Translate this page into:
A new case of imatinib-induced drug reaction with eosinophilia and systemic symptoms
Correspondence Address:
Ines Lahouel
Department of Dermatology, Farhat Hached Hospital, 4000 Sousse
Tunisia
| How to cite this article: Saidi W, Lahouel I, Laarif M, Aounallah A. A new case of imatinib-induced drug reaction with eosinophilia and systemic symptoms. Indian J Dermatol Venereol Leprol 2017;83:224-226 |
Sir,
Imatinib mesylate is an inhibitor of tyrosine kinases. It is approved for the treatment of chronic myeloid leukemia. The most commonly reported adverse reactions are maculopapular eruptions and periorbital edema. Severe adverse reactions are seen in 5% of patients.[1] We were able to find only three previous reports of drug-induced reaction with eosinophilia and systemic symptoms (DRESS) caused by imatinib.[2],[3],[4]
A 53-year-old woman was diagnosed with chronic myeloid leukemia. Initially, the patient had been treated with hydroxyurea along with allopurinol, since December 27, 2013. On March 13, 2014, hydroxyurea and allopurinol were stopped and imatinib, 400 mg daily was introduced. Nineteen days after starting imatinib, the patient presented with fever and a generalized rash associated with oral erosions, facial oedema [Figure - 1] and cervical and axillary lymphadenopathy. There had been no history of any other recent drug intake. The laboratory findings were notable for leukocytosis, with a white blood cell count up to 18,700/µL and eosinophilia of 10,100/µL. Her creatine phosphokinase level was elevated at 576 IU/L. She had increased levels of urea (10 mmol/L) (normal = 3.3-7) and creatinine (102 µmol/L) (n = 53-90). The liver enzyme levels were normal. The serological tests for Epstein-Barr virus, human herpesvirus 6 and human immunodeficiency virus were negative. Skin biopsy showed a discrete parakeratosis, acanthosis, significant spongiosis, dermal edema and a perivascular inflammatory infiltrate consisting of mainly eosinophils and lymphocytes [Figure - 2] and [Figure - 3].
 |
| Figure 1: Erythematous facial edema |
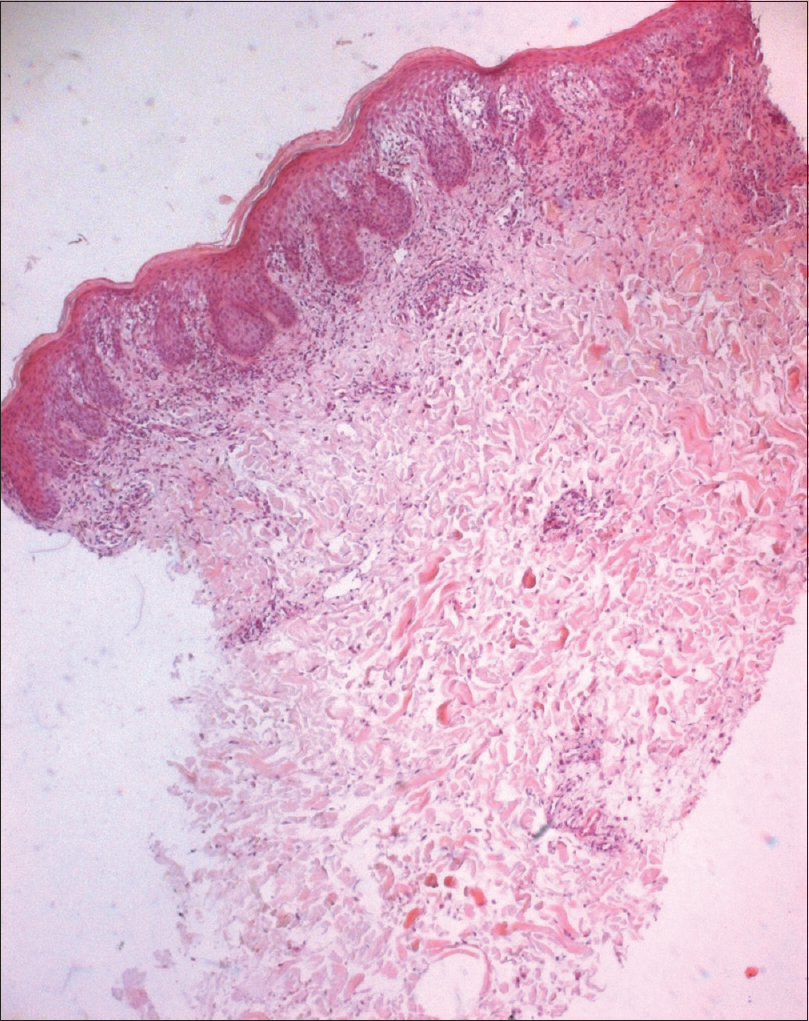 |
| Figure 2: Parakeratosis, acanthosis, spongiosis with an inflammatory infiltrate (H and E, ×400) |
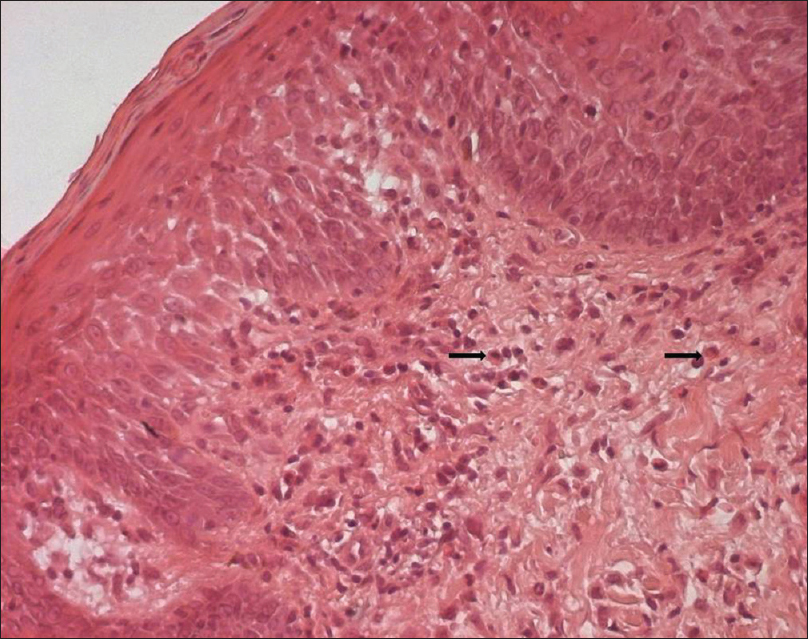 |
| Figure 3: Parakeratosis, acanthosis, spongiosis with an inflammatory infiltrate consisting of lymphocytes and mainly eosinophils (arrow) (H and E, ×1000) |
The patient was diagnosed to have drug-induced reaction with eosinophilia and systemic symptoms (DRESS) syndrome, based on the following criteria: rash on more than 50% of her body, lymphadenopathy, eosinophilia, muscular and renal involvement. Imatinib was stopped and the patient was prescribed oral antihistamines and topical steroids. Improvement was noted with decreased erythema on the 4th day. The rash disappeared completely within 2 weeks. Renal function tests, muscle enzymes and eosinophil level normalized by the 8th day after discontinuation of imatinib. The patient was changed to an alternative anti-chronic myeloid leukemia medication, nilotinib. Reintroduction or desensitization was not attempted as drug-induced reaction with eosinophilia and systemic symptoms (DRESS) syndrome is considered a severe form of drug reaction.
Cutaneous toxicity due to imatinib is, in most cases, mild. Severe responses were reported in 5% of patients.[1] The most commonly reported adverse responses are maculopapular eruptions, periorbital edema and the less common ones include Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, hypopigmentation, lichenoid reaction, pityriasis rosea and Sweet syndrome.[1]
We were able to find three previous reports of drug-induced reaction with eosinophilia and systemic symptoms (DRESS) caused by imatinib.[2],[3],[4] The three patients had chronic myeloid leukemia. Drug-induced reaction with eosinophilia and systemic symptoms usually occurs within two to 6 weeks of introducing the drug.[3] In our patient, the rash appeared 19 days after introducing imatinib; hydroxyurea and allopurinol were stopped 3 weeks before the onset of the eruption. The mean plasma half-life of allopurinol ranges from 1 to 2 h. Allopurinol is metabolized to oxypurinol whose plasma half-life ranges from 13.6 to 29 h. After five plasma half-lives (maximum 6 days) of stopping the drug, allopurinol and its metabolites are eliminated from the body. The mean plasma half-life of hydroxyurea is short (6 h). This makes it unlikely that allopurinol or hydroxyurea were the cause of the drug eruption.
The clinical improvement and disappearance of eosinophilia were observed after stopping imatinib. According to the objective causality assessment by the Naranjo probability scale, imatinib-induced drug-induced reaction with eosinophilia and systemic symptoms was probable in our case.[5]
Drug-induced reaction with eosinophilia and systemic symptoms (DRESS) is a severe idiosyncratic reaction. Its physiopathology is not clear. Viral agents are suspected as triggering factors.[3] In our case, the viral serology did not show any active infection.
Most rashes due to imatinib are self-limiting and do not require discontinuation of treatment.[1] Oral antihistamines and topical steroids are sufficient in most cases. In two reports of drug-induced reaction with eosinophilia and systemic symptoms (DRESS) caused by imatinib, the course was favorable a few days after cessation of the drug.[2],[3] Only one case required systemic steroids and the patient was treated with oral prednisolone 1 mg/kg/day.[4] In our case, because of the absence of severe visceral involvement, we opted for topical steroids. The rash disappeared completely within 2 weeks. Le Nouail et al. also reported that the rash and associated manifestations improved within 12 days after introduction of topical steroids, as seen in our case.[3]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Scheinfeld N. Imatinib mesylate and dermatology part 2: A review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol 2006;5:228-31.
[Google Scholar]
|
| 2. |
Goldman J, Duval-Modeste AB, Lambert A, Contentin N, Courville P, Musette P, et al. Imatinib-induced DRESS. Ann Dermatol Venereol 2008;135:393-6.
[Google Scholar]
|
| 3. |
Le Nouail P, Viseux V, Chaby G, Billet A, Denoeux JP, Lok C. Drug reaction with eosinophilia and systemic symptoms (DRESS) following imatinib therapy. Ann Dermatol Venereol 2006;133:686-8.
[Google Scholar]
|
| 4. |
Kumar M, Mandal PK, Dolai TK, Bhattacharrya M. Imatinib causing drug rash with eosinophilia and systemic symptoms: A rare cutaneous reaction. Indian Dermatol Online J 2014;5 Suppl 2:S120-2.
[Google Scholar]
|
| 5. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
Fulltext Views
4,507
PDF downloads
2,858





