Translate this page into:
A modified two-step treatment for actinomycetoma
2 Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
3 Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India
4 Department of Mycology, National Institute of Communicable Diseases, New Delhi, India
Correspondence Address:
M Ramam
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi - 110 029
India
| How to cite this article: Ramam M, Bhat R, Garg T, Sharma VK, Ray R, Singh M K, Banerjee U, Rajendran C. A modified two-step treatment for actinomycetoma. Indian J Dermatol Venereol Leprol 2007;73:235-239 |
Abstract
Background: Combination antibiotic regimens are effective in the treatment of actinomycetoma but many treatment schedules require supervised parenteral therapy for prolonged periods. We describe a schedule that includes parenteral medication in an initial, short phase followed by a longer phase of oral medication. Methods: Sixteen patients with clinically diagnosed mycetoma, who did not show any evidence of a fungal etiology, were treated presumptively for actinomycetoma. Evidence of actinomycotic infection was found on microscopy of granules / discharge and / or histopathological examination in eight (50%) patients. The treatment consisted of an intensive phase (Step 1) with gentamicin, 80 mg twice daily, intravenously and cotrimoxazole, 320/1600 mg twice daily orally for four weeks. This was followed by a maintenance phase with cotrimoxazole and doxycycline, 100 mg twice daily till all sinuses healed completely. The treatment was continued for 5-6 months. Results: Treatment response was assessed monthly. At the end of the intensive phase, there was a significant improvement in all 16 patients. Nine patients who continued the maintenance phase of the regimen had complete healing of sinuses with marked reductions in swelling and induration in 2.4 � 1.7 months. Maintenance treatment was continued for a mean of 9.1 � 4.3 months in these patients. Six patients have remained free of disease activity during a follow-up period of 11.1 � 4.2 months after treatment was stopped. Two patients developed leucopenia and thrombocytopenia necessitating withdrawal of cotrimoxazole.Conclusion: This regimen was effective in treating actinomycetoma. The short duration of the phase requiring parenteral therapy makes it convenient to administer.




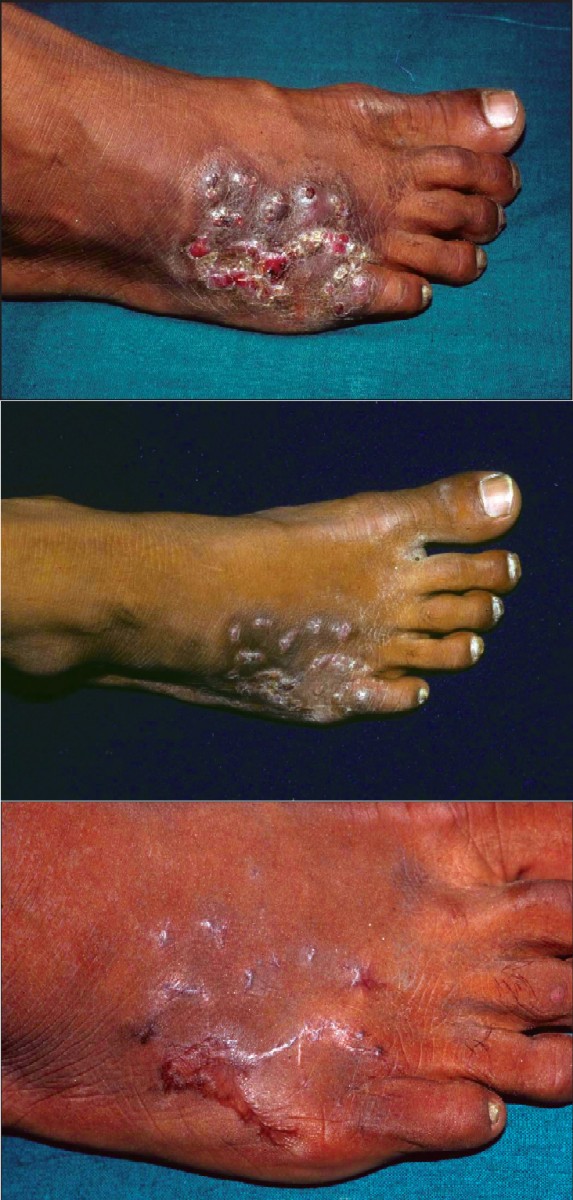 |
| Figure 1a: Actinomycetoma, before treatment Figure 1b: The same patient in Figure 1a, with actinomycetoma at the end of intensive phase of modified regimen Figure 1c: The same patient in Figure 1a, with actinomycetoma after 5.5 months of maintenance therapy |
 |
| Figure 1a: Actinomycetoma, before treatment Figure 1b: The same patient in Figure 1a, with actinomycetoma at the end of intensive phase of modified regimen Figure 1c: The same patient in Figure 1a, with actinomycetoma after 5.5 months of maintenance therapy |
Mycetomas are chronic infections of the skin and subcutaneous tissues that may be caused by true fungi (eumycetoma) or by filamentous bacteria (actinomycetoma). Actinomycetoma are commoner than eumycetoma worldwide and around 75% of mycetomas are actinomycotic in parts of India. [1] Actinomycetoma and eumycetoma have the same clinical picture: the affected part, usually an extremity, demonstrates swelling, induration and sinuses that discharge pus with or without granules composed of colonies of the organism. Treatment of mycetomas can be difficult. Eumycetoma may be unresponsive to antifungal therapy. [2] Actinomycetoma respond to antibiotic therapy but prolonged treatment is necessary. [3]
We previously reported a two-step schedule in the treatment of actinomycotic mycetomas, consisting of penicillin, gentamicin and cotrimoxazole in the initial intensive phase of 5-7 weeks, followed by a maintenance phase with amoxicillin and cotrimoxazole till 2-5 months after complete healing. [4] The treatment schedule required the administration of four injections of penicillin and two of gentamicin daily in the intensive phase. Other workers have reported the effectiveness of combinations of an aminoglycoside and cotrimoxazole without penicillin. [3],[5] As the omission of penicillin from the two-step schedule would make it easier to administer the initial phase, we studied the records of patients who received this modified treatment schedule which also substituted doxycycline for amoxicillin in the second phase.
Methods
The records of 21 patients who presented with a mycetoma to the dermatology department of the All India Institute of Medical Sciences, New Delhi, India between August 2000 and October 2003 were evaluated. The clinical diagnosis of mycetoma was based on the presence of the characteristic clinical features viz, swelling, induration and discharging sinuses with or without granules [Figure - 1]a. All patients were admitted to hospital for baseline evaluation and initiation of treatment. Radiographs were obtained to assess involvement of the underlying bone. The affected part was bandaged in gauze for 48 hours to obtain any discharged granules.
Gram-stained smears and 10% potassium hydroxide preparations of sinus discharge and granules, wherever present, were examined microscopically in order to identify the causative organisms. Sinuses were biopsied for histopathological examination and bacterial and fungal cultures. Cultures were also carried out with sinus discharge. All patients with a mycetoma in whom a fungus was not detected were presumptively diagnosed as having actinomycetoma. Pretreatment investigations included a complete hemogram, renal and liver function tests, urine examination, chest radiographs and audiometry. The treatment consisted of two steps described in [Table - 1].
One patient received doxycycline instead of cotrimoxazole in the last two weeks of the intensive phase since she developed cytopenias. Another patient received oral amoxicillin (500 mg, thrice daily) instead of cotrimoxazole since she developed cytopenias after two weeks of maintenance phase therapy. Further, this patient′s maintenance treatment was discontinued after 3.5 months, two weeks after complete healing as she reported amenorrhea and was found to be pregnant on testing. Patients were assessed every four weeks for clinical response to treatment and those who missed their follow-up visits were mailed reminders. At every visit, reductions in swelling, induration and discharge and healing of sinuses were noted. Clinical photographs were also obtained.
Results
Twenty-one patients with mycetomas were seen during the study period. Three of these patients showed fungal elements on microscopy of grains and / or biopsy, one was found to have nephrolithiasis and could not be given gentamicin and cotrimoxazole and another was later found to have scrofuloderma. Data for the remaining 16 patients was analyzed. The patients′ ages ranged from 16 to 72 years (mean = 33.3±15.9 years) and 14 were males. All patients resided in northern India. The mycetomas had been present for periods ranging from two months to 19 years (median =3 years) at the time of presentation. All had received various medications previously, including cotrimoxazole, dapsone, tetracyclines, streptomycin, penicillin, antifungal and antitubercular therapy for varying durations. Responses to these treatments were partial and / or temporary. Surgical treatment (below-knee amputation and excision, one patient each) was also followed by recurrences.
In nine (56.3%) patients, an ankle, a foot and / or a sole were affected. Two patients had involvement of a leg or thigh and two patients of their buttocks or flanks [Table - 2]. One patient each had involvement of the lower back, a shoulder and arm and a below-knee amputation stump. Yellowish granules were noted in the sinus discharge of two (12.5%) patients. Radiographs showed bone involvement with lytic areas, sclerosis and/or osteopenia and periosteal reaction in nine (56.3%) patients. Actinomycotic organisms were seen in the sinus discharge in five (31.3%) patients. Histopathologic sections showed colonies of organisms in three (18.8%) patients. A mix of acute and chronic inflammation with an infiltrate of lymphocytes and histiocytes and neutrophilic microabscesses, as well as granulation tissue was seen in 12 patients. Epithelioid cell granulomas were identified in two patients. In all, actinomycetes were seen on microscopy of granules/discharge and/or histopathologic examination in eight (50%) patients. The causative organism could not be isolated on culture in any of the cases.
A significant clinical response occurred by the end of the intensive phase in all patients, with mild to marked reductions in swelling, induration and/or sinus discharge [Figure - 1]b. Four patients were lost to follow-up after the intensive phase. Three others discontinued follow-up after 2-5 weeks of maintenance phase therapy and had not healed at their last visit. Of the nine patients who continued to follow-up during the maintenance phase, all had complete healing of all sinuses and marked reductions in swelling and induration in 0.75-6 months (mean = 2.4 ± 1.7 months) [Figure - 1]c. The maintenance treatment was continued for a total duration of 3.5-16 months (mean = 9.1 ± 4.3 months) in these nine patients. Treatment was given for 5-6 months after remission in all patients except two patients who returned for follow-up visits after taking the treatment on their own for 16 months. Two patients have not followed up after completion of therapy. Of the seven patients who followed up after treatment, six have remained disease-free for an additional period of 5-16 months (mean = 11.1 ± 4.2 months) while one developed a single fresh sinus 31 months after treatment was stopped. This lesion was excised and revealed a mixed cell infiltrate in the dermis suggestive of a mycetoma though no organism was seen. He has been well since the excision. The treatment and follow-up of the patients is summarized in [Table - 2].
Two patients developed cytopenias, which recovered after cotrimoxazole was withdrawn (total WBC and platelet counts of 2,700/mm 3 and 16,000/mm 3 respectively in one patient and 2,200/mm 3 and 59,000/mm 3 in the other). Other adverse events that could be related to the treatment were transient tinnitus with normal audiometry findings while on gentamicin in one patient and one episode of microscopic hematuria (1-2 RBCs/ high-power field) with normal renal function tests, transient nausea and vomiting and occasional abdominal pain in the maintenance phase in another patient. The patient who had inadvertently received treatment during pregnancy underwent a cesarean section for cephalopelvic disproportion; the baby was seven months old and apparently healthy when the patient was last seen. Her mycetoma was inactive when evaluated 13 months after stopping the treatment.
Discussion
Several antibiotics have been used in the treatment of actinomycetoma. Cotrimoxazole, dapsone, streptomycin, sulfadoxine-pyrimethamine, rifampicin and amoxicillin-clavulanic acid have all been found effective. [3],[5],[6],[7],[8] In addition, in vitro sensitivity of human isolates of actinomycetes to norfloxacin, ciprofloxacin [1] and linezolid [8] has been demonstrated. Combined antibiotic therapy is preferable to monotherapy to avoid drug resistance and to eradicate residual infection. [9] Some combinations that have been found useful are cotrimoxazole and streptomycin, [3] cotrimoxazole and amikacin, [5] cotrimoxazole and dapsone, [10] cotrimoxazole and penicillin, [11] dapsone and ampicillin, tetracycline or chloramphenicol, [7] dapsone and amikacin [12] and dapsone and streptomycin. [10] Surgery may be required in patients unresponsive to medical therapy alone. Repeated debulking procedures in combination with medical therapy are preferred to amputation.
A microbiologic diagnosis of actinomycetoma by the demonstration of organisms was established in eight (50%) patients. In the remainder, the diagnosis was based on clinico-pathological findings. Faced with the choice of continuing to perform laboratory tests to document the diagnosis or empirically initiating treatment, we chose the latter course for these patients with long-standing disease. In one case, we were proved to have missed the diagnosis of tuberculosis. All the other patients responded to treatment. If attempts to document the diagnosis microbiologically fail, a combination of suggestive clinical and histological features may justify therapy in clinical practice.
A combination of an aminoglycoside and cotrimoxazole has been used with good results. Mahgoub treated 81 patients with streptomycin and cotrimoxazole for 4-24 months; 66 patients were cured or greatly improved. [3] Welsh et al used a combination of amikacin and cotrimoxazole in 13 patients. Amikacin was administered in cycles of 21 days for 1-3 cycles with intervals of 15 days between cycles while cotrimoxazole was administered continuously for 35-105 days. All patients achieved remission with this regimen with most patients requiring two cycles (42 days) of amikacin and 70 days of cotrimoxazole therapy. [5]
Previously, we used a two-step regimen consisting of an intensive phase with penicillin, gentamicin and cotrimoxazole for 5-7 weeks, followed by maintenance therapy with amoxicillin and cotrimoxazole. [4] All seven patients treated with this regimen responded rapidly during the intensive phase and the six patients who completed therapy healed completely. The omission of intravenous penicillin in the present series of patients reduced the daily number of injections to two without any apparent reduction in efficacy. Outpatient or day care treatment may hence be feasible in this phase. We used the aminoglycoside for a shorter period than other workers to reduce the need for parenteral drugs and close monitoring for adverse effects. We chose to use gentamicin in the initial phase because it is currently five times cheaper than amikacin in India. All three drugs used in the regimen are easily available and relatively inexpensive, an important consideration in the treatment of this disorder, which predominantly affects poor people from rural farming communities.
The duration of treatment required to cure actinomycetoma is not clearly defined. Prolonged treatment is recommended to prevent relapses. [3] We continued maintenance therapy with cotrimoxazole and doxycycline for an arbitrarily fixed period of 5-6 months after clinical remission. There was a good response in all patients who continued to follow-up in the maintenance phase. In addition to healing of sinuses and reduction in swelling and induration, patients reported improvements in the function of their affected limbs. In one of our patients, treatment was curtailed because of pregnancy but her mycetoma stayed in clinical remission even though she received only 3.5 months of maintenance therapy. It appears that a shorter course of therapy may suffice in some patients.
Adverse events occurred in six (37.5%) patients. In four patients, side effects were mild and did not necessitate cessation of therapy. Two patients developed cytopenias, which reversed on stopping cotrimoxazole. The hematological side effects of cotrimoxazole may occur due to either trimethoprim or sulfamethoxazole. Trimethoprim-induced cytopenias are more frequent, dose-dependent, usually mild and easily reversed while those due to sulfamethoxazole are rare, idiosyncratic and may be severe. Regular monitoring of the hemogram is helpful in early identification of this adverse effect.
The cause of the relatively high dropout rate could not be ascertained. Five of the seven patients who were lost to follow-up during therapy lived in towns remote from our hospital and this may have contributed to their inability to report for their appointments.
In conclusion, a two-step schedule consisting of a short, intensive phase followed by a longer period of oral therapy, appears to be effective in the treatment of actinomycetoma. The short phase requires parenteral therapy and close supervision while the later oral therapy phase requires less frequent visits and monitoring. The need for compliance with the prolonged treatment needs to be impressed upon patients. It may be possible to use the schedule on an outpatient basis.[13]
| 1. |
Chaudhuri BN, Maiti PK, Sil J. Antibiotic sensitivity patterns of actinomycetes isolated from patients with actinomycetomas. Indian J Med Res 1997;105:162-6.
[Google Scholar]
|
| 2. |
McGinnis MR. Mycetoma. Dermatol Clin 1996;14:97-104.
[Google Scholar]
|
| 3. |
Mahgoub ES. Medical management of mycetoma. Bull World Health Organ 1976;54:303-9.
[Google Scholar]
|
| 4. |
Ramam M, Garg T, D'Souza P, Verma KK, Khaitan BK, Singh MK, et al. A two-step schedule for the treatment of actinomycotic mycetomas. Acta Derm Venereol 2000;80:378-80.
et al. A two-step schedule for the treatment of actinomycotic mycetomas. Acta Derm Venereol 2000;80:378-80.'>[Google Scholar]
|
| 5. |
Welsh O, Sauceda E, Gonzalez J, Ocampo J. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol 1987;17:443-8.
[Google Scholar]
|
| 6. |
Gomez A, Saul A, Bonifaz A, Lopez M. Amoxicillin and clavulanic acid in the treatment of actinomycetomas. Int J Dermatol 1993;32:218-20.
[Google Scholar]
|
| 7. |
Mahaisavariya P, Chaiprasert A, Sivayathorn A, Khemngern S. Deep fungal and higher bacterial skin infections in Thailand: Clinical manifestations and treatment regimens. Int J Dermatol 1999;38:279-84.
[Google Scholar]
|
| 8. |
Gugnani HC, Suselan AV, Anikwe RM, Udeh FN, Ojukwu JO. Actinomycetoma in Nigeria. J Trop Med Hyg 1981;84:259-63.
[Google Scholar]
|
| 9. |
Fahal AH. Mycetoma: A thorn in the flesh. Trans R Soc Trop Med Hyg 2004;98:3-11.
[Google Scholar]
|
| 10. |
Chavez G, Estrada R, Bonifaz A. Perianal actinomycetomas experience of 20 cases. Int J Dermatol 2002;41:491-3.
[Google Scholar]
|
| 11. |
Khatri ML, Al-Halali HM, Fouad Khalid M, Saif SA, Vyas MC. Mycetoma in Yemen: Clinoco-epidemiologic and histopathologic study. Int J Dermatol 2002;41:586-93.
[Google Scholar]
|
| 12. |
Sharma N, Mendiratta V, Sharma RC, Hemal U, Verma M. Pulse therapy with amikacin and dapsone for the treatment of actinomycotic foot: A case report. J Dermatol 2003;30:742-7.
[Google Scholar]
|
| 13. |
Fahal AH, Hassan MA. Mycetoma. Br J Surg 1992;79:1138-41.
[Google Scholar]
|
Fulltext Views
14,835
PDF downloads
1,783





