Translate this page into:
A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts
2 Department of Pharmacology, Rampurhat Government Medical College, Rampurhat, West Bengal, India
3 Department of Biochemistry, Medical College and Hospital, Kolkata, India
4 Department of Dermatology, Bankura Sammilani Medical College, Bankura, West Bengal, India
Correspondence Address:
Nilay Kanti Das
Department of Dermatology, Bankura Sammilani Medical College, Kenduadihi, Bankura - 722 102, West Bengal
India
| How to cite this article: Chandra S, Sil A, Datta A, Pal S, Das NK. A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts. Indian J Dermatol Venereol Leprol 2019;85:355-366 |
Abstract
Background: Present day therapeutic modalities for viral warts are mostly ablative in nature, limited by high recurrence rates and are unsuitable for numerous lesions. Immunotherapy has the potential to overcome these limitations.
Aims: This study aimed at comparing efficacy and safety of and quality of life changes with intradermal purified protein derivative (PPD) of tuberculin antigen and Mycobacterium w (Mw) vaccine in immunotherapy of warts.
Methods: Patients with multiple (≥5) warts were randomized (1:1) into two groups (PPDand, Mw vaccine groups). Fortnightly, 0.1 ml of either medicine was injected intradermally over the deltoidregion till complete resolution or a maximum of six doses. Patients were followed-up for another 3 months for recurrence.
Results: Sixty-four participants received either PPD or Mw vaccine. The number of warts were comparable at baseline (P = 0.089, Mann–Whitney test), and reduced significantly with treatment in both groups (P < 0.001, Friedman's ANOVA), as seen from the fourth follow-up onwards with Mw and fifth follow-up onwards with PPD (P < 0.05, Post hoc Dunn's test). Intergroup comparison showed significantly more (P < 0.05, Mann–Whitney test) reduction with Mw than PPD at the sixth and seventh follow-up. The size of warts also reduced significantly (P < 0.001) in both groups from the third follow-up onwards. Complete remission was more (P = 0.539, Fischer's exact test) in the Mw group (68.8%) than the PPD group (50%); and was significantly higher (P = 0.049, Mann–Whitney test) in patients having shorter duration of warts. Adverse events were significantly more (P < 0.001) with Mw including ulceration (50%), discharge (15.6%), pain-swelling-induration and scar at the injection site (97% each), whereas some of those receiving PPD noted erythema and scaling at the injection site (18.8%), and post-inflammatory hyperpigmentation (12.5%). No recurrence was seen till the end of the study.
Limitation: Unicentric trial.
Conclusion: Intradermal injection of Mw vaccine was more effective but had a higher incidence of adverse effects compared to PPD of tuberculin antigen in patients with warts.
Introduction
Management of verrucae is often frustrating to the patient and the physician alike.[1] Unfortunately, even with years of medical literature on this subject, high-quality evidence for efficacy is lacking for almost all treatments. Primary treatment modalities for verrucae include ablative therapies, e.g. topical chemical cautery, cryotherapy, electrocautery, excision, bleomycin sulfate injection and laser vaporization, but none gives a guarantee of cure, and recurrence and scarring are common.[2],[3] These destructive modalities are designed to remove visible lesions; nonvisible infected tissues are not targeted. Moreover, in patients with numerous lesions, there is no effect on lesions other than the treated ones, resulting in repeated and long-drawn treatment sessions.
Immunotherapy in warts utilizes the ability of the immune system to mount a delayed-type hypersensitivity response to various antigens in wart tissue. It has been found to be associated with the production of Th1 cytokines which activate cytotoxic and natural killer cells to eradicate the HPV infection. This clears not only treated warts but also distant warts, unlike conventional therapies.[4]
Antigens such as BCG, PPD, candida, mumps, MMR and Mycobacterium w (Mw) vaccine as well as interferons have shown promise as immunotherapeutic agents.[3] Purified protein derivative (PPD) is an extract of Mycobacterium tuberculosis used for testing exposure to tuberculin protein. It is an especially promising immunotherapeutic agent in countries where vaccination against tuberculosis is performed routinely.[5]Mw vaccine contains killed nonpathogenic, saprophytic, cultivable, atypical mycobacterium belonging to Runyon Group IV, which has now been renamed Mycobacterium indicus pranii.[6] Due to its strong immunogenic and immunomodulatory actions, it has found place in treatment of both genital[7] and extragenital warts.[6]
India is endemic for tuberculosis, with a high prevalence rate (249 per 100,000 population). It is estimated that 40% of the Indian population is infected with M. tuberculosis.[8] Considering this along with the practice of routine immunization against tuberculosis, it can be argued that the Indian population is already sensitized to mycobacterial antigens; this was our rationale in choosing two mycobacteria-derived antigens for immunotherapy.
This study was undertaken to evaluate efficacy, safety and tolerability of, and quality of life changes with intradermal tuberculin PPD versus those with Mw vaccine in the treatment of multiple warts in an Indian setting. The study was planned to provide evidence to clinicians about which modality to adopt or which immunotherapeutic antigen to use when confronted with a patient having numerous cutaneous warts.
Methods
The study was carried out as a double-blind, randomized, controlled parallel-group trial of tuberculin PPD versus Mw vaccine in the Dermatology OPD of Medical College, Kolkata. Institutional Ethics Committee permission was taken and trial was registered with CTRI (registration number CTRI/2015/03/005433). Adult consenting patients (18–65 years) of either sex suffering from multiple viral warts (≥5 in number) were included. Pregnant, lactating women, patients immunosuppressed due to drug or disease, those suffering from liver or kidney diseases, and those with mucosal, ulcerated, inflamed or genital warts were excluded.
Randomization and blinding
The participants were randomized equally in a 1:1 ratio (unstratified) into two treatment groups by a computer-generated random number table using WINPEPI software. Randomization was concealed by the sequentially numbered opaque sealed envelope (SNOSE) technique. Insulin syringes were each prepared with 0.1 ml of trial medicines and packed in envelopes by a departmental nurse not associated with the trial, making both the treating physician and the participants blind to the treatment received.
Study medications
- Tuberculin PPD (10 TU/0.1 ml) – the formulation marketed by Span Diagnostics Private Limited, Gujarat, India, (Tuberculin Diluted; Batch no. 4000013401, Mfg. Dt: 07-2014, Exp. Dt: 10-2015) was utilized.
- Mycobacterium w vaccine formulation marketed by Cadila Pharmaceuticals, Licenced by National Institute of Immunology, New Delhi, India, (Immuvac; Batch no. 14001; Mfg. Dt: 12-2014, Exp. Dt: 11-2016) was utilized.
Both medications were purchased from the respective company for the trial by the investigator.
Sample size
The sample size was 29 patients of viral warts in each treatment group considering a superiority trial. Sample size was calculated considering complete clearance of warts of 83% with Mw vaccine[6] and 50% with tuberculin PPD,[7] with 80% power and 0.05 probability of Type I error. Considering a possible 10% dropout rate, this translated to a recruitment target of 32 subjects in each group or 64 subjects overall.
Visits and follow-ups
The study was carried out for 18 months. Each participant received intradermal injections (PPD or Mw) every fortnight for a total of 6 doses; then the patient was advised to come for follow-up every month for 3 months to assess recurrence. The first participant was included on May 2014 and recruitment was continued till January 2015. Follow-up of the last recruited participant was done till April 2015.
At the screening visit, history was recorded and a clinical examination was done. Tuberculin (Mantoux) test was done on all patients with 0.1 ml of 10 TU PPD and the reading was taken after 72 h. Baseline laboratory values of hemoglobin, total leucocyte count, differential counts, platelet count, erythrocyte sedimentation rate, fasting blood sugar, serum urea, serum creatinine and liver function tests were recorded.
The baseline visit was scheduled 3 days after the screening visit. Erythema and induration of the tuberculin test was recorded by measuring the maximum horizontal diameter. Number and size of warts at presentation were recorded in a pre-tested case-record-form, and the vernacular version of the Dermatology Life Quality Index (DLQI) form was filled by the patient. The investigational product was injected into the left arm ( first dose), and patients were asked to telephone the investigator and report immediately if any untoward reactions occurred.
Treatment visits were scheduled at week 2, 4, 6, 8, and 10 with subsequent follow-up visits at week 14, 18, and 22 weeks. At each fortnightly visit, intradermal injections were administered into alternate arms as per the randomization for a total of six doses or less in case of complete resolution. Efficacy and safety parameters were tested at every visit. The size of warts was estimated by measuring the largest diameter of the largest wart with a ruler. Laboratory investigations were repeated at week 10. The DLQI was reassessed at week 22.
Study parameters
Efficacy parameters were reduction in the total number of lesions, decrease in thesize of existing lesions, and physicians' and patients' global assessments of disease activity improvement on a five-point Likert scale (from 0 to 4).[9] Safety parameters recorded were the adverse events reported by participants or elicited by clinicians and the changes in laboratory parameters after active treatment. Quality of life was assessed by vernacular version of DLQI questionnaire administered at baseline and at the study end.[10]
Statistical analysis
Continuous variables were compared between groups by independent samples t-test and within group by paired t-test. Mann–Whitney U-test and Wilcoxon's test was carried out for unpaired and paired nonparametric data. Categorical data were compared between groups by Chi-square test or Fisher's exact test, as appropriate. Freidman's analysis of variance (ANOVA) followed by post-hoc Dunn's test was carried out with nonparametric data for within group repeated measures comparisons. Efficacy analysis was done on a modified intention-to-treat basis for the 64 subjects reporting for at least two post-baseline follow-up visits. Missing values were dealt with by the last observation carried forward strategy. Subgroup analysis was planned taking the presence of a BCG immunization scar as the grouping variable. Pre and posttreatment laboratory data were obtained from patients who had come for at least five follow-ups. This included 29 patients of PPD group and 30 patients of Mw group. For analysis of adverse effects, all patients who had received at least one dose of the injection were considered, i.e. all 32 patients each from the PPD group and M w group.
Results
Of the 105 participants screened, 64 were randomized equally into the treatment groups. The flow of study participants is depicted in [Figure - 1].
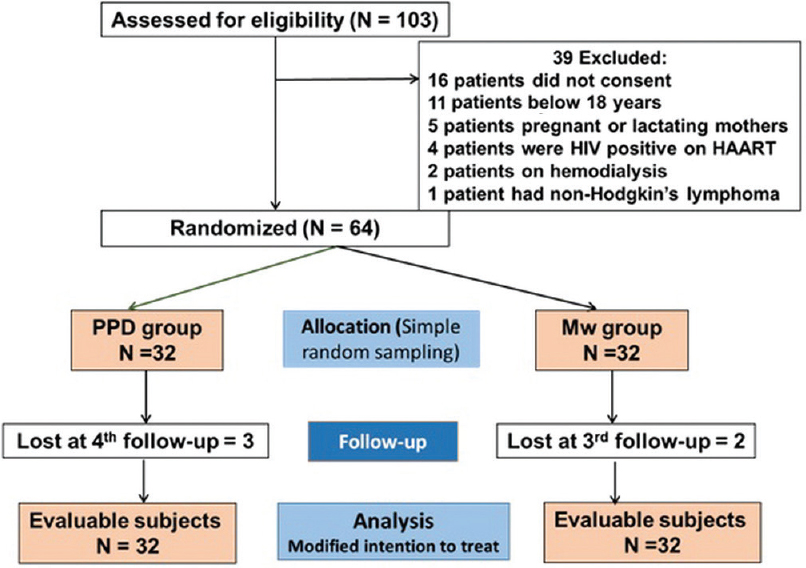 |
| Figure 1: Flowchart of study participants |
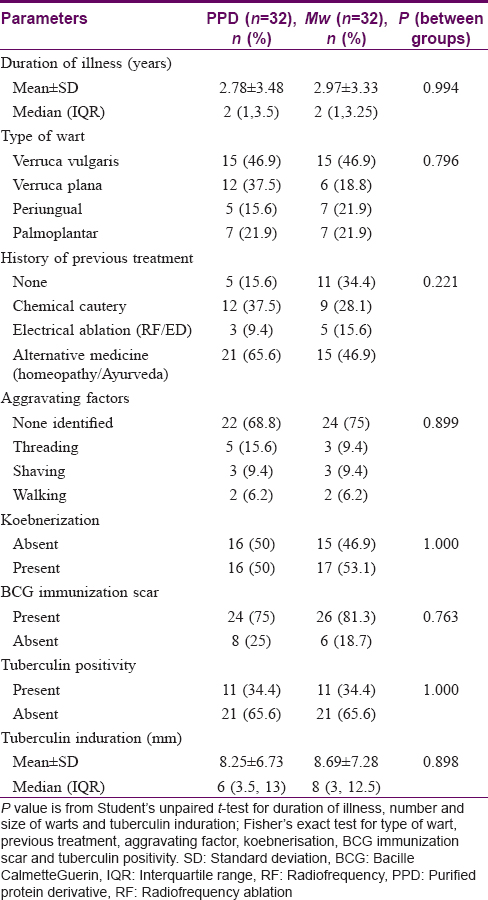
Clinicodemographic data of study participants are highlighted in [Table - 1]. Both groups were found to be comparable on those parameters.
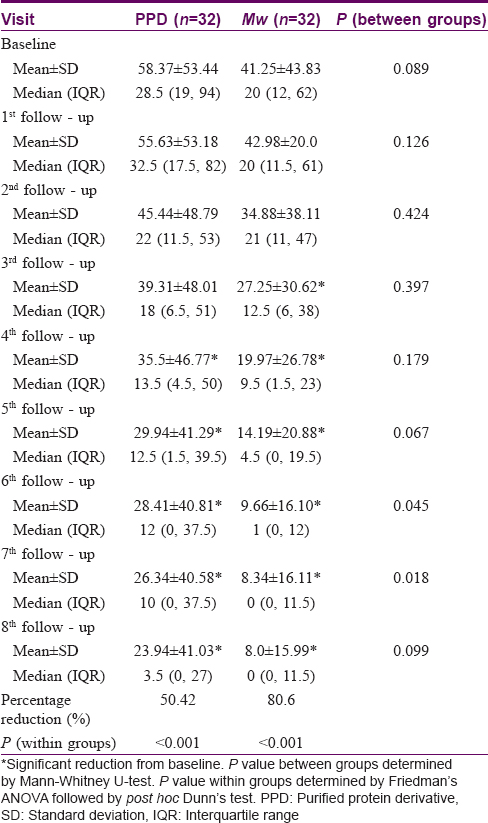
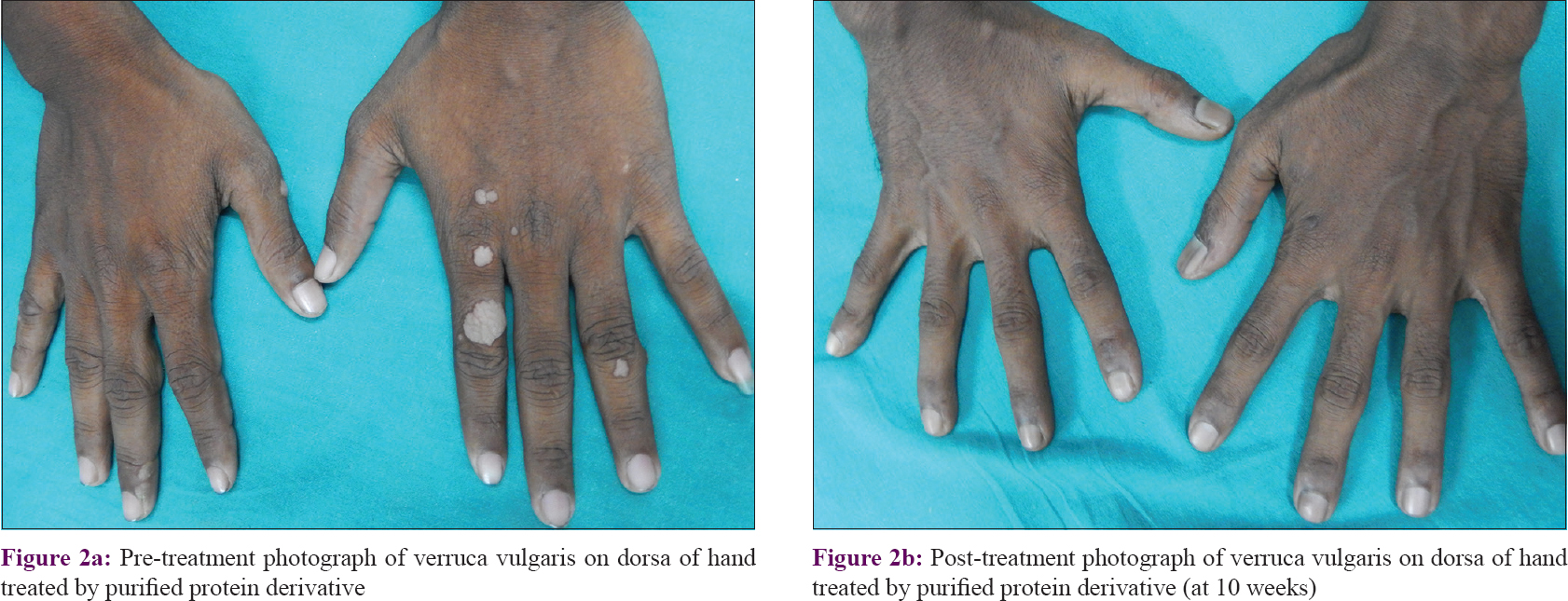
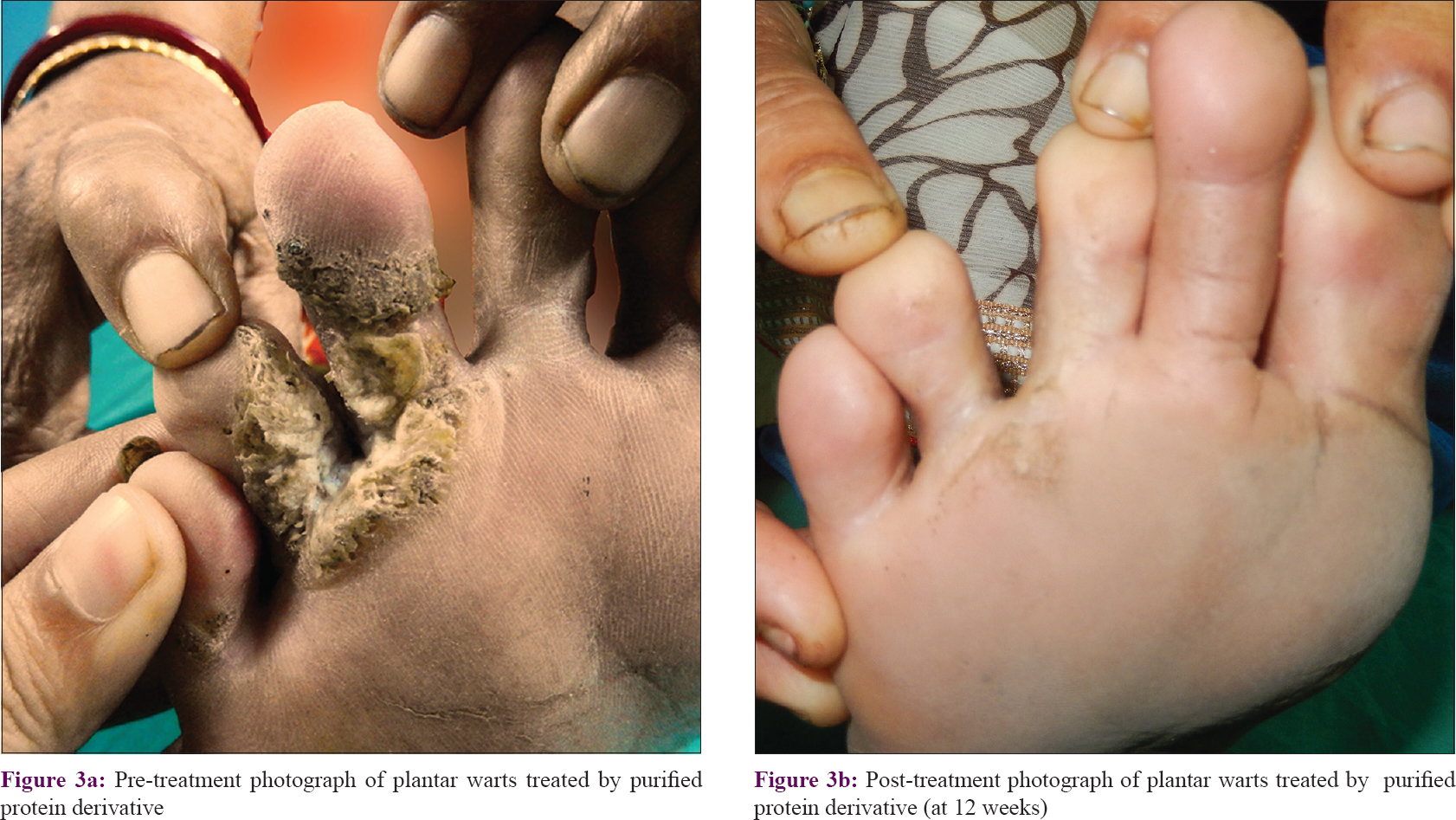
The number of warts in the PPD group was 58.37 ± 53.44 while in the Mw group it was 41.25 ± 43.83 at baseline, and they were comparable (P = 0.089). Reduction in wart numbers started from the first follow-up itself in both groups; however, the reduction was significant (P < 0.001) from the fourth follow-up onwards in the PPD group and third follow-up onwards in the Mw group, and this decline was maintained till the end of the study. Intergroup comparison revealed significantly more reduction in the Mw group than the PPD group at 6th and 7th follow-up visits, but this difference was not noted in the next visit. Changes in the number of warts during 3 months of active treatment (baseline to fifth follow-up) and 3 months of posttreatment follow-up (sixth, seventh, and eighth follow-ups) are depicted in [Table - 2] and [Figure - 2]a, [Figure - 2]b, [Figure - 3]a and [Figure - 3]b. The number of warts declined significantly (P < 0.001) in the active treatment phase in both treatment arms in the initial 3 months, and there was a decrease in the number of warts posttreatment during the sixth to eighth follow-up visits. With treatment, PPD achieved 50.4% and Mw, 80.6% reduction in the total number of warts from their respective baseline values [Table - 3].
 |
| Figure 2: |
 |
| Figure 3: |
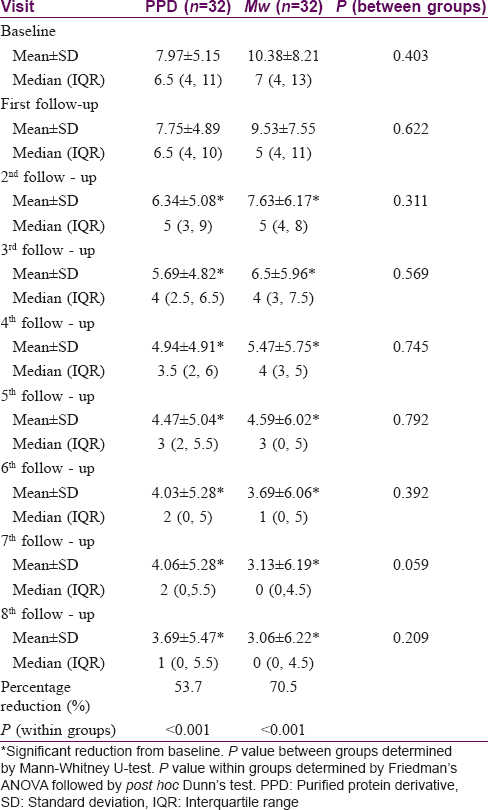
The two groups were comparable at baseline with regard to the size of the warts (P = 0.403), with the average size of the largest wart in the PPD group being 7.9 ± 5.1 cm and Mw group being 10.4 ± 8.2 cm. There was a significant reduction (P < 0.001) in size from the third follow-up onwards in both groups, which was persistent till the end of the study. Intergroup comparison showed significantly more (P = 0.059) reduction in wart size in Mw group in the seventh follow-up, however, the difference was not maintained till the end of study. With PPD, patients achieved 53.7% and with Mw 70.5% reduction in wart size from their respective baseline values [Table - 3].
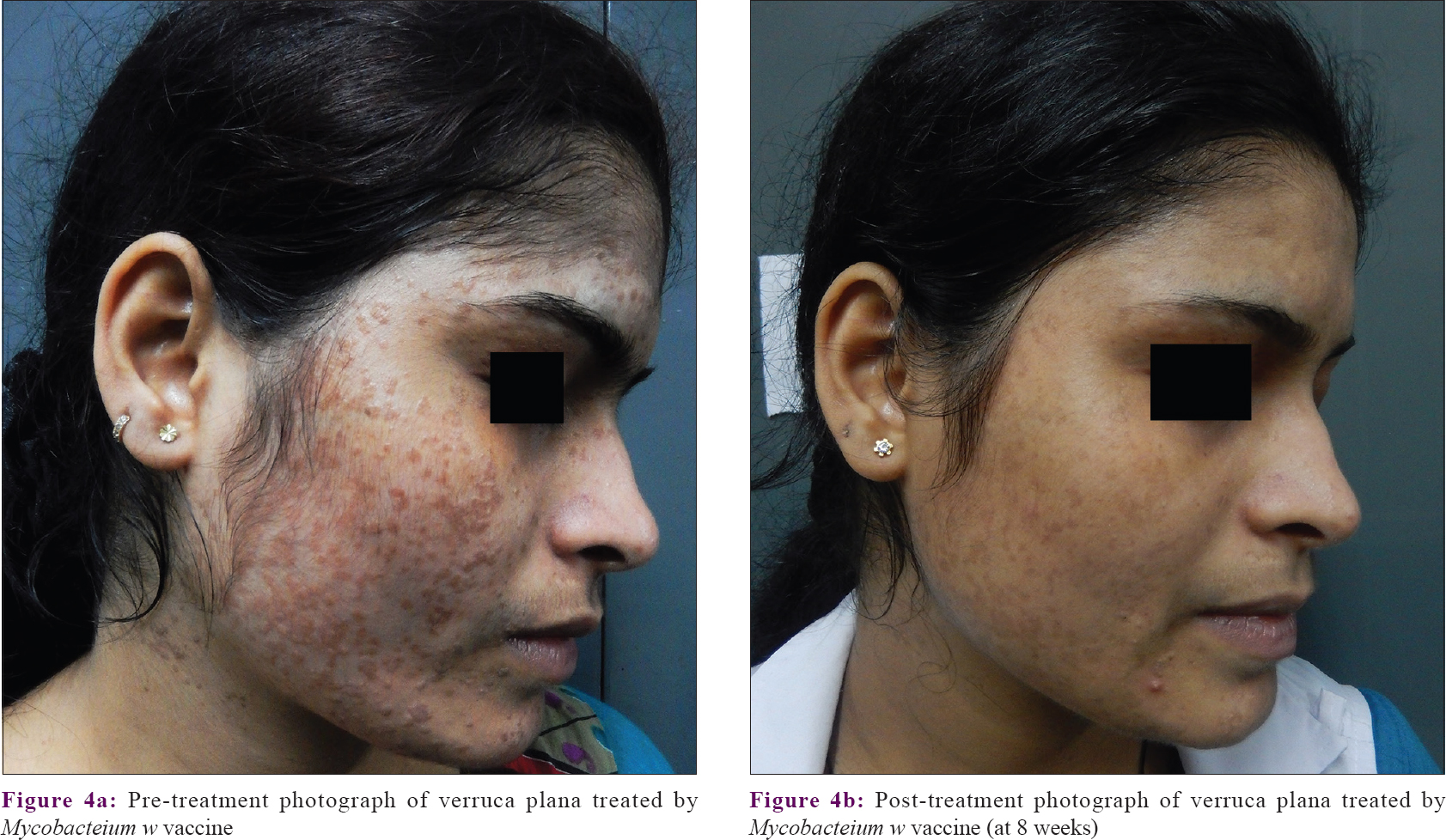
Assessment of disease severity by the physician showed that at baseline the disease was severe in both treatment arms (Physicians' global assessment of disease activity improvement scale value at baseline was 0, since the disease was most severe). Severity reduced significantly (P < 0.001) in in both groups from the first follow-up onwards. Comparison between the treatment groups showed that decrease in disease severity was significantly more with Mw vaccine than with PPD at the sixth, seventh, and eighth follow-up visits. Patients' global assessment of disease activity improvement scale also showed similar results. [Figure - 4]a and [Figure - 4]b.
 |
| Figure 4: |
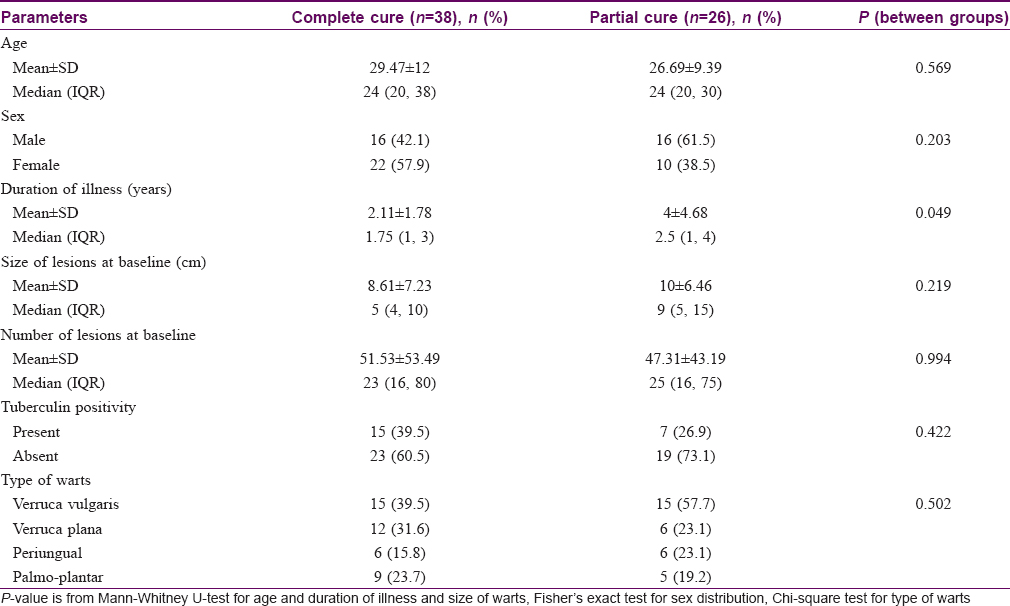
Out of a total of 64 patients, 38 achieved complete clearance of their warts. Complete resolution of warts was seen from the fourth follow-up onwards with PPD and third follow-up onwards with Mw, with 50% (16/32) of patients achieving complete remission in the former group compared to 68.8% (22/32) of patients in the latter group (P = 0.539). It was seen that age, sex, type and baseline number, size of warts and tuberculin positivity status were comparable between complete and partial responders; however, patients having shorter durations of warts achieved complete clearance significantly more (P = 0.049) than those having warts for longer durations [Table - 4].
A BCG immunization scar was present in 24 (75%) patients in the PPD group whereas 26 (81.3%) had a BCG scar in the Mw group [Table - 1]. Subgroup analysis with BCG immunization scar as a grouping variable showed no significant change in the reduction of number of warts between the two subgroups at any follow-up (P = 0.872 in PPD group; P = 0.231 in Mw group at final visit). Tuberculin reactivity status too did not have any effect on the reduction of the number of warts either in the PPD group (P = 0.276 at the last follow-up) or the Mw group (P = 0.399).
Quality of life (assessed by DLQI) was comparable (P = 0.114) in both treatment arms at baseline. The DLQI improved significantly from baseline at the end of the study with both PPD (P < 0.001) and Mw (P < 0.001), though intergroup comparison showed no difference between the two treatment groups (P = 0.583) at the end of the study.
Adverse events were encountered in 31.3% (10/32) of patients in the PPD group and all patients in the Mw group, and the difference was statistically significant (P < 0.001). In the PPD group, 12.5% (4/32) of patients developed hyperpigmentation at the site of the lesions after receiving five injections, which persisted till the end of study; 18.8% (6/32) of patients developed transient mild erythema and scaling at injection site after four doses, which subsided within 2–3 weeks without treatment; one patient reported a sudden onset of pruritus with erythema over the warts after receiving three doses of injection which was treated with antihistamines for 7 days, followed by rapid regression of the wart lesions. In the Mw group, almost all patients developed an indurated nodule at the injection site after every dose which healed with scarring [Figure - 5]a and [Figure - 5]b. 37.5% (12/32) patients' nodules were painful requiring tablet paracetamol (650 mg) twice daily till resolution; 34.4% (11/32) patients additionally developed ulceration of the nodule, and in 12.5% patients (4/32) there was a discharge from the injection site nodule. The patients with ulceration with or without discharge were treated with a standard atypical mycobacterial drug regimen comprising tablet doxycycline (100 mg) twice daily + tablet ofloxacin (200 mg) twice daily + tablet azithromycin (500 mg) once daily till healing of their ulcer. Self-limiting erythema and scaling over the injection sites occurred in one patient after three doses and one reported pruritus and erythema over the warts after receiving two injections that was followed by rapid clearance of the lesions over 10 days.
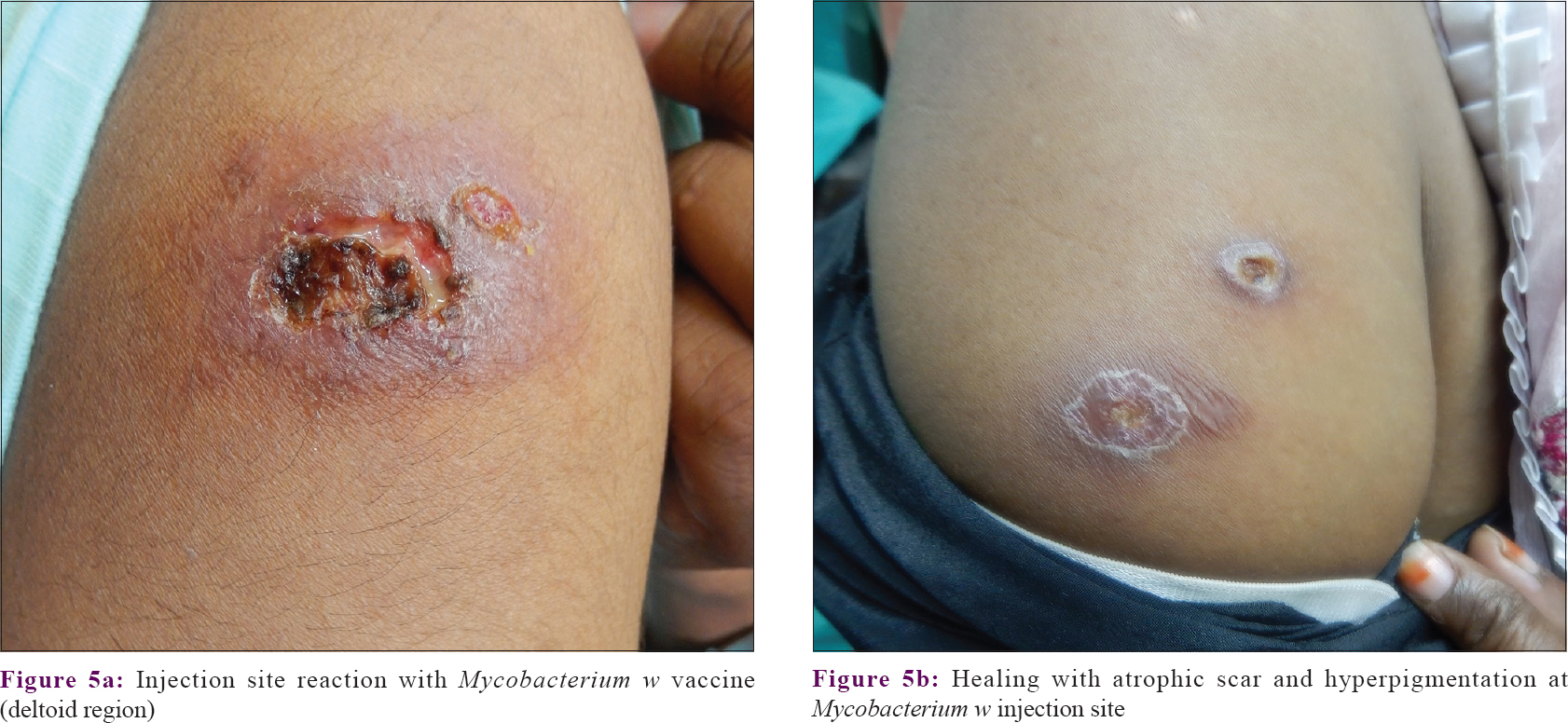 |
| Figure 5: |
Laboratory parameters were within normal limits and comparable between the two groups.
Discussion
The most troublesome factor in the management of warts is the high recurrence rate of at least 30% after apparently successful treatment, possibly by recrudescence of the virus from the surrounding tissue reservoir.[3] In the present study, 21 and 8 patients respectively had recurrences after chemical cautery and electrosurgery. Immunotherapy for warts addresses the limitations of conventional ablative therapy as it enhances the cell mediated immunity and helps clear the virus-infected tissues, irrespective of whether they are visible or not. In this sense, immunotherapy can target lesions situated remotely from the site of its administration, making it a better option in multiple warts, warts on inaccessible or difficult-to-treat sites (like sub- or periungual regions) and in cosmetically sensitive areas (such as the face). Various agents like PPD,[11] BCG vaccine,[12],[13] MMR vaccine,[4] and Candida vaccine,[14] have been used for immunotherapy of warts in the past with favorable results.
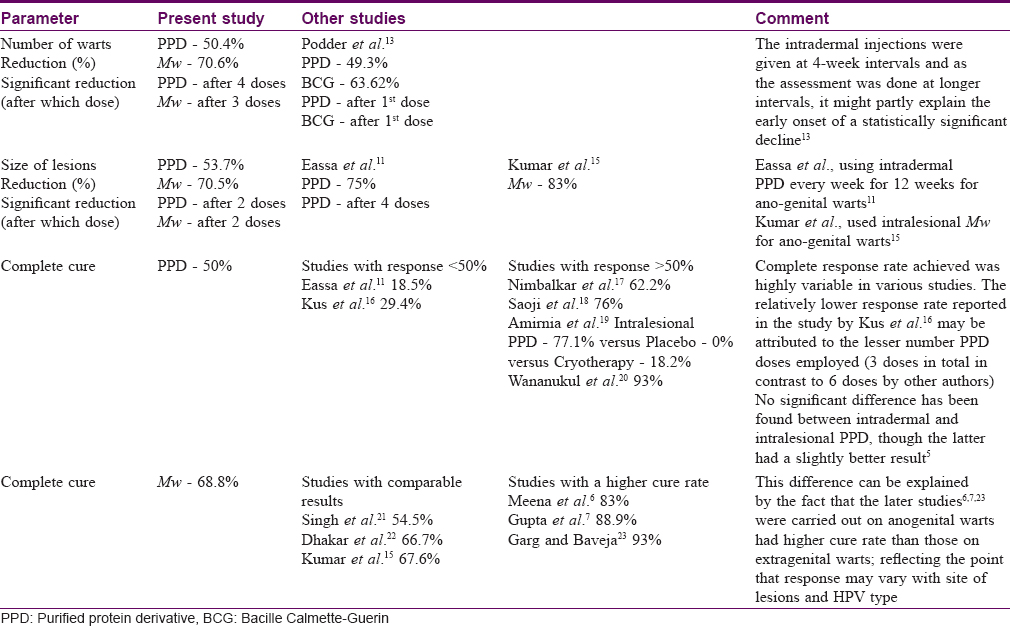
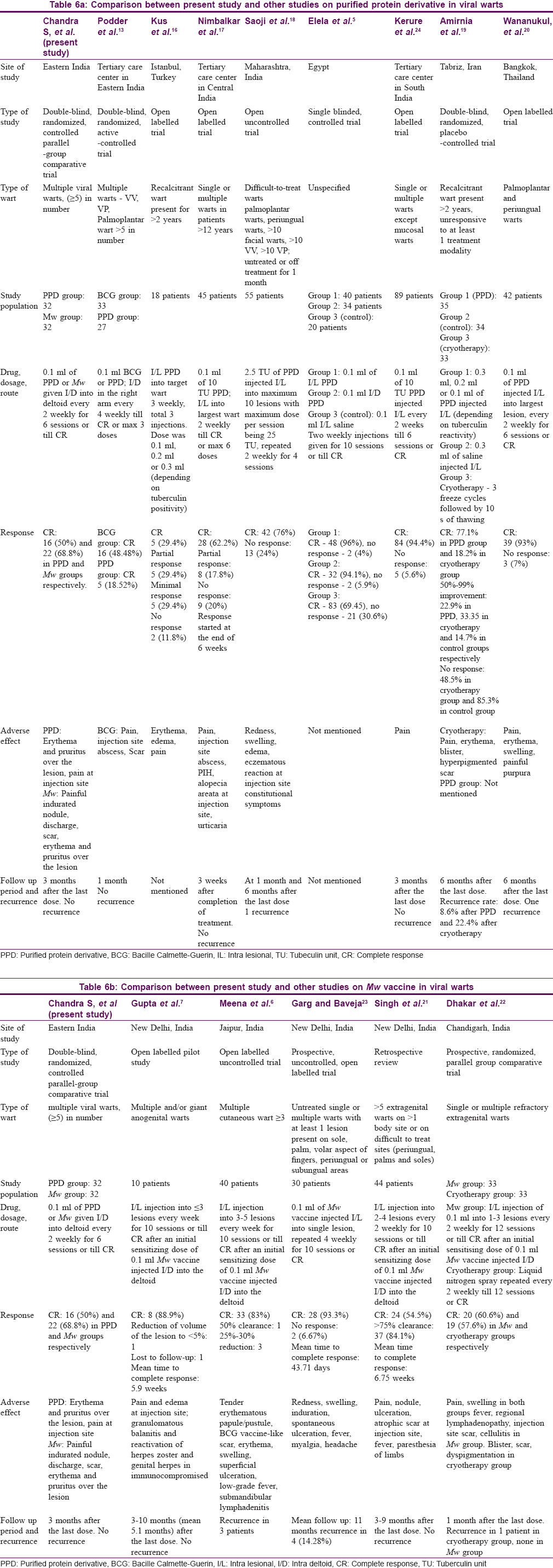
In the present study, there were significant reductions in the number and size of warts in both groups (PPD and Mw arms) over 3 months of active treatment and another 3 months of follow-up, demonstrating that both the study medications were effective in wart treatment. The results of other similar studies with respect to reduction in the number of warts, size of warts and complete clearance of warts are shown in [Table - 5]. [Table - 6]a and [Table - 6]b respectively show a comparison between our study and other studies on PPD and Mw vaccine in viral warts. Our study obtained complete cure in 15.6% (5/32) and 34.4% (11/32) patients in PPD and Mw groups, respectively, at the end-of-treatment visit; this increased to 50% (16/32) and 68.8% (22/32) in the respective groups at the final visit (3 months after end of treatment). With respect to complete clearance, though achieved early with Mw, the final difference in outcome was not statistically significant (P = 0.203). This highlights the fact that immune enhancement and clinical effects persist beyond the treatment schedule, and patients need to be counseled that the improvement may continue and that in case of partial response one should wait for at least 3 months before resorting to other treatment modalities.
While analyzing the prognostic factors to complete resolution, our study demonstrated a significantly better response only in lesions of shorter duration, a finding in line with other studies.[5],[25] This might be due to virus-specific immune apathy in long-standing lesions. Other factors such as age, sex, baseline size and number of lesions, type of warts, presence of BCG immunization scar and tuberculin positivity had no correlation with response rates. Some previous studies have found a positive correlation between younger age and a better respone[25] while one (Elela et al.) found a better response with older age.[5] Another study reported better response in patients with strongly positive tuberculin test.[11] Our study did not find such associations.
We found significant improvement in physicians' and patients' global assessment of disease activity and DLQI in both groups. This might reflect the frustration with the disease and other therapeutic modalities tried before, leading to physicians' and patients'satisfaction with reductions achieved both despite adverse events, which were generally mild.
Injection site reactions were probably due to local immunological responses against the injected antigen and the subsequent elaboration of cytokines leading to an inflammatory reaction. It was found in both the groups, occurring in almost all patients receiving the Mw vaccine compared to only 6 patients receiving PPD. These side-effects have been commonly documented by other authors as well.[11],[16],[17],[18]
Our study indicates that immunotherapy with either Mw vaccine or PPD are potentially effective therapeutic modalities for treatment of warts. The route of injection though being intradermal were nonetheless effective in both the treatment arms. They might be especially useful in Indian settings where the population is widely sensitized to mycobacteria.[26],[27] The phenomenon of immunological uplift is supported by the fact that resolution of warts continues even after the scheduled dosage of injections., and patients opting for immunotherapy need to be counseled about this favorable fact. The Mw vaccine may provide a slightly more rapid benefit, but otherwise the choice between the Mw vaccine and PPD would be guided by the availability and affordability of the agents. It is also to be highlighted that nodule with ulceration at the injection site and scarring are universal with Mw vaccine; thus preprocedural patient counseling is important.
Conclusion
Immunotherapeutic antigens PPD and Mw vaccine are effective and safe in the treatment of multiple warts in the Indian setting. Mw vaccine, though more effective, is associated with more adverse events. Our study provides strong evidence (level Ib) that intradermal immunotherapy has potential for consideration as first-line therapy in patients having multiple cutaneous warts and to replace conventional ablative methods as the treatment of choice in such patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgment
The authors acknowledge the guidance of Prof. D. Bandyopadhyay, Head of Department; and the support obtained from faculty, residents and staff of Department of Dermatology, Medical College, Kolkata.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ciconte A, Campbell J, Tabrizi S, Garland S, Marks R. Warts are not merely blemishes on the skin: A study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol 2003;44:169-73.
[Google Scholar]
|
| 2. |
Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, candida, and trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol 2005;141:589-94.
[Google Scholar]
|
| 3. |
Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol 2011;77:261-3.
[Google Scholar]
|
| 4. |
Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol 2010;24:1166-70.
[Google Scholar]
|
| 5. |
Elela IM, Elshahid AR, Mosbeh AS. Intradermal vs. intralesional purified protein derivatives in treatment of warts. Golf J Deramatol Venereol 2011;18:21-6.
[Google Scholar]
|
| 6. |
Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol 2013;149:237-9.
[Google Scholar]
|
| 7. |
Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol 2008;22:1089-93.
[Google Scholar]
|
| 8. |
TB India 2012. Revised National TB Control Program, Annual Status Report. New Delhi, India: Central TB Division, Directorate General of Health Services Ministry of Health and Family Welfare; 2012.
[Google Scholar]
|
| 9. |
Bonifati C, Berardesca E. Clinical outcome measures of psoriasis. Reumatismo 2007;59 Suppl 1:64-7.
[Google Scholar]
|
| 10. |
Finlay AY. Quality of life indices. Indian J Dermatol Venereol Leprol 2004;70:143-8.
[Google Scholar]
|
| 11. |
Eassa BI, Abou-Bakr AA, El-Khalawany MA. Intradermal injection of PPD as a novel approach of immunotherapy in anogenital warts in pregnant women. Dermatol Ther 2011;24:137-43.
[Google Scholar]
|
| 12. |
Sharquie KE, Al-Rawi JR, Al-Nuaimy AA, Radhy SH. Bacille Calmette-Guerin immunotherapy of viral warts. Saudi Med J 2008;29:589-93.
[Google Scholar]
|
| 13. |
Podder I, Bhattacharya S, Mishra V, Sarkar TK, Chandra S, Sil A, et al. Immunotherapy in viral warts with intradermal Bacillus Calmette-Guerin vaccine versus intradermal tuberculin purified protein derivative: A double-blind, randomized controlled trial comparing effectiveness and safety in a tertiary care center in Eastern India. Indian J Dermatol Venereol Leprol 2017;83:411.
[Google Scholar]
|
| 14. |
Signore RJ. Candida albicans intralesional injection immunotherapy of warts. Cutis 2002;70:185-92.
[Google Scholar]
|
| 15. |
Kumar P, Dar L, Saldiwal S, Varma S, Datt Upadhyay A, Talwar D, et al. Intralesional injection of Mycobacterium w vaccine vs. imiquimod, 5%, cream in patients with anogenital warts: A randomized clinical trial. JAMA Dermatol 2014;150:1072-8.
[Google Scholar]
|
| 16. |
Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol 2005;19:515-6.
[Google Scholar]
|
| 17. |
Nimbalkar A, Pande S, Sharma R, Borkar M. Tuberculin purified protein derivative immunotherapy in the treatment of viral warts. Indian J Drugs Dermatol 2016;2:19-23.
[Google Scholar]
|
| 18. |
Saoji V, Lade NR, Gadegone R, Bhat A. Immunotherapy using purified protein derivative in the treatment of warts: An open uncontrolled trial. Indian J Dermatol Venereol Leprol 2016;82:42-6.
[Google Scholar]
|
| 19. |
Amirnia M, Khodaeiani E, Fouladi DF, Masoudnia S. Intralesional immunotherapy with tuberculin purified protein derivative (PPD) in recalcitrant wart: A randomized, placebo-controlled, double-blind clinical trial including an extra group of candidates for cryotherapy. J Dermatolog Treat 2016;27:173-8.
[Google Scholar]
|
| 20. |
Wananukul S, Chatproedprai S, Kittiratsacha P. Intralesional immunotherapy using tuberculin PPD in the treatment of palmoplantar and periungual warts. Asian Biomed 2009;3:739-43.
[Google Scholar]
|
| 21. |
Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacteriumindicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol 2014;80:509-14.
[Google Scholar]
|
| 22. |
Dhakar AK, Dogra S, Vinay K, Sarangal R, Kanwar AJ, Singh MP, et al. Intralesional Mycobacterium w vaccine versus cryotherapy in treatment of refractory extragenital warts: A randomized, open-label, comparative study. J Cutan Med Surg 2016;20:123-9.
[Google Scholar]
|
| 23. |
Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg 2014;7:203-8.
[Google Scholar]
|
| 24. |
Kerure AS, Nath AK, Oudeacoumar P. Intralesional immunotherapy with tuberculin purified protein derivative for verruca: A study from a teaching hospital in South India. Indian J Dermatol Venereol Leprol 2016;82:420-2.
[Google Scholar]
|
| 25. |
Shaheen MA, Salem SA, Fouad DA, El-Fatah AA. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: A comparative clinical and immunological study. Dermatol Ther 2015;28:194-200.
[Google Scholar]
|
| 26. |
Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012;3:2-6.
[Google Scholar]
|
| 27. |
Pai M, Rodrigues C. Management of latent tuberculosis infection: An evidence-based approach. Lung India. 2015;32:205–207.
[Google Scholar]
|
Fulltext Views
5,664
PDF downloads
2,399





