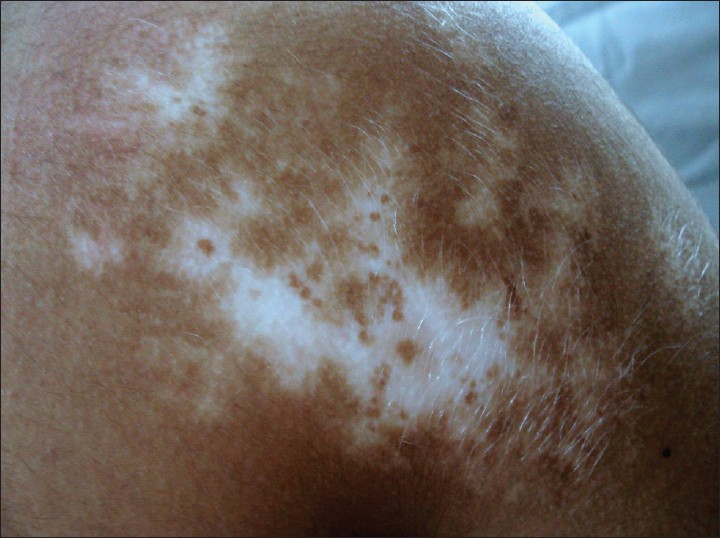
The margins of the lesion were completely irregular in 77 (40.9%) [Figure - 6], partly irregular and partly smooth in 84 (44.6%) [Figure - 7] patients and completely smooth margins in 27 (14.36%) patients.
 |
| Figure 7: Segmental vitiligo showing partly smooth and partly irregular margins |
Islands of normal/hyperpigmented skin within the lesion or segment [Figure - 8] were recorded in 176 patients and were present in 115 patients. There were 11 patients in whom these islands appeared without treatment. In the remaining patients, it could not be ascertained whether the islands were present de novo or appeared following treatment.
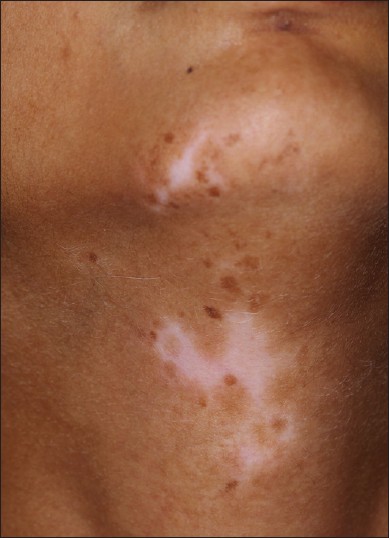 |
| Figure 8: Segmental vitiligo showing island of normo- and hyperpigmented macules within the segment |
Koebnerization was noted in 10 (5.3%), and 8 of these patients had a progressive course of disease at the time of presentation.
Aggravating factors
None of the patients noted factors like sunlight, trauma, emotional stress, or any other apparent factor which precipitated or aggravated the disease. One patient admitted to using a cap to cover leucotrichia on her scalp. Other patients did not use any method to camouflage their lesions.
Family history
There were 25 patients (13.2%) who had history of a family member being affected. Seven had vitiligo in 1 st degree relative, 10 had in 2 nd degree relative, and 9 had in 3 rd degree or beyond. The type of vitiligo could not be ascertained by history.
Other associated diseases
One patient each had alopecia areata and hypothyroidism. There was no history of early onset diabetes mellitus, thyroid abnormality, pernicious anemia, or Addison′s disease in any patient. None of the patients had halo nevi. Bone tuberculosis, congenital deafness, corneal opacity, chronic suppurative otitis media, type 2 diabetes, molluscum contagiosum, febrile convulsions, Pott′s spine, squint, facial hemiatrophy with ichthyosis vulgaris with endocrinopathy was seen in one patient each. Other segmental disorders like segmental lichen planus were present in 1 and nevus depigmentosus in 2 patients.
Associated contact leucoderma
None of the patients attributed the depigmentation due to contact with rubber, chemical, or plastic.
Discussion
Segmental vitiligo constitutes 5 [8] -27.9% [5] of all cases of vitiligo. In a study on 450 patients of vitiligo at AIIMS, New Delhi, segmental vitiligo was seen in 6.7% cases. An additional 1.7% had lesions of segmental vitiligo along with other types of vitiligo (mixed vitiligo). [9] Previous reports have described certain clinical features of segmental vitiligo. It is a disease of childhood; the incidence of segmental vitiligo is significantly more in children (19%) as compared to adults (5%). [10] The mean age of onset reported previously was 15.6 years, [11] which was similar to 12 years in this study. We found that at the time of presentation, approximately half of the patients had progressive disease, which is consistent with other reports that in most patients, disease stabilizes by 6 months to 1 year irrespective of treatment and disease progression halts faster than non-segmental vitiligo. [11],[12] This ′self-limiting′ nature of the disease reduces the requirement of aggressive systemic therapy at the onset of the disease.
Koga and Tango have described type A and type B vitiligo as non-segmental and segmental, respectively, and they noted progression from type B (segmental) to type A (non-segmental) vitiligo in 2.2% (3/134 patients). [5] In a case series of 127 patients with segmental vitiligo, Ezzedine et al found that 26 (20.4%) progressed from segmental vitiligo to non-segmental vitiligo. [13] In this study, progression from segmental vitiligo to another type of vitiligo was seen in only 10% patients and most developed focal vitiligo. Hence, a patient can be counseled that it is less likely that his disease will spread to other parts of the body, thus reducing psychological burden.
The pattern of the lesions is what characterizes segmental vitiligo, yet there is no consensus regarding it. Dermatomal pattern has been mentioned most commonly [5],[11],[14] and is given in the definition of segmental vitiligo in many reputed dermatology books. Some authors have suggested segmental vitiligo to follow Blashcko′s lines. [14],[15],[16],[17] Specific patterns have been described on the face, which do not follow dermatomal, blaschkoid, or acupuncture lines and have been revised recently. [18],[19] The recognition of visual patterns is intrinsically subjective and difficult to automate or objectivize. [20] This is a particular problem because in some anatomical areas, such as the back and abdomen, there is considerable overlap between dermatomes and Blaschko lines.
In our study, predominance of Blaschkoid pattern (49.4%) and occasional case of phylloid (1%) and checkerboard pattern (0.5%) suggest cutaneous mosaicism, and the convincing dermatomal pattern only in very small number (3.1%) questions the basis of neural theory in the pathogenesis of segmental vitiligo. We noted that many patterns went unclassified emphasizing that more work is needed in this area.
The co-existence of generalized and segmental vitiligo in some patients may be an indicator that mixed vitiligo represents type 2 mosaicism of Happle. [21],[22],[23],[24] In keeping with other disorders demonstrating type 2 mosaicism, we have noted that when both types of vitiligo (segmental and non-segmental) occur together, depigmentation and leucotrichia tend to be more prominent in the segmental lesions than in the non-segmental lesions. Also, it was observed by the authors that in the same patient, segmental lesions did not respond as well to medical treatment as the focal lesions in accordance with the theory that the segmental lesions of type 2 mosaicism are more severe than the non-segmental type. [21],[22],[23]
The lower incidence of family history of vitiligo and coexisting autoimmune disorders in segmental vitiligo as compared to non-segmental vitiligo has been reported previously and was observed in this study also (13.2% and 1%, respectively.) [5],[11],[12],[25],[26] This further suggests that autoimmunity may not be an important pathway for pathogenesis of segmental vitiligo. The incidence of halo nevi is lower in segmental vitiligo (10.2%) [26] and has been considered to be a marker for progression of segmental vitiligo to non-segmental vitiligo. [13] However, we did not observe halo nevi in any patient of segmental vitiligo including those progressing to mixed vitiligo.
Leucotrichia is a consistent feature of segmental vitiligo, and two studies by Lee et al, have reported leucotrichia to be present in all patients of segmental vitiligo, especially with the use of a digital portable microscope. [27],[28] In this study, leucotrichia was present in majority (86.1%), but not in all patients. One reason was that in patients with segmental vitiligo at an anatomical site where hair is absent, such as dorsae of hands and feet, leucotrichia cannot be evaluated and we had 14 such patients. The advantage of digital portable microscope over close naked eye examination in evaluation of leucotrichia has not been compared; however, one can assume that the difference may not be too much. Leucotrichia in the entire lesion was seen in 37.2% patients, and Lee et al have reported that such patients with leucotrichia of > 90% of lesional hair show poor response to medical therapy. [28] Leucotrichia extending beyond the lesion of vitiligo has not been reported previously; [27],[28] however, in this study, leucotrichia extending beyond the margins was seen in 64.8% patients. Irregular margins and islands of normal pigmented or hyperpigmented skin within the depigmented macule or segment were other important findings seen in 85.5% and 65.3% patients, respectively.
Various definitions and terminologies have been used for segmental vitiligo. In the dermatosurgery literature, unilateral vitiligo has been used as a synonym for segmental vitiligo. [25] While unilaterality is an important criterion, it is not the sole criterion. Rarely, segmental vitiligo can cross the midline, [6],[18] occur in two contralateral segments [6] or be associated with a symmetrical, generalized vitiligo. [9],[13] Further, all vitiligo that begins with a single lesion will be unilateral until other lesions develop rendering unilaterality as an inadequate single criterion for this type of disease. [29] Similarly, limited extent of involvement as a sole defining characteristic may not be enough to diagnose segmental vitiligo as generalized vitiligo may be localized to one body part for variable periods early in their evolution. Therefore, a combination of various features described in this study is helpful to diagnose segmental vitiligo and hence, differentiate it from early lesions of non-segmental vitiligo.
Few limitations of the study are lack of accurate large templates of Blaschko′s lines and dermatomal patterns and objective assessment of extent of leucotrichia.
Conclusion
We propose that segmental vitiligo is a specific subtype of vitiligo, which is distinct from non-segmental vitiligo including focal vitiligo. It cannot be diagnosed on the basis of a single criteria or definition. A combination of features such as early onset; unilateral lesion usually not crossing midline; patterned; having prominent leucotrichia extending beyond the lesion; irregular margins and islands of normal or hyperpigmented macules characterize segmental vitiligo. Pattern of the lesion is probably the most important criteria which by itself can strongly suggest the diagnosis of segmental vitiligo. However, more the number of features in an individual, more sure one can be of the diagnosis. Early recognition of segmental vitiligo can help to reassure the patient that it usually does not spread to other parts of the body, stabilizes early, and responds very well to surgical methods with probably a lower risk of recurrence. Recognition of this type of vitiligo would clearly be important in prognostication and choice of treatment modality.
| 1. |
Lerner AB. Vitiligo. J Invest Dermatol 1959;32:285-310.
[Google Scholar]
|
| 2. |
Halder RM, Taliaferro SJ. Vitiligo. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Lefell DJ, editors. Fitzpatrick's Dermatology in General Medicine, 7 th ed. New York: McGraw-Hill; 2008. p. 836-81.
th ed. New York: McGraw-Hill; 2008. p. 836-81.'>[Google Scholar]
|
| 3. |
Handa S, Dogra S. Epidemiology of childhood vitiligo: A study of 625 patients from North India. Pediatr Dermatol 2003;20:207-10.
[Google Scholar]
|
| 4. |
Das SK, Majumdar PP, Chakraborty R, Majumdar TK, Haldar B, Rao CD. Studies on vitiligo I. Epidemiological profile in Calcutta, India. Genet Epid 1985;2:71-8.
[Google Scholar]
|
| 5. |
Koga M, Tango T. Clinical features and course of type A and type B vitiligo. Br J Dermatol 1988;118:223-8.
[Google Scholar]
|
| 6. |
Hann SK. Clinical features of segmental Vitiligo. In: Hann SK, Nordlund JJ, editors. Vitiligo, 1 st ed. UK: Blackwell Science; 2000. p. 49-60.
[Google Scholar]
|
| 7. |
Kathuria S, Khaitan BK, Ramam M, Sharma VK. Segmental vitiligo: A randomized controlled trial to evaluate efficacy and safety of 0.1% tacrolimus ointment vs 0.05% fluticasone propionate cream. Indian J Dermatol Venereol Leprol 2012;78:68-73.
[Google Scholar]
|
| 8. |
Handa S, Pandhi R, Kaur I. Vitiligo: A retrospective comparative analysis of treatment modalities in 500 patients. J Dermatol 2001;28:461-6.
[Google Scholar]
|
| 9. |
Khaitan BK, Pasricha JS, Sood A. clinical profile of 450 Indian vitiligo patients. American Academy of Dermatology 61 st annual meeting 2003. San Francisco.
[Google Scholar]
|
| 10. |
Halder RM, Grimes PE, Cowan CA, Enterline JA, Chakrabarti SG, Kenney JA. Childhood vitiligo. J Am Acad Dermatol 1987;16:948- 54.
[Google Scholar]
|
| 11. |
Hann SK, Lee HJ. Segmental vitiligo: Clinical findings in 208 patients. J Am Acad Dermatol 1996;35:671-4.
[Google Scholar]
|
| 12. |
Mazereeuw-Hautier J, Bezio S, Mahe E, Bodemer C, Eschard C, Viseux V, et al. Segmental and nonsegmental childhood vitiligo has distinct clinical characteristics: A prospective observational study. J Am Acad Dermatol 2010;62:945-9.
[Google Scholar]
|
| 13. |
Ezzedine K, Diallo A, Léauté-Labrèze C, Séneschal J, Prey S, Ballanger F, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol 2012;166:39-44.
[Google Scholar]
|
| 14. |
Taïeb A, Picardo M; VETF Members. The definition and assessment of vitiligo: A consensus report of the Vitiligo European Task Force. Pigment Cell Res 2007;20:27-35.
[Google Scholar]
|
| 15. |
Happle R. "Zosteriform" lichen planus: The bizarre consequences of a misnomer. Acta Derm Venereol 1998;78:300.
[Google Scholar]
|
| 16. |
Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol 1994;32:157-90.
[Google Scholar]
|
| 17. |
Taïeb A, Morice-Picard F, Jouary T, Ezzedine K, Cario-André M, Gauthier Y. Segmental vitiligo as the possible expression of cutaneous somatic mosaicism: Implications for common non-segmental vitiligo. Pigment Cell Melanoma Res 2008;21:646-52.
[Google Scholar]
|
| 18. |
Hann SK, Chang JH, Lee HS, Kim SM. The classification of segmental vitiligo on the face. Yonsei Med J 2000;41:209-12.
[Google Scholar]
|
| 19. |
Kim DY, Oh SH, Hann SK. Classification of segmental vitiligo on the face: Clues for prognosis. Br J Dermatol 2011;164:1004-9.
[Google Scholar]
|
| 20. |
Weibel L, Harper JI. Linear morphoea follows Blaschko's lines. Br J Dermatol 2008;159:175-81.
[Google Scholar]
|
| 21. |
Happle R. New aspects of cutaneous mosaicism. J Dermatol 2002;29:681-92.
[Google Scholar]
|
| 22. |
Happle R. Segmental type 2 manifestation of autosome dominant skin diseases. Development of a new formal genetic concept. Hautarzt 2001;52:283-7.
[Google Scholar]
|
| 23. |
Happle R. Superimposed segmental manifestations of polygenic skin disorders. J Am Acad Dermatol 2007;57:690-9.
[Google Scholar]
|
| 24. |
Poblete-Gutiérrez P, Wiederholt T, König A, Jugert FK, Marquardt Y, Rübben A, et al. Allelic loss underlies type 2 segmental Hailey-Hailey disease, providing molecular confirmation of a novel genetic concept. J Clin Invest 2004;114:1467-74.
[Google Scholar]
|
| 25. |
Barona MI, Arrunategui A, Falabella R, Alzate A. An epidemiological case-control study in a population with vitiligo. J Am Acad Dermatol 1995;33:621-5.
[Google Scholar]
|
| 26. |
Ezzedine K, Diallo A, Léauté-Labrèze C, Mossalayi D, Gauthier Y, Bouchtnei S, et al. Multivariate analysis of factors associated with early-onset segmental and nonsegmental vitiligo: A prospective observational study of 213 patients. Br J Dermatol 2011;165:44-9.
[Google Scholar]
|
| 27. |
Lee DY, Park JH, Lee JH, Yang JM, Lee ES. Is segmental vitiligo always associated with leukotrichia? Examination with a digital portable microscope. Int J Dermatol 2009;48:1262.
[Google Scholar]
|
| 28. |
Lee DY, Kim CR, Park JH, Lee JH. The incidence of leukotrichia in segmental vitiligo: Implication of poor response to medical treatment. Int J Dermatol 2011;50:925-7.
[Google Scholar]
|
| 29. |
Hann SK, Chung HS, Park YK. Epidemiologic case-control study in patients with vitiligo. J Am Acad Dermatol 1997;36:282-3.
[Google Scholar]
|
Fulltext Views
14,381
PDF downloads
3,238





