Translate this page into:
A non-healing oral ulcer as a manifestation of systemic tuberculosis in an immunocompetent man
2 Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
4 Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Rahul Mahajan
Department of Dermatology and Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Bhatia R, Mahajan R, Arava S, Singh S, Kandasamy D. A non-healing oral ulcer as a manifestation of systemic tuberculosis in an immunocompetent man. Indian J Dermatol Venereol Leprol 2017;83:238-241 |
Sir,
Oral ulcers can occasionally be the initial manifestation of a systemic illness. We report the case of a young immunocompetent male with a non-healing oral ulcer as the presenting feature of systemic tuberculosis.
A 29-year-old man presented to us with an oral ulcer for nine months. It first appeared over the upper labial mucosa, and then gradually increased in size to involve the hard palate. There was history of loosening of the teeth and difficulty in eating food. He denied smoking or chewing tobacco. Oral examination revealed a non-tender, ulcerated plaque over the hard palate measuring 5 cm × 3.5 cm, which extended to the upper labial mucosa. The surface was irregular, with a “cobblestoned” appearance [Figure - 1]a. On palpation, there was no bleeding or induration at the base. A lymph node was enlarged at the right submandibular region. It was mobile, firm and measured 1.5 cm in diameter. There was no warmth, erythema or tenderness over the lymph node. The patient had a BCG vaccination scar. The systemic examination revealed no obvious abnormalities. The patient was investigated with the differential diagnoses of Wegener's granulomatosis, squamous cell carcinoma, deep fungal infection, pyostomatitis vegetans, Crohn's disease and tuberculosis. The laboratory findings were within normal limits, except for an elevated erythrocyte sedimentation rate of 86 mm/hour. The c-ANCA and HIV antibody tests were negative. The panoramic radiograph of the maxillofacial region was normal. Two biopsies from the edge of the ulcer showed mixed cell infiltrates while the third biopsy from the edge of the ulcer on the anterior portion of hard palate revealed epithelioid cell granulomas with no acid fast bacilli [Figure - 2]. Fine needle aspiration cytology from the right submandibular node showed a granulomatous infiltrate. The chest X-ray showed patchy opacities in the upper lobes of lungs. Computed tomography scan revealed nodular opacities with areas of cavitation in the upper lobes of the lungs and diffuse circumferential thickening of the cecum, ascending colon and hepatic flexure extending to the terminal ileum [Figure - 3]. Biopsies from the lung and colon showed epithelioid cell granulomas with areas of necrosis [Figure - 4]. Tissue cultures for bacteria, mycobacteria and fungus were negative. The biopsies were subjected to a polymerase chain reaction to detect mycobacteria, which was negative. The tuberculin skin test was strongly positive (20 mm × 25 mm). A provisional diagnosis of tuberculosis involving the intestines, lungs and oral cavity was made, and antitubercular therapy was started. There was significant improvement in the ulceration and induration within a month of treatment, thereby confirming our diagnosis [Figure - 1]b.
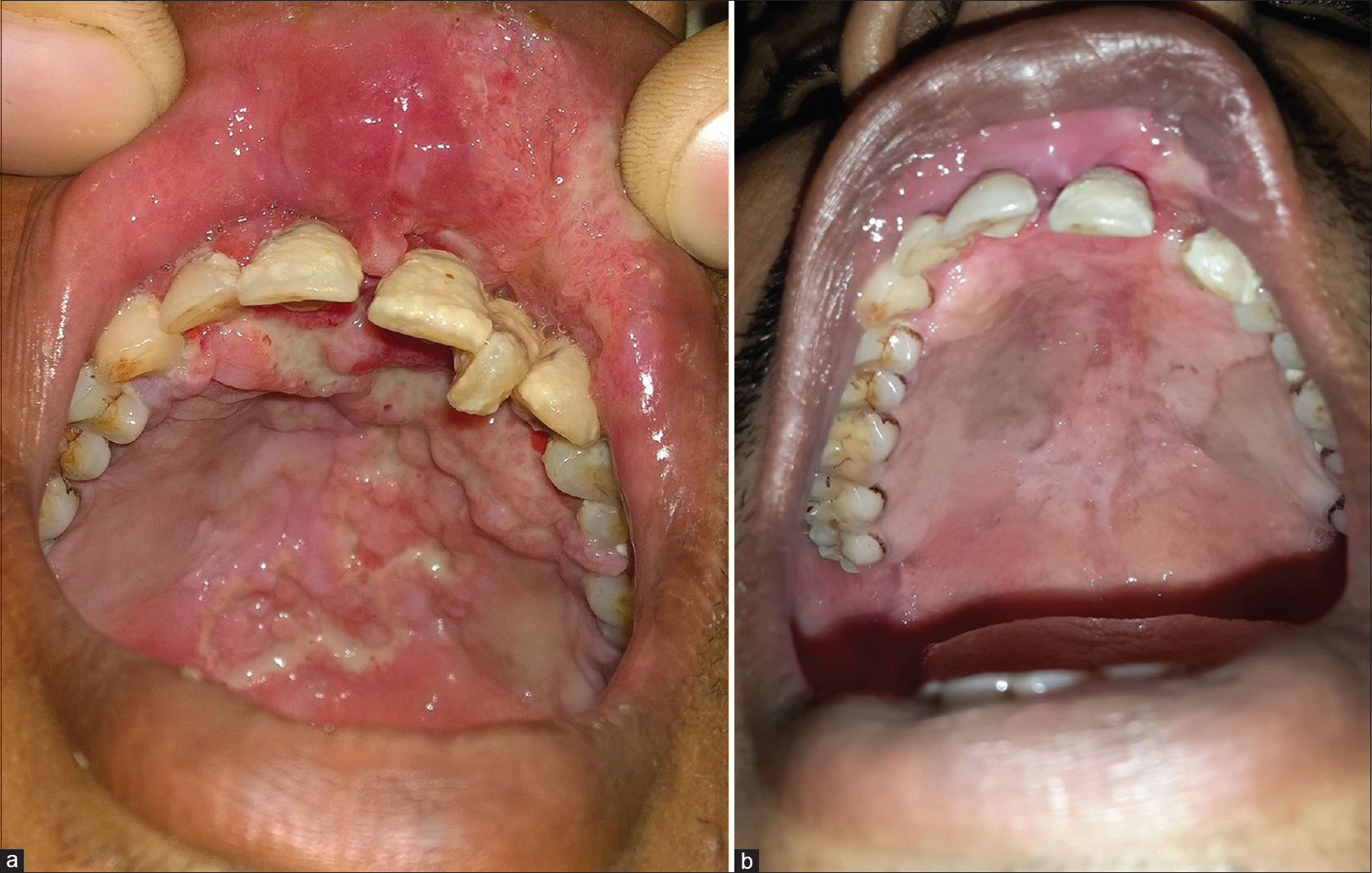 |
| Figure 1: (a) Baseline photograph showing ulceration of the hard palate with areas of necrotic slough, labial hypertrophy and loosening of the upper second incisor. (b) Complete resolution of the ulcer and labial hypertrophy four weeks after initiation of antitubercular treatment |
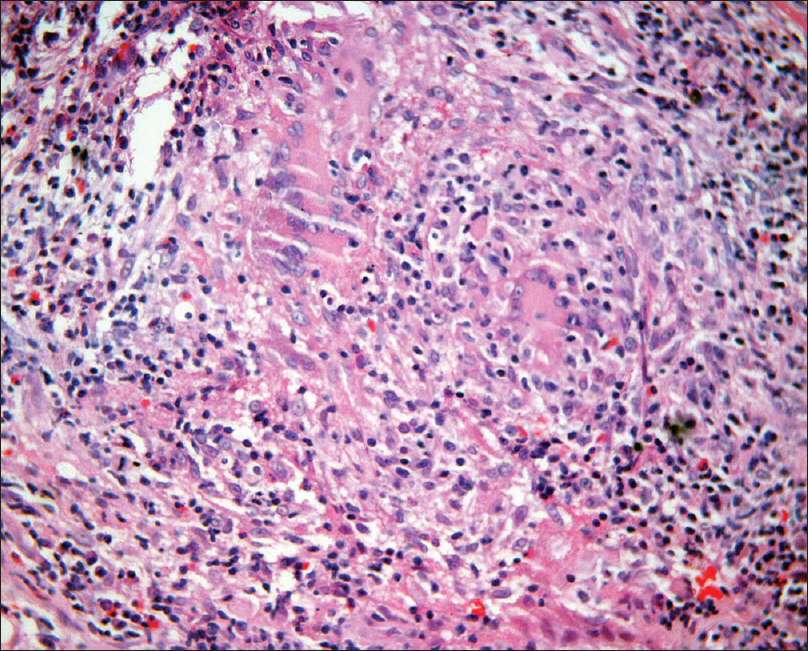 |
| Figure 2: Oral mucosal biopsy showing dense dermal mixed cell infiltrate of lymphocytes, plasma cells, histiocytes and epithelioid cells along with Langhans giant cells (H and E, ×400) |
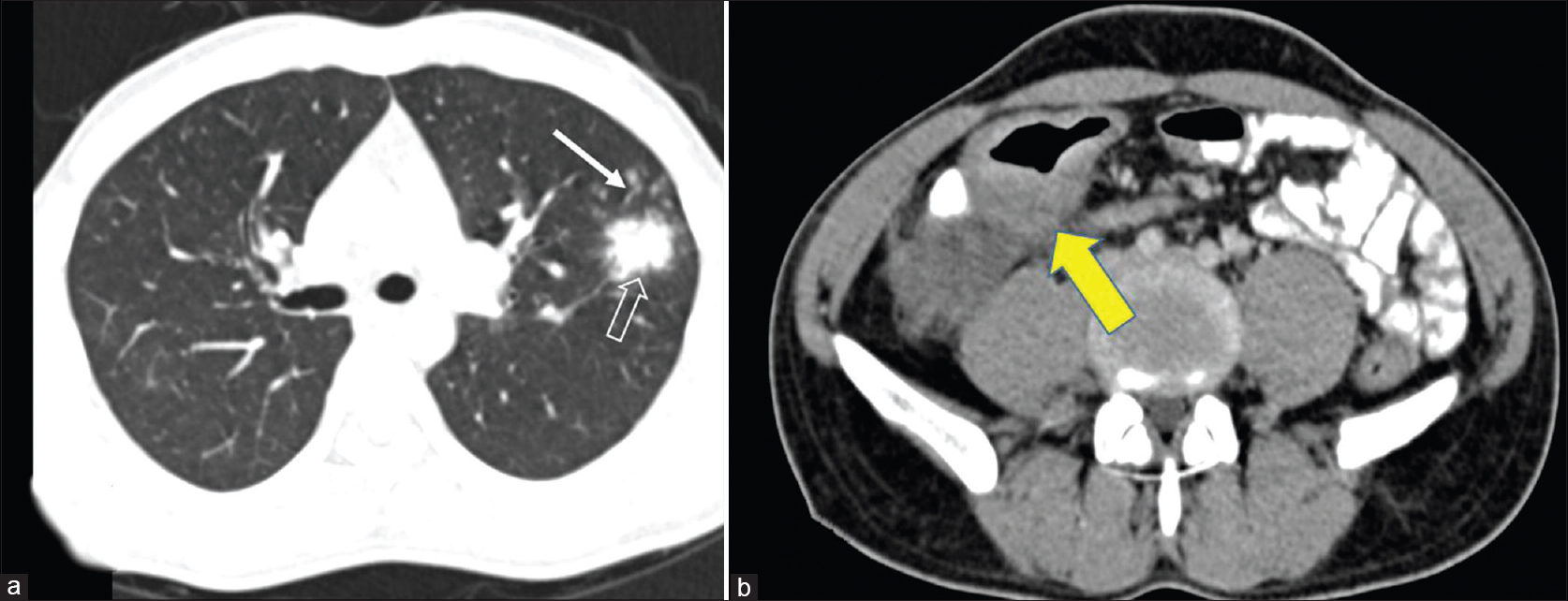 |
| Figure 3: (a) Contrast-enhanced computed tomography scan of the chest showing an area of parenchymal infiltration in the left lobe (outlined arrow) and surrounding multiple centrilobular nodules (solid arrow). (b) Thickening of the ileocecal junction (yellow arrow) with a few enlarged mesenteric nodes. These features are consistent with active tuberculosis |
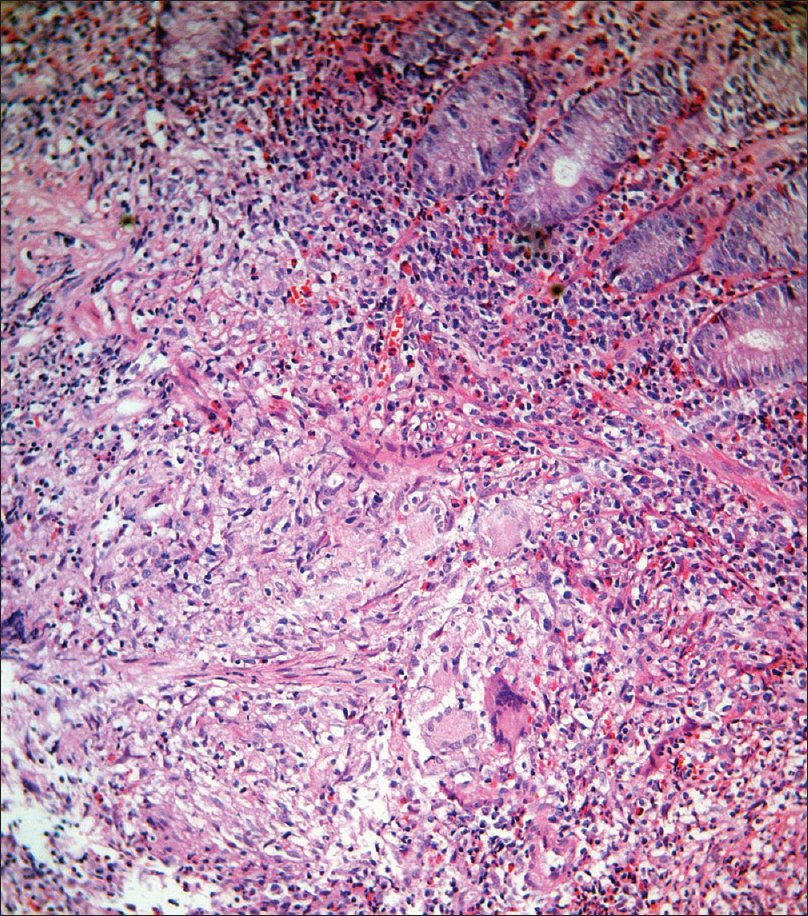 |
| Figure 4: Dense infiltrate of epithelioid cells, Langhans giant cells, eosinophils and neutrophils in the submucosa of the colon (H and E, ×400) |
Oral tuberculosis can be either primary or secondary. It is quite uncommon with an incidence of only 0.1–0.4%.[1],[2] It typically occurs in the setting of immunosuppression. Primary oral tuberculosis usually affects children, while secondary oral involvement usually occurs in the middle and older age groups.[3],[4] The tongue is most commonly affected, followed by the palate, buccal mucosa and lips. The salivary glands, tonsils and mandibular ridge are less involved.[5] The oral manifestations of tuberculosis are varied and include ulcers, nodules, fissures and osteomyelitis of the jaw.[6] Painless, non-healing oral ulcers with irregular edges and a minimally indurated base are the most common finding, as in our case. However, such a clinical picture can also be seen in syphilis, Wegener's granulomatosis, histoplasmosis, actinomycosis and squamous cell carcinoma. Orificial tuberculosis presents as multiple, small, superficial and painful ulcers in the setting of immunosuppression. Numerous bacilli are found on Ziehl–Neelsen staining. We did not consider this diagnosis in our patient as the ulcer was painless and he was immunocompetent.
Repeated biopsies may be needed for the diagnosis of in tuberculosis. It has been reported that a single biopsy from the oral mucosa may not show characteristic findings, especially in early disease.[7] Attempts to isolate the organism from oral mucosal biopsies usually have a low yield.[1],[8] In our case, only the third biopsy revealed epithelioid cell granulomas. The inability to detect the organism in the lungs, oral cavity or gastrointestinal tract could be explained by the low sensitivity of microscopy in patients with less advanced disease.[9] Taken together, sensitive broth-based culture media for Mycobacterium tuberculosis and nucleic acid amplification tests (like polymerase chain reaction) help to detect 95–98% of multibacillary cases, and 48–53% of paucibacillary cases. However, bacteriological confirmation is still not achieved in 10–20% of cases, and in these cases the diagnosis is based solely on the clinical or radiological improvement with treatment.[10],[11]
Incipient tuberculosis is a type of well-contained asymptomatic tuberculosis in apparently immunocompetent individuals. It shows well-formed granulomas and lack of bacilli due to the good immune status of the host. It is distinguished from latent tuberculosis on the basis of radiological evidence of disease and a high risk of progression in incipient tuberculosis.[12] Asymptomatic pulmonary and intestinal tuberculosis have been described in isolation.[13],[14] Our patient may be a case of incipient or early tuberculosis which was detected because of the oral ulcer, like the asymptomatic cases of systemic tuberculosis described previously. The diagnosis of tuberculosis was confirmed on the basis of good response to antitubercular treatment.
We report this case due to its unusual presentation. Non-healing oral ulcers were the first presentation of intestinal and pulmonary tuberculosis in an apparently healthy, immunocompetent man. A search for an underlying focus of tuberculosis should be made in case of non-healing oral ulcers, especially in endemic countries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Verma A, Mann SB, Radotra B. Primary tuberculosis of the tongue. Ear Nose Throat J 1989;68:718-20.
[Google Scholar]
|
| 2. |
Prada JL, Kindelan JM, Villanueva JL, Jurado R, Sánchez-Guijo P, Torre-Cisneros J. Tuberculosis of the tongue in two immunocompetent patients. Clin Infect Dis 1994;19:200-2.
[Google Scholar]
|
| 3. |
Prabhu SR, Daftary DK, Dholakia HM. Tuberculous ulcer of the tongue: Report of case. J Oral Surg 1978;36:384-6.
[Google Scholar]
|
| 4. |
Shafer WG, Hine MK, Levy BM. Bacterial infection of the oral cavity. In: Shafer WG, Hine MK, Levy BM, editors. Textbook of Oral Pathology. 6th ed. Philadelphia: W.B. Saunders Company; 2011. p. 313-7.
[Google Scholar]
|
| 5. |
Vaid S, Lee YY, Rawat S, Luthra A, Shah D, Ahuja AT. Tuberculosis in the head and neck – A forgotten differential diagnosis. Clin Radiol 2010;65:73-81.
[Google Scholar]
|
| 6. |
Kiliç A, Gül U, Gönül M, Soylu S, Cakmak SK, Demiriz M. Orificial tuberculosis of the lip: a case report and review of the literature. Int J Dermatol 2009;48:178-80.
[Google Scholar]
|
| 7. |
Hashimoto Y, Tanioka H. Primary tuberculosis of the tongue: Report of a case. J Oral Maxillofac Surg 1989;47:744-6.
[Google Scholar]
|
| 8. |
Prabhu SR, Sengupta SK. Bacterial infections due to mycobacteria. In: Prabhu SR, Wilson DF, Daftary DK, Johnson NW, editors. Oral Diseases in the Tropics. 1st ed. Delhi: Oxford University Press; 1993. p. 195-202.
[Google Scholar]
|
| 9. |
WHO. Diagnostics for Tuberculosis: Global Demand and Market Potential. Geneva, Switzerland: WHO; 2006.
[Google Scholar]
|
| 10. |
Hanna BA. Laboratory diagnostics. In: Rom WN, Garay SM, editors. Tuberculosis. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2004. p. 163-76.
[Google Scholar]
|
| 11. |
Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet 2003;362:887-99.
[Google Scholar]
|
| 12. |
Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: Defining early disease states in the context of host immune response. J Infect Dis 2011;204 Suppl 4:S1179-86.
[Google Scholar]
|
| 13. |
Singhaniya SB, Barpande SR, Bhavthankar JD. Oral tuberculosis in an asymptomatic pulmonary tuberculosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;111:e8-10.
[Google Scholar]
|
| 14. |
Sato S, Yao K, Yao T, Schlemper RJ, Matsui T, Sakurai T, et al. Colonoscopy in the diagnosis of intestinal tuberculosis in asymptomatic patients. Gastrointest Endosc 2004;59:362-8.
[Google Scholar]
|
Fulltext Views
7,819
PDF downloads
2,117





