Translate this page into:
A novel homozygous frameshift mutation in DSG1 gene in an Indian consanguineous family with severe dermatitis, multiple allergies and metabolic wasting syndrome
Corresponding author: Dr. Sahana M. Srinivas, Department of Pediatric Dermatology, Indira Gandhi Institute of Child Health, Bengaluru, Karnataka, India. sahana.bmc@gmal.com
-
Received: ,
Accepted: ,
How to cite this article: Srinivas SM, Basavanaik P, Gowdra A. A novel homozygous frameshift mutation in DSG1 gene in an Indian consanguineous family with severe dermatitis, multiple allergies and metabolic wasting syndrome. Indian J Dermatol Venereol Leprol 2021;87:410-6.
Sir,
Severe dermatitis, multiple allergies and metabolic wasting syndrome (OMIM #615508), also known as congenital erythroderma with palmoplantar keratoderma, hypotrichosis and hyper-immunoglobulin E, is a recently recognized autosomal recessive genodermatosis caused by homozygous or compound heterozygous loss of function mutation in desmoglein1 gene (DSG1) or heterozygous missense mutation in desmoplakin gene (DSP). 1,2 It was first described in 2013 by Samuelov et al.1 It is clinically characterized by congenital ichthyosiform erythroderma, palmoplantar keratoderma, failure to thrive, multiple allergies, increased serum immunoglobulin E levels, hypotrichosis, recurrent infections and other systemic abnormalities like metabolic wasting, malabsorption, esophagitis, cardiac defects, microcephaly and developmental delay. Till date, approximately 14 cases of severe dermatitis, multiple allergies and metabolic wasting syndrome from 10 families have been reported in literature with varying phenotypes. Herein, we describe a rare case of severe dermatitis, multiple allergies and metabolic wasting syndrome in an Indian consanguineous family with a novel homozygous frameshift mutation, in exon 15 of DSG1gene and identical mutation in a heterozygous state in the parents.
A 4-year-old term boy, first born to a second degree consanguineously married couple of Indian origin, presented with erythema and scaling all over the body from 7thday of life with recurrent respiratory infections, failure to thrive, metabolic wasting and developmental delay. Both the parents had mild palmoplantar keratoderma. He had Cushingoid features, short stature with height less than 3rd percentile (69.6cm) and bone age of 2 years along with infantile face, voice and microphallus with a stretch penile length of 2.5 cm (<2 standard deviation for age) which were suggestive of growth hormone deficiency. Cutaneous examination showed diffuse erythema with scaling all over the body [Figure 1a]. Well-defined hyperkeratotic scaly plaques were present on the extensor aspect of both the knee joints, upper and lower limbs [Figure 1b]. Ill-defined hyperkeratosis with accentuation was present on the flexures and perianal region [Figure 1c]. Scalp showed mild scaling and hypotrichosis [Figure 1a]. Diffuse palmoplantar keratoderma was present [Figures 2a and b]. Investigations revealed increased serum immunoglobulin E levels (>2000 IU/mL) and growth hormone stimulation test confirmed severe growth hormone deficiency. Light microscopy of the hair shaft was normal. Histopathological examination revealed orthokeratosis, hypergranulosis, subtle acantholysis and a few scattered dyskeratotic cells in the epidermis [Figure 3]. Differential diagnoses considered were Netherton syndrome, non-bullous ichthyosiform erythroderma, trichothiodystrophy, ichthyosis hypotrichosis syndrome and epidermal growth factor receptor deficiency.
Genetic testing was done in the proband and his parents after taking written informed consent. Clinical exome sequencing report of the proband revealed homozygous 2 base pair (AG) deletion in exon 15 of DSG1 gene located on chromosome 18,c.2601_2602delAG (NM_001942.3:g. 28934754_28934755delAG) where amino acid arginine was replaced by serine at 867th codon position that resulted in a frameshift mutation and premature truncation of protein 9 amino acids downstream to codon 867 (p.Arg867Serfs*9; ENST00000257192.4) [Figure 4]. This DSG1 variant has not been reported in Human Gene Mutation Database Professional 2018.4, Single Nucleotide Polymorphism Database, 1000 genomes, Exome Aggregation Consortium and our internal databases. We also excluded it from 300 normal healthy Indians.The in-silico prediction of the variant is damaging by MutationTaster (www.mutationtaster.org). Protein Variation Effect Analyzer, (http://provean.jcvi.org) predicted it to be deleterious with a score of -4.3. This variant is not associated with nonsense-mediated mRNA decay and it is highly conserved across species.3 This mutant variant of the index case was validated by Sanger sequencing [Figure 5a].The Sanger sequence report of both father and mother revealed an identical mutation in a heterozygous state [Figures 5b and c]. Fortunately, his asymptomatic sister did not have the mutation.
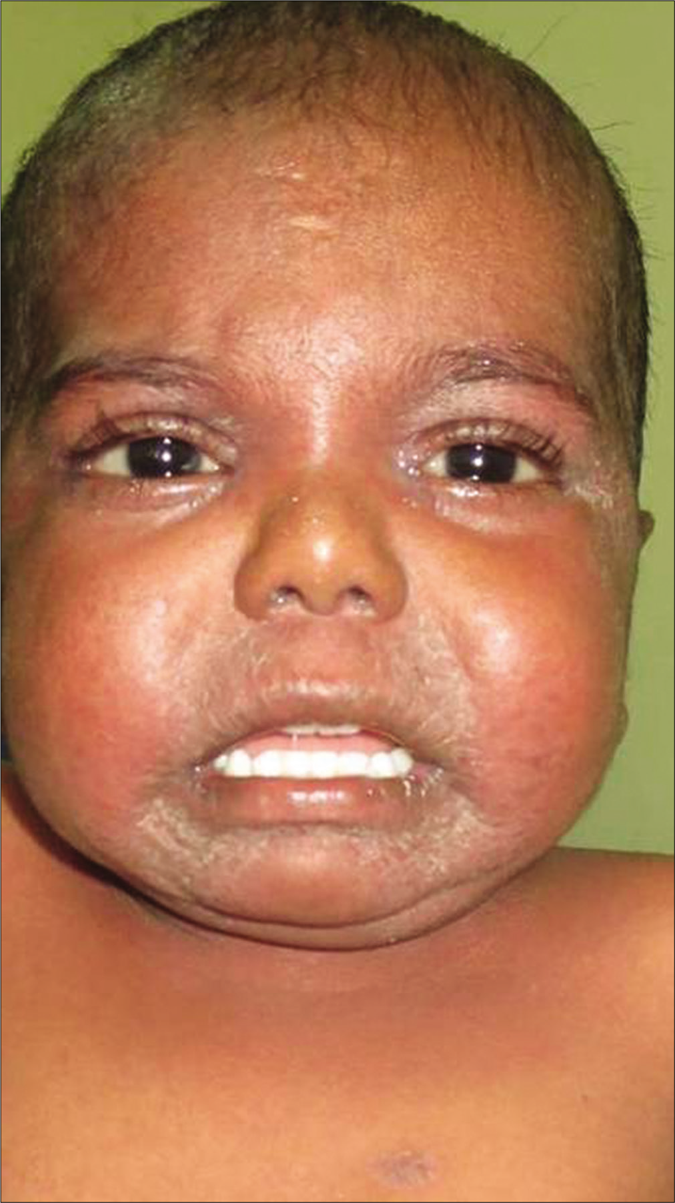
- Diffuse erythema with scaling on face and chest along with scant hair on the scalp

- Well-defined hyperkeratotic plaques present on the knee joint
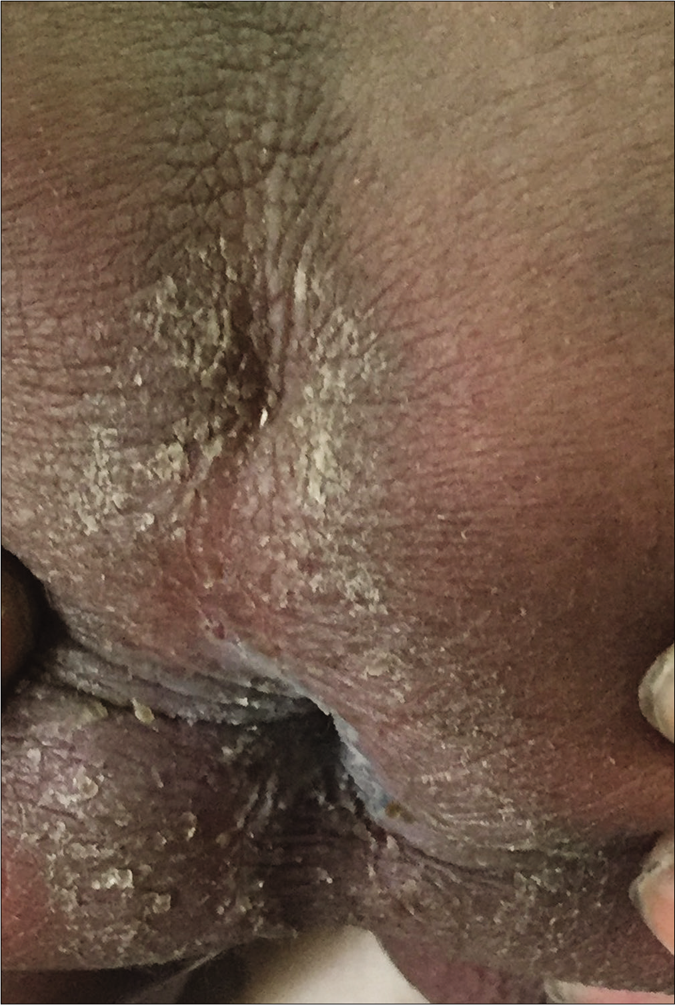
- Perianal scaling with hyperkeratosis

- Diffuse keratoderma present on palms
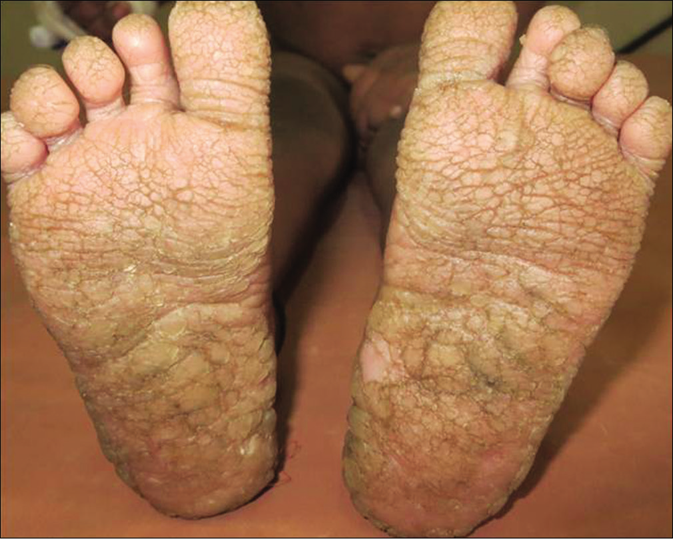
- Diffuse plantar keratoderma
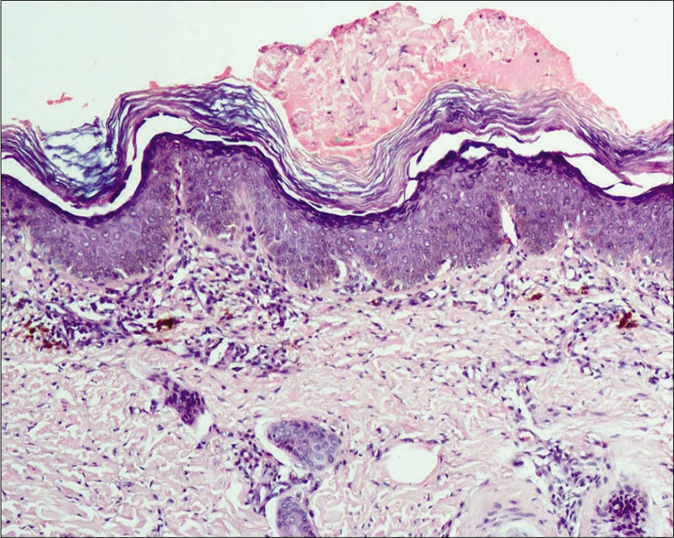
- Epidermis showing orthokeratosis, hypergranulosis, subtle acantholysis and a few scattered dyskeratotic cells (H and E,×100)
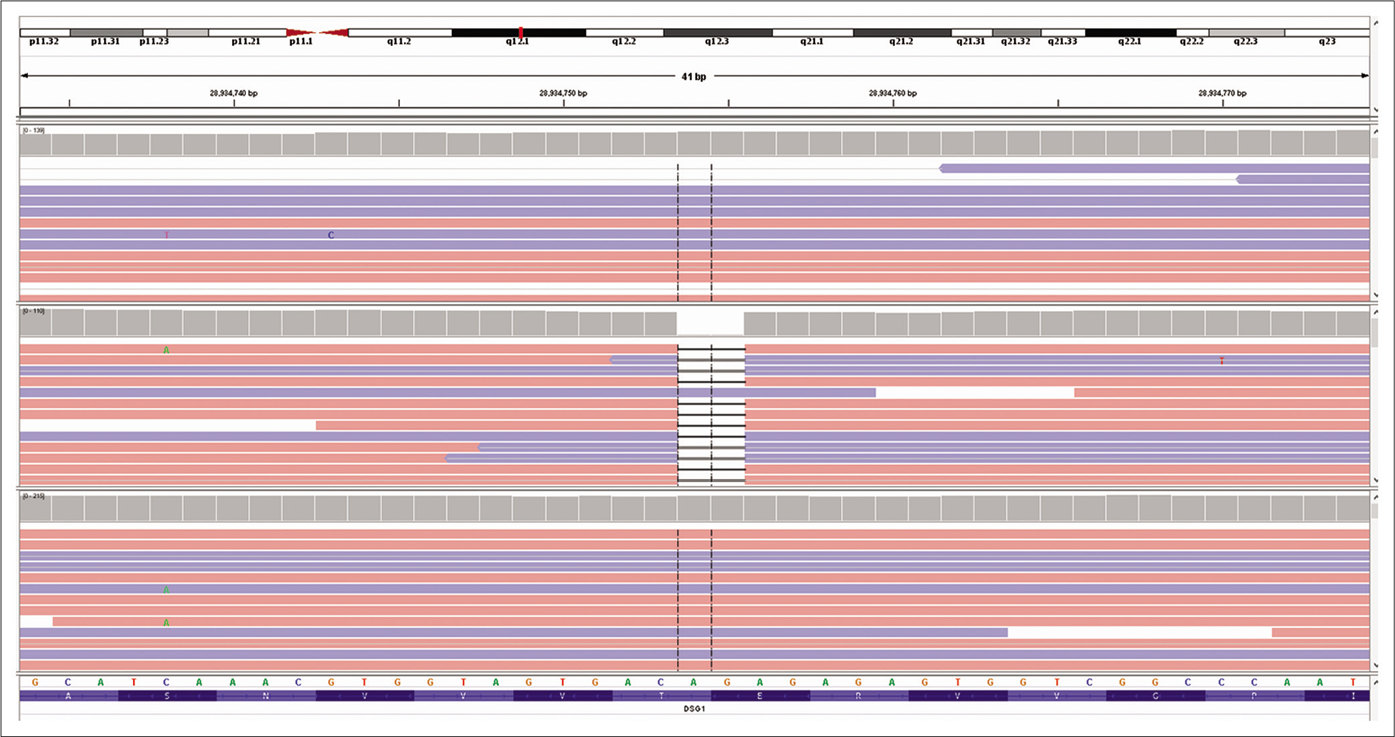
- Excerpt of next-generation sequencing data of the proband visualized with the integrative genomic viewer showing homozygous 2 base pair deletion in exon 15 of desmoglein1 gene located on chromosome 18 compared with two control sequences
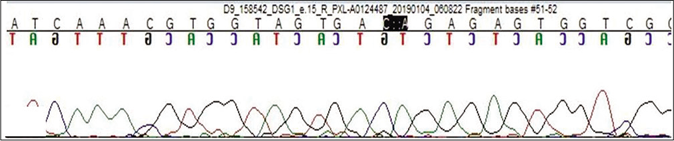
- Sequence chromatogram and alignment to the reference sequence showing the variation in exon 15 of the desmoglein1 gene (chr18:28934754_28934755delAG; c.2601_2602delAG; p.Arg867SerfsTer9) in the proband
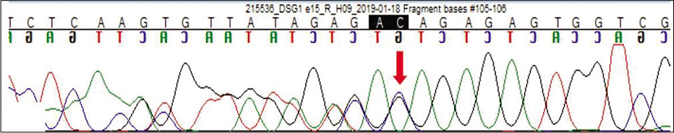
- Sequence chromatogram and alignment to the reference sequence showing the identical variation in heterozygous carrier father indicated by the red arrow
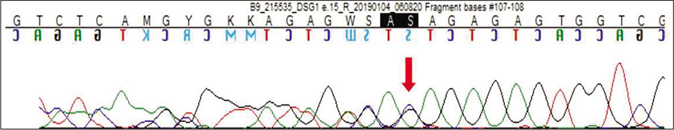
- Sequence chromatogram and alignment to the reference sequence showing the identical variation in heterozygous carrier mother indicated by the red arrow
Desmosomal cadherin DSG1 is a component of intercellular desmosome junctions; it has a central role in the pathogenesis of pemphigus foliaceus, bullous impetigo, staphylococcal scalded skin syndrome and an inherited skin disorder – striate palmoplantar keratoderma (OMIM #148700).1 Monoallelic DSG1 mutation results in striate palmoplantar keratoderma whereas biallelic loss of function mutation results in severe life-threatening severe dermatitis, multiple allergies and metabolic wasting syndrome.4 This mutation results in complete loss of protein function by the cytoplasmic accumulation of the abnormal protein which cannot be transported to the cell surface. Heritable barrier abnormality with subsequent immune cascade is a possible cause.This results in increased expression of various genes coding for cytokines associated with allergic diseases.5 The role of atopy and allergies in the pathogenesis of severe dermatitis, multiple allergies and metabolic wasting syndrome is not well-established. Hence, further investigations are required to know the exact etiology and pathomechanism in producing this phenotypic heterogeneity.
The clinical phenotype and genotype of all 10 families reported in the literature have been described in Table 1.1,2,4-7 Currently, 10 pathogenic variants in 14 cases in two genes DSG1 and DSP have been described in literature: homozygous nonsense-2, splice site-4 and frameshift mutations-3 in DSG1 gene and heterozygous missense-1 in DSP gene [Figure 6].1,2,4-7 Cardiac abnormality, food allergies, hypoalbuminemia, microcephaly and esophageal abnormalities were not seen in our case. The differential diagnosis of severe dermatitis, multiple allergies and metabolic wasting syndrome with salient features has been tabulated in Table 2. Several therapeutic approaches like topical corticosteroids, topical tacrolimus and oral retinoids (acitretin 0.5mg/kg) have been tried with partial improvement of ichthyosis and palmoplantar keratoderma. Our case was treated with bland emollients and wet wrap therapy. Parents were not willing to start oral retinoids. Long-term prognosis depends on the systemic involvement.
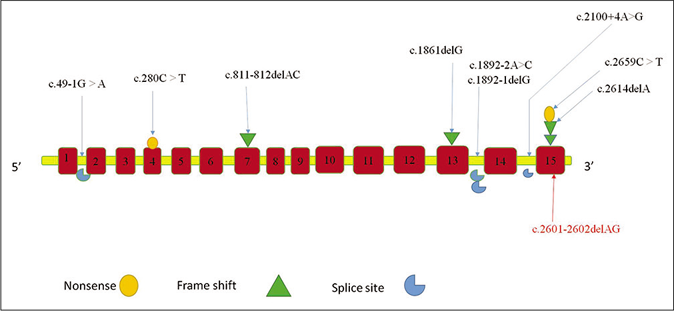
- Mutation spectrum of recessive mutations in the desmoglein1gene causing severe dermatitis, multiple allergies and metabolic wasting syndrome as described in literature till date.The novel mutation in our study is represented in red
| Study | Samuelov L, et al. | Has C, et al. | McAleer MA, et al. | Cheng R, et al. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | ||||||||||||||||||||
| 2013 | 2015 | 2015 | 2016 | |||||||||||||||||
| Case1 (family 1) | Case2 (family 1) | Case3 (family 2) | Case4 (family 3) | Case5 (family 4) | Case 6 (family 5) | Case 7 (family 5) | ||||||||||||||
| Age/sex | 7 years/female | 3 years/female | 9 months/female | 17 years/female | 6 years/male | 13 years/female | 7 years/male | |||||||||||||
| Consanguinity | + | + | + | − | − | + | + | |||||||||||||
| Congenital erythroderma | + | + | + | + | + | + | + | |||||||||||||
| PPK | + | + | + | + | + | + | + | |||||||||||||
| Hair/nails | Hypotrichosis | Hypotrichosis | Hypotrichosis | Curly hair | Hypotrichosis | Curly hair | N | |||||||||||||
| Multiple allergies | + | + | + | + | + | − | − | |||||||||||||
| Metabolic wasting | + | + | + | − | − | − | + | |||||||||||||
| Other Systemic involvement | Hypernatremia, recurrent infections, eosinophilic esophagitis, GERD, malabsorption, microcephaly, VSD, PS | Hypernatremia, recurrent infections, eosinophilic esophagitis, GERD, malabsorption, microcephaly, VSD, PS | Recurrent infections, growth retardation | − | Macrocephaly, developmental delay, nystagmus, keratitis | − | Recurrent infections, growth retardation | |||||||||||||
| Family history | PPK in parents | PPK in parents | PPK in parents/2 siblings death | Focal PPK in parents | N | PPK in parents/sibling death | PPK in parents/sibling death | |||||||||||||
| Ig E levels | Raised | Raised | Raised | Raised | Raised | Raised | N | |||||||||||||
| Gene/mutation | HOM/DSG1/c. 49-1G>A | HOM/DSG1/c. 49-1G >A | HOM/DSG1/C.1861delG | HOM/DSG1/c. 2659C>T | HET/DSP/c. 6208G>A | HOM/DSG1/c. 1892-1delG | HOM/DSG1/c. 1892-1delG | |||||||||||||
| Study | Schlipf NA et al. | Danescu S, et al. | JYW Lee, et al. | Shahar Taiber et al. | Present case | |||||||||||||||
| Year | ||||||||||||||||||||
| 2016 | 2016 | 2017 | 2018 | 2019 | ||||||||||||||||
| Case 8 (family 6) | Case 9 (family 6) | Case 10 (family 7) | Case 11 (family 8) | Case 12 (family 9) | Case 13 (family 10) | Case 14 (family 10) | Case 15 (family 11) | |||||||||||||
| Age/sex | 27 years/male | 11years/male | 2 years/female | 19 years/female | 2.5 years/male | 2 months/female | 17 years/female | 4 years/male | ||||||||||||
| Consanguinity | − | − | − | + | + | + | + | + | ||||||||||||
| Congenital erythroderma | + | + | + | + | + | + | + | + | ||||||||||||
| PPK | + | + | + | + | + | + | + | + | ||||||||||||
| Hair/nails | N | N | Curly | N | Hypotrichosis | N | N | Hypotrichosis | ||||||||||||
| Multiple allergies | + | + | + | − | − | + | + | − | ||||||||||||
| Metabolic wasting | − | − | − | − | − | − | − | + | ||||||||||||
| Other Systemic involvement | − | − | − | − | − | − | − | Recurrent respiratory infections, failure to thrive, metabolic wasting, short stature, developmental delay, growth hormone deficiency | ||||||||||||
| Study | Schlipf NA et al. | Danescu S, et al. | JYW Lee, et al. | Shahar Taiber et al. | Present case | |||||||||||||||
| Year | ||||||||||||||||||||
| 2016 | 2016 | 2017 | 2018 | 2019 | ||||||||||||||||
| Case 8 (family 6) | Case 9 (family 6) | Case 10 (family 7) | Case 11 (family 8) | Case 12 (family 9) | Case 13 (family 10) | Case 14 (family 10) | Case 15 (family 11) | |||||||||||||
| Family history | Mother - PPK | Mother- PPK | Father- PPK | N | Plantar keratoderma in parents | PPK in parents | PPK in parents | PPK in parents | ||||||||||||
| Ig E levels | Raised | Raised | Raised | Raised | N | Raised | Raised | Raised | ||||||||||||
| Gene/mutation | HOM/DSG1/c. 2614delA | HOM/DSG1/c. 2614delA | Compound HET/DSG1/c. 811_812delAC | HOM/DSG1/c. 1892-2A>C | HOM/DSG1/c. 280C>T | HOM/DSG1/c. 2659C>T | HOM/DSG1/c. 2659C>T | HOM/DSG1/c. 2601-2602delAG | ||||||||||||
+: Present, -: Absent, N: Normal, GERD: Gastroesophageal reflux disease, VSD: Ventricular septal defect, PS: Pulmonary stenosis, GH: Growth hormone deficiency, HOM: Homozygous, HET: Heterozygous, DSG: Desmoglein, DSP: Desmoplakin, PPK: Palmoplantar keratoderma
| Disorder | Gene | Mode of inheritance | Clinical features |
|---|---|---|---|
| Atopic dermatitis | FLG | AD | Pruritus, eczematous reactions along with characteristic distribution, erythroderma in childhood, features of diagnostic criteria by Hanifin and Rajka. No systemic involvement |
| Hyperimmunoglobulinemia E syndrome | STAT3/DOCK8 | AD/AR | Atopic dermatitis/erythroderma, cutaneous staphylococcal infections, severe viral infections, sinopulmonary abscess, recurrent bronchitis, pneumonia, emphysema, bronchiectasis, abscess, coarse facies, dental and skeletal abnormalities |
| Netherton syndrome | SPINK 5 | AR | Generalized scaling, erythroderma, ichthyosis linearis circumflexa, failure to thrive, diarrhea, atopic features, trichorrhexis invaginata |
| Nonbullous ichthyosiform erythroderma | TGM1/ABCA 12, ALOXE3, ALOX12B, CYP4F22 | AR | At birth as collodion membrane, generalized erythema, scaling, ectropion, alopecia, rarely intrauterine growth retardation and failure to thrive |
| Trichothiodystrophy | ERCC2, ERCC3, GTF2H5, C7Orf11 | AR | Congenital ichthyosiform erythroderma, brittle hair, impaired intelligence, short stature, decreased fertility, photosensitivity, “tiger tail” hair shaft defect on polarized microscopy |
| Ichthyosis hypotrichosis syndrome | ST14 | AR | Generalized ichthyosis with sparing of face, palms and soles, alopecia of scalp, eyebrows and eyelashes, follicular atrophoderma, photophobia, hypohidrosis, dental abnormalities |
| EGFR deficiency | EGFR | AR | Generalized erythema with scaling, pustules, alopecia, failure to thrive, diarrhea, high IgE, hypernatremia, recurrent bronchiolitis |
| ADAM17 deficiency | ADAM17 | AR | Erythroderma, failure to thrive, short stature, malabsorption, recurrent infections, cardiomyopathy |
| NISCH syndrome | CLDN1 | AR | Generalized scaling, sparing of skin folds, palms and soles, curly hair, frontotemporal cicatricial alopecia, sclerosing cholangitis, hepatomegaly, oligodontia |
AD: Autosomal dominant, AR: Autosomal recessive, FLG: Filaggrin, STAT3 gene: Signal transducer and activator of transcription 3, DOCK8: Dedicator of cytokinesis, SPINK5: Serine Peptidase Inhibitor Kazal Type 5, TGM1: Transglutaminase 1, ALOXE3: Archidonate Lipoxygenase 3, CYP4F22: Cytochrome P450 Family 4 Subfamily F Member 22, GTF2H5: General Transcription Factor IIH Subunit 5, ST14: Suppressor of Tumorigenicity 14 protein, EGFR: Epidermal growth factor receptor gene, NISCH: Neonatal ichthyosis-sclerosing cholangitis , CLDN1: Claudin 1
In conclusion, our described case of severe dermatitis, multiple allergies and metabolic wasting syndrome of Indian origin presented with characteristic clinical features with a novel homozygous frameshift mutation in the DSG1gene. Growth hormone deficiency with microphallus was unique in our case. This study expands the spectrum of the DSG1 mutation database and emphasizes the important role played by DSG1 in maintaining epidermal integrity, signal transduction pathways and keratinocyte differentiation. DSG1 mutation screening needs to be done in all suspected cases of severe dermatitis, multiple allergies and metabolic wasting syndrome.
Limitations of the study: Functional assays have not been done due to logistic reasons. Further studies need to be done to understand the exact pathogenesis of severe dermatitis, multiple allergies and metabolic wasting syndrome. Change in a phenotypic presentation in our case may be due to the different position of the mutation loci and epigenetic modifiers with multiple environmental factors.
Acknowledgment
We the authors would like to thank Medgenome Labs LTD for performing genetic analysis for our patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet. 2013;45:1244-8.
- [CrossRef] [PubMed] [Google Scholar]
- Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N terminal plakin domain of desmoplakin. J Allergy Clin Immunol. 2015;136:1268-76.
- [CrossRef] [PubMed] [Google Scholar]
- Heterozygous truncating variants in POMP escape nonsense mediated decay and cause a unique immune dysregulatory syndrome. Am J Hum Genet. 2018;102:1126-42.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of desmoglein 1 associated with palmoplantar keratoderma, dermatitis and multiple allergies. Br J Dermatol. 2015;172:257-61.
- [CrossRef] [PubMed] [Google Scholar]
- Homozygous desmoglein1 gene mutations and severe dermatitis, multiple allergies and metabolic wasting (SAM syndrome) Ann Dermatol Venereol. 2015;142:798-9.
- [Google Scholar]
- SAM syndrome is characterized by extensive phenotypic heterogeneity. Exp Dermatol. 2018;27:787-90.
- [CrossRef] [PubMed] [Google Scholar]
- Homozygous acceptor splice site mutation in DSG1 disrupts plakoglobin localization and results in keratoderma and skin fragility. J Dermatol Sci. 2018;89:198-201.
- [CrossRef] [PubMed] [Google Scholar]





