A pilot study to determine Neisseria gonorrhoeae–Chlamydia trachomatis coinfection rates in symptomatic patients attending STI Clinics, New Delhi, India
Corresponding author: Dr. Seema Sood, Professor, Department of Microbiology, 2nd Floor Teaching Block, All India Institute of Medical Sciences, New Delhi - 110 029, India. eemalsood@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Aravinda A, Sood S, Chaudhry R, Kapil A, Sharma PK, Gupta S. A pilot study to determine Neisseria gonorrhoeae–Chlamydia trachomatis coinfection rates in symptomatic patients attending STI Clinics, New Delhi, India. Indian J Dermatol Venereol Leprol 2022;88:367-71.
Abstract
Background:
Neisseria gonorrhoeae and Chlamydia trachomatis are the two most prevalent bacterial sexually transmitted infections. For over two decades, treatment guidelines have recommended empirical co-treatment for N.gonorrhoeae and C.trachomatis as symptoms overlap and co-infection is common. Studies from India estimating the same are limited and mostly based on conventional techniques.
Aim and Objective:
The aim of this study was to determine the frequency of N.gonorrhoeae and C.trachomatis coinfection using nucleic acid amplification tests. Further, we assessed the utility of pus cell estimation in Gram stained smears as a screening tool for inclusion of samples for molecular diagnosis.
Methods:
This was a prospective study conducted at two tertiary care hospitals; 100 patients (55 females and 45 males) with genitourinary discharge attending STI clinics were recruited, and endocervical or urethral swabs were collected. PCRs for N.gonorrhoeae and C.trachomatis were put up. In addition, microscopy and culture for gonococcus was performed followed by antimicrobial susceptibility testing. Statistical analysis was performed using the SPSS 16 software.
Results:
N.gonorrhoeae infection was more common than C.trachomatis. A total of 14 patients were positive by PCR (9 males and 5 females) for gonococcus. However, culture was positive only in 8 male patients. PCR for C.trachomatis was positive in 9 (4 males and 5 females) and the co-infection rate was 5%. The sensitivity and negative predictive value of pus cell estimation was 100% for males and 64% and 94.6% respectively for females. All isolates were susceptible to extended spectrum cephalosporins and azithromycin.
Limitation:
The sample size of the study was small.
Conclusion:
Frequency of N.gonorrhoeae/C.trachomatis coinfection in symptomatic STI patients is low. Coinfection is considerably overestimated and necessary confirmation of etiological diagnosis could reduce widespread empirical administration of broad-spectrum antibiotics.
Keywords
Chlamydia trachomatis
coinfection
Neisseria gonorrhoeae
polymerase chain reaction
Introduction
Chlamydia trachomatis and Neisseria gonorrhoeae are the most common causes of bacterial sexually transmitted infections reported worldwide. In developing countries like ours, these infections still remain underreported. As symptoms of N.gonorrhoeae/C.trachomatis overlap making specific clinical diagnosis difficult, cotreatment is recommended under the syndromic approach in resource-limited settings like ours. However, one consequence of this has been the emergence of antibiotic resistance in genital tract pathogens and flora.1
There are indications that the frequency of N.gonorrhoeae/C. trachomatis coinfection may be considerably over-estimated, at least in some circumstances.2 The cotreatment strategy was proposed at a time when chlamydial culture was performed only in research settings.3 Over the past two decades, diagnostic testing for Chlamydia trachomatis has improved dramatically. Therefore, the present pilot study was undertaken to determine the frequency of N.gonorrhoeae/C. trachomatis coinfection in symptomatic men and women attending STI clinics in New Delhi, using nucleic acid amplification tests (NAATs).
Methods
A total of 100 consecutive genitourinary discharge patients attending the outpatient departments of the All India Institute of Medical Sciences (AIIMS) and PGIMER Dr. Ram Manohar Lohia Hospital, New Delhi, from January to November 2015 were included. Males presenting with urethral discharge and females with cervical/vaginal discharge/lower abdominal pain of age 15–60 years were enrolled. Patients currently on antibiotics and menstruating women were excluded. Written informed consent was obtained from the patients or from the guardians if age less than 18 years. As there was no separate ethical approval committee, permission for sample collection was obtained from the Medical Superintendent of the Institute and the the same was upoladed.
Endocervical (female) and urethral (male) swabs were collected in triplicate. Sampling was done following standard protocol after a period of urine holding. In males milking of urethra was done whenever required. Immediate consecutive sampling was done by inserting swabs 1 cm deep and uniform each time. Swab-1 was used for Gram-staining. Immediate plating on to both selective and nonselective media (modified Thayer Martin medium and chocolate agar) were done using Swab-2. Swab-3 was used for DNA extraction (Qiagen Sciences Inc., USA) for polymerase chain reaction (PCR) assay and same order was followed in all cases. As PCR is a highly sensitive test method it is assumed not to have affected the results as it being tested on the final swab. Sampling and the slide assesessment was done by the same investigator (s) in all patients
Based on the number of polymorphs, the smears were categorized as negative, 1+ (<1/oil immersion field, 2+ (1–5/oil immersion field), 3+ (6–30/oil immersion field), or 4+ (>30/oil immersion field).4 Smears showing intracellular Gram-negative diplococci were considered positive for Neisseria gonorrhoeae, while those with extracellular Gram-negative diplococci especially from endocervical samples were considered to be of doubtful significance.
Presumptive identification of N.gonorrhoeae was performed based on colony morphology, Gram reaction, oxidase, and superoxol tests and confirmed by the rapid carbohydrate utilization test. Susceptibility testing was performed using the Calibrated Dichotomous Sensitivity protocol (disc diffusion and E-test) and the strains were categorized as susceptible (S), less susceptible (LS), resistant (R), or of decreased susceptibility (DS).5 β-Lactamase production was detected using nitrocefin discs (BD, USA).
PCR targeting the opa-gene was performed for N.gonorrhoeae. In-house primers and PCR conditions previously standardized in our laboratory were used in our study.6 The forward primer 5’ CGG TGC TTC ATC ACC TTA G 3’ and reverse primer 5’ GGA TTC ATT TTC GGC TCC TT 3’ were used. A 188-bp amplified product was obtained in positive samples. World Health Organization N.gonorrhoeae reference strains C and F were used as controls PCR targeting the cryptic plasmid of C.trachomatis was performed, for which primers designed by Claas e t al. (1991) and reaction conditions standardized previously in our laboratory were used in the study.7,8 The forward primer 5’GGA CAA ATC GTA TCT CGG’3 and reverse primer 5’ GAA ACC AAC TCT ACG CTG 3’ were used. A 517-bp amplified product was obtained in positive samples. The control DNA for C. trachomatis was from a positive patient sample.
The chance of PCR inhibition in samples was checked by PCR for β-globin gene (internal control).
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated and statistical analysis was performed using SPSS-16 software.
Results
Of the 100 patients recruited, 55 were females and 45 males. They were essentially of a younger age group with 67% being between 18 and 34 years old. All patients had a complaint of urethral/vaginal discharge either alone (79%,n=79) or associated with dyspareunia/pruritus/both (21%,n=21). About 31% of the males(n=14) and 5% of the females(n=3) complained of dysuria and lower abdominal pain respectively. One among the male patients was a known HIV sero-positive case.
Neisseria gonorrhoeae infection
Polymorphonuclear cells (grade ≥1) on the Gram stain were seen in 48% (20 males and 28 females), 27% (11 males and 16 females) showed Gram-negative diplococci and 8% (all males) were culture-positive. The antimicrobial susceptibility testing results of gonococcal isolates are compiled in Table 1. A total of 14 (9 males and 5 females) were positive by opa-gene based PCR. The gel picture shows the result of PCR in three cases [Figure 1].
| Antibiotics | Disc diffusion (%) | MIC (%) | ||||
|---|---|---|---|---|---|---|
| Resistant | Less susceptible | Susceptible | Resistant | Less susceptible | Susceptible | |
| Penicillin | 5 (62.5) PPNG: 3 (37.5) | 3 (37.5) | 0 | 5 (62.5) | 3 (37.5) | 0 |
| Nalidixic acid | 8 (100) | 0 | 0 | ND | ND | ND |
| Ciprofloxacin | 8 (100) | 0 | 0 | 2 (25) 6 (75) HLR | 0 | 0 |
| Tetracycline | TRNG: 5 (62.5) | Not TRNG: 3 (37.5) | TRNG: 5 (62.5) CMRNGT: 2 (25) | 1 (12.5) | 0 | |
| Spectinomycin | 0 | 0 | 8 (100) | 0 | 0 | 8 (100) |
| Azithromycin | 0 | 0 | 8 (100) | 0 | 0 | 8 (100) |
| Ceftriaxone | 0 | DS: 0 | 8 (100) | 0 | 0 | 8 (100) |
| Cefpodoxime | 0 | DS: 0 | 8 (100) | ND | ND | ND |
PPNG: penicillinase-producing N. gonorrhoeae; HLR: high-level resistance; TRNG: plasmid mediated tetracycline-resistant N. gonorrhoeae;
CMRNGT: chromosomally mediated tetracycline resistant N. gonorrhoeae; DS: decreased susceptibility; ND: not done. N. gonorrhoeae: Neisseria gonorrhoeae
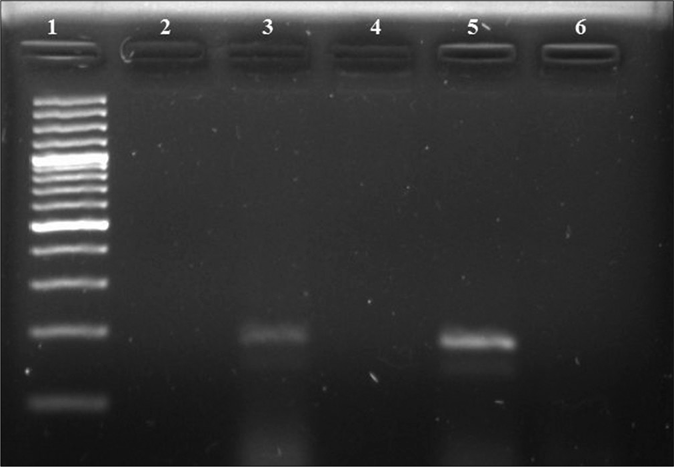
- Polymerase chain reaction results of opa gene. Lane 1: 100-bp DNA ladder; Lane 2: negative control; Lane 3: positive clinical sample (188 bps); Lanes 4, 6: negative clinical samples; Lane 5: positive control (N. gonorrhoeae WHO reference strain C) (188 bps)
Using PCR in addition to the eight culture-positive patients, six other patients (one male and five females) were identified. Validity statistics for PCR for N.gonorrhoeae in males with reference to the culture were as follows: sensitivity, specificity, positive predictive value and negative predictive value were 100% [59.8%–100% with 95% confidence interval CI)], 97.3% (84.2%–99.9% with 95% CI), 88.9% (50.7%–99.4% with 95% CI), and 100% (87.9%–100% with 95% CI) respectively. As no female was positive by gonococcal culture, we could not compare PCR with the same. An overall comparison of conventional tests with PCR for gonococcus is provided in Table 2.
| Test | Male (n=45), n(%) | Female (n=55), n(%) |
|---|---|---|
| Gram-negative diplococci in Gram stained smear | 11 (24.44) | 16 (29.09) |
| Culture positivity | 8 (17.77) | 0 |
| opa-based PCR | 9 (20) | 5 (9.09) |
PCR: polymerase chain reaction
As PCR provides enhanced diagnosis of gonorrhoea, we further assessed the utility of microscopy as a screening tool for sample inclusion for molecular diagnosis; the results are summarized in Table 3.
| Microscopy grade | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Pus cells ≥2+ (male) | 100 | 89.89 | 69.2 | 100 |
| Pus cells ≥2+ (female) | 60 | 70 | 16.7 | 94.6 |
NPV: negative predictive value; PPV: positive predictive value; 2+: 1-5/oil immersion field
Chlamydia trachomatis infection
Out of 100 patients, 9 (4 males and 5 females) were positive by a cryptic-plasmid-based PCR. The gel picture of the PCR result for three cases is shown in Figure 2.
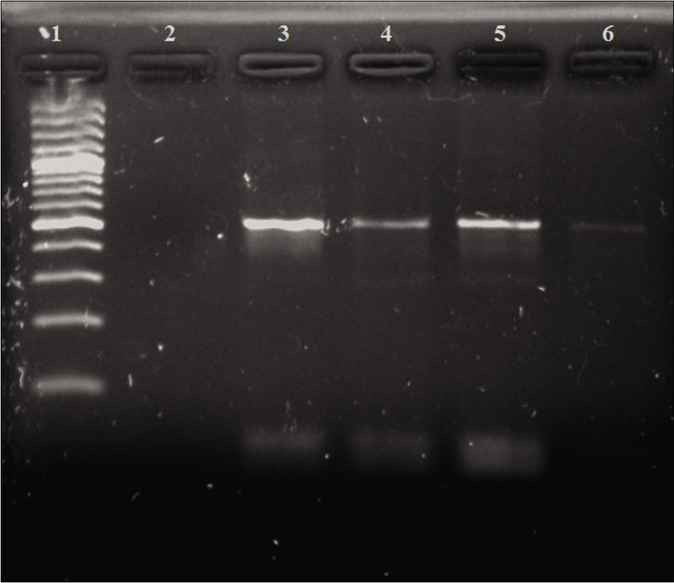
- Polymerase chain reaction results of cryptic plasmid gene. Lane 1: 100-bp DNA ladder; Lane 2: negative control; Lane 3: positive control (517 bps); Lanes 4–6: positive clinical samples (517 bps)
Neisseria gonorrhoeae/Chlamydia trachomatis coinfection
Based on the results of PCR targeting the opa gene for N. gonorrhoeae and cryptic plasmid for C. trachomatis, overall coinfection was seen in five (three females and two males) cases.
Discussion
Even with good quality control measures, the sensitivity of gonococcal culture (the gold standard) may range from 85% to 95%. Opa-based PCR has proven to be very useful due to its multicopy nature enhancing the sensitivity and reducing the risk of false-negatives.9,10 In this study, the clinical sensitivity of conventional culture methods for the detection of Neisseria gonorrhoeae in female patients was found to be low (five false negatives). This is not surprising as it has already been shown that culture results are interfered with especially by the mixed microflora of the female genital tract. Introduction of NAAT s has achieved significant progress in diagnosis of gonorrhoea in women because of their low detection limit, and high specificity (95%–100%) and sensitivity (95%).11 Because of their high accuracy, these are increasingly considered as the gold standard for diagnosis and detection in varied samples.12
In our study, it was found that including samples with grade ≥2+ of pus cells in the urethral swab for molecular diagnosis was highly useful as a screening tool in males, with a 100% sensitivity. Meanwhile, in females, the sensitivity was low (60%) and its utility is questionable. However, it increases the yield of PCR from 9.1% to 16.7% in females, thereby optimizing its use in resource-limited settings. It is to be highlighted that with the emergence of decreased susceptibility to ceftriaxone and resistance to azithromycin, we will be at a loss of therapeutic options for gonorrhoea. Therefore, in developing countries like ours, initial screening of samples with a simple bedside technique followed by a conventional PCR may be a judicious approach for STI laboratories having conventional PCR machines.
Bala et al. in 2013 reported an increasing trend in decreased susceptibility towards ceftriaxone in N.gonorrhoeae from South East Asian region countries from 2009 to 2011 but a decline thereafter in 2012.13 Resistance to spectinomycin (0.6%–10.5%) and azithromycin (<5%) was also reported at few centers from Bhutan and India. Although the number of isolates in our study was small, none of them exhibited decreased susceptibility to extended spectrum cephalosporins or resistance to azithromycin.
Tissue culture (gold standard) for chlamydial diagnosis is too expensive, of low sensitivity, and too laborious for routine diagnostic practice.14 PCR has been well-evaluated with an overall sensitivity and specificity of 90% and 99–100%, respectively.15 The advantages of a plasmid-targeted PCR are the intrinsic amplification caused by its multicopy nature (7–10), presence in >99% of the strains, and relative stability. The earlier study by Sood et al. had found that the in-house PCR used by us would detect all cases, including new-variant C. trachomatis with a specificity as high as 98.84%.7
Limited studies of coinfection with N. gonorrheae and C. trachomatis are available from our country, which are mostly based on conventional techniques only, including the retrospective 6-year study by Bala et al., reporting an overall coinfection rate of 1.1%.16 Among NAAT-based studies on coinfection, Bhalla et al. in 2007 detected 7.7%, Sachdev et al. reported 18% in symptomatic women, and Sonkar et al. a single case in 335 symptomatic females.17-19 Among the overseas studies, Tapsall and Kinchington from Sydney in 1995 found coinfection rates of 3.5% in males and 17.6% in females using conventional techniques.20 A multicentric cross-sectional study from Brazil by Barbosa et al. on men attending STI clinics reported a coinfection prevalence of 4.4% in first-catch urine samples using Cobas Amplicor CT/NG.1 The discrepancy in the frequencies reported can be explained by the difference in the sociodemographic profile of the study population, sampling, and the techniques used for identification.
A similar study by Arif et al. in 2017 undertaken in genitourinary discharge syndrome also demonstrated wide variation in syndrome-based and laboratory based etiological diagnosis and infective etiology could be established in only 20% of the patients. Other recognised pathogens include Mycoplasma genitalium, Ureaplasma urealyticum, Trichomonas vaginalis, Herpes simplex virus type 1 and 2 etc.21 Besides these, emerging pathogens have been identified in recent studies include adenoviruses and Sneathia spp. Most of these agents are amenable to anti-microbial therapy, but since they are not specifically looked for, their true prevalence in the causation of urethritis remains unknown.22
It would seem that though presumptive etiological diagnosis based on clinical manifestations eliminates treatment delays, but it is often inaccurate. In low-resource settings like ours, inexpensive PCR assays can be used in conjunction with syndromic diagnosis to increase diagnostic sensitivity and specificity, especially in females and may be considered in framing new guidelines for the management of these patients.
Limitations
Limitation of our study was the small sample size, it being a pilot study undertaken as a part of postgraduate thesis work.
Conclusion
In the present pilot study, we had performed both coventional culture techniques and sensitive PCR tests. It was found that out of 100 genitourinary discharge patients, 9 had only gonorrhoea, 4 had only a chlamydia infection, 5 had coinfection, and 82 patients were negative for both. But all these patients had been co-treated for N. gonorrhoeae/C.trachomatis as a part of syndromic case management. We would like to conclude that coinfection is considerably overestimated. Necessary confirmation of etiological diagnosis could reduce widespread empirical administration of broad-spectrum antibiotics, in turn containing the emergence and spread of antibiotic resistance.
Acknowledgement
The authors thank Mr. Rajendra Singh, Laboratory Technician, STD Laboratory, Department of Microbiology, AIIMS, for his invaluable assistance. They are thankful to Dr. Aruna Mittal, formerly Scientist F, IOP, SJH, New Delhi, for kindly gifting the control DNA for C. trachomatis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Prevalence of Neisseria gonorrhoeae and Chlamydia trachomatis infection in men attending STD clinics in Brazil. Rev Soc Bras Med Trop. 2010;43:500-3.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with genital chlamydial and gonococcal infection in males. Genitourin Med. 1993;69:393-396.
- [CrossRef] [PubMed] [Google Scholar]
- Genital Chlamydia trachomatis: an update. The Indian journal of medical research. ;138(3):303-316.
- [Google Scholar]
- Microbiology AS for Clinical Microbiology Procedures Handbook (2nd ed). Washington DC: American Society of Microbiology; 2007. p. :3.2.1.16.
- [Google Scholar]
- A Manual for Medical and Veterinary Laboratories 2011, Modified In: The CDS Disc Method of Antibiotic Sensitivity Testing (Calibrated Dichotomous Sensitivity Test) (6th ed). Randwick, Australia: The Prince of Wales Hospital, outh Eastern Area Laboratory Services, Australia; 2012.
- [Google Scholar]
- Evaluation of an opa gene based nucleic acid amplification test for detection of Neisseria gonorrhoeae in urogenital samples in North India. Epidemiol Infect. 2012;140:2110-6.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study for diagnosis of genital Chlamydia trachomatis infections by polymerase chain reaction among symptomatic Indian women. Indian J Dermatol Venereol Leprol. 2012;78:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic Value of the Polymerase Chain Reaction for Chlamydia Detection as Determined in a Follow Up Study. Journal of Clinical Microbiology 1991 Jan:42-45.
- [CrossRef] [PubMed] [Google Scholar]
- Nucleic acid amplification tests (NAATs) for gonorrhoea diagnosis in women: Experience of a tertiary care hospital in North India. Indian J Med Res. 2014;140:649-52.
- [Google Scholar]
- Evaluation of opa based real time PCR for detection of Neisseria gonorrhoeae. Sex Transm Dis. 2005;32:199-202.
- [CrossRef] [PubMed] [Google Scholar]
- Nucleic acid amplification testing for Neisseria gonorrhoeae: An ongoing challenge. J Mol Diagn. 2006;8:3-15.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a rapid antigen detection test for Neisseria gonorrhoeae in urine sediment for diagnosis of gonococcal urethritis in males. J Infect Chemother. 2004;10:208-11.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South East Asia region between 2009 and 2012: A retrospective analysis. Sex Transm Infect. 2013;89(Suppl 4):iv28-35.
- [CrossRef] [PubMed] [Google Scholar]
- Chlamydia trachomatis (trachoma, perinatal infections, lymphogranuloma venerum and other genital infections) In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases (7th ed). Philadelphia: Churchill Livingstone Elsevier; 2010.
- [CrossRef] [Google Scholar]
- Development and clinical evaluation of a polymerase chain reaction test for detection of Chlamydia trachomatis. J Clin Microbiol. 1992;30:2122-8.
- [CrossRef] [PubMed] [Google Scholar]
- Gonorrhoea and its co infection with other ulcerative, non ulcerative sexually transmitted and HIV infection in a regional STD centre. Indian J Med Res. 2011;133:346-9.
- [Google Scholar]
- Coinfection of Neisseria gonorrhoeae and Chlamydia trachomatis in symptomatic and asymptomatic women in India: Implications in reproductive health. Sex Transm Infect. 2011;87:A282.
- [CrossRef] [Google Scholar]
- Prevalence and co infection study of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis among symptomatic women using PCR assay. BMC Infect Dis. 2014;14(Suppl 3):P5.
- [CrossRef] [PubMed Central] [Google Scholar]
- Simultaneous detection of Neisseria gonorrhoeae and Chlamydia trachomatis by PCR in genitourinary specimens from men and women attending an STD clinic. J Commun Dis. 2007;39:1-6.
- [Google Scholar]
- The frequency of co infection with Neisseria gonorrhoeae and Chlamydia trachomatis in men and women in Eastern Sydney. Pathology. 1996;28:84-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of syndromic case management and polymerase chain reaction based diagnostic assays for treatment of Neisseriagonorrhoeae, Chlamydia trachomatis and genital mycoplasmas in patients of genitourinary discharge. Indian J Med Microbiol. 2017;35:286-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial Vaginosis-Associated Bacteria in Men: Association of Leptotrichia/Sneathia spp. With Nongonococcal Urethritis. Sex Transm Dis. 2013;40(12):944-949.
- [CrossRef] [PubMed] [Google Scholar]






