Translate this page into:
A randomized comparative study of the efficacy of topical latanoprost versus topical betamethasone diproprionate lotion in the treatment of localized alopecia areata
Corresponding author: Dr. Sanjeev Handa, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector-12, Chandigarh - 160 012, India. handa_sanjeev@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Bhat S, Handa S, De D. A randomized comparative study of the efficacy of topical latanoprost versus topical betamethasone diproprionate lotion in the treatment of localized alopecia areata. Indian J Dermatol Venereol Leprol 2021;87:42-8.
Abstract
Background:
Topical corticosteroids are the standard therapy for the treatment of alopecia areata. Recently, topical latanoprost has been found effective in the treatment of eyelash alopecia areata.
Objectives:
The objective of this study was to compare the efficacy of topical latanoprost ophthalmic solution (group 1) with that of topical betamethasone diproprionate lotion (group 2) in the treatment of localized alopecia areata.
Methods:
This was a single-centre, randomized, two-armed, parallel-group efficacy trial. Fifty consecutive patients with localized alopecia areata were randomized in a 1:1 ratio to receive either topical latanoprost 0.005% ophthalmic solution or topical betamethasone diproprionate 0.05% lotion. Of these 50 patients, 44 patients (21 in group 1 and 23 in group 2) completed the treatment protocol.
Results:
The percentage reduction in area involved with alopecia areata at 16 weeks (primary outcome) was lower in latanoprost vs. betamethasone group (median [interquartile range], 11.1 [0–99.1] vs. 100% [13.6–100], P = 0.02). Significantly lesser patients in the latanoprost group had a complete response to treatment as compared to the betamethasone group (6 [24%] vs. 14 [56%], P = 0.02). The median (interquartile range) hair regrowth score was significantly lower in the latanoprost vs. the betamethasone group (1 [0–4.5] vs. 5 [1–5], P = 0.02). Subjects in the betamethasone group showed a more rapid reduction in the involved area.
Limitations:
Short duration of treatment and follow-up were limitations of this study.
Conclusion:
Our results suggest that topical latanoprost 0.005% ophthalmic solution is less effective but safer than topical betamethasone dipropionate 0.05% lotion in the treatment of localized alopecia areata (clinicaltrials.gov: NCT02350023).
Keywords
Betamethasone
corticosteroid
latanoprost
localized alopecia areata
a prostaglandin analogue
Introduction
Alopecia areata is an autoimmune disease. It presents clinically as well-demarcated, round or oval, completely bald, smooth surfaced patch (es) of non-scarring alopecia.1 Although it usually affects the scalp, any hair-bearing area can be involved.
The treatment of alopecia areata is a challenge.2 There is a lack of evidence-based data for various therapeutic modalities for alopecia areata. Among the various treatment options, topical corticosteroids are considered as standard therapy for the treatment of localized alopecia areata.3 The serendipitous discovery of eyelash hypertrichosis induced by latanoprost in patients treated for glaucoma has led to many clinical and animal model research studies aimed at exploring the potential of this therapeutic modality in alopecia areata.4,5 Besides, the side effect profile of latanoprost is much better than topical corticosteroids. Therefore, the successful use of latanoprost in alopecia areata, if proved, could add a safer option to topical corticosteroids. The present study is aimed at assessing the efficacy of topical latanoprost 0.005% ophthalmic solution in localized scalp alopecia areata and to compare its efficacy as monotherapy with topical corticosteroid monotherapy, i.e., betamethasone dipropionate 0.05% lotion.
Methods
This was a single-centere randomized two-arm parallel-group efficacy trial conducted at the dermatology outpatient department of the Postgraduate Institute of Medical Education and Research, Chandigarh, India between January 2013 and June 2014. The study received ethical approval from the Institutional Ethics Committee (9121/PG-2Trg/12/8073) of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. All patients provided written informed consent. The trial is registered at clinicaltrials. gov (NCT02350023).
Patient selection
Consecutive patients with localized alopecia areata attending the dermatology clinic of this institute were enrolled in this study. Patients were eligible for inclusion in the study if they met the following criteria: (a) presence of five or fewer patches of alopecia areata, involving less than 40% scalp area and (b) stable disease without the appearance of a new patch or increase in the size of the existing patch for at least 15 days. Patients with any of the following were excluded: (a) presence of ophiasis; (b) patients who have received topical or oral treatment for alopecia areata in the past 1 month; (c) any other coexisting hair disorder diagnosed based on clinical history and examination (viz., trichotillomania androgenetic alopecia, telogen effluvium); (d) presence of any contraindication for topical corticosteroids (local skin infections, skin atrophy) or latanoprost (dermatitis); (e) pregnant and lactating women and (f) unwilling to provide informed consent.
Study protocol
All patients were subjected to thorough history taking and clinical examination at baseline. Potassium hydroxide mount of clipped hair, thyroid function tests and test for the presence of antinuclear antibody by indirect immunofluorescence was performed for each patient before starting treatment. Women of childbearing potential also underwent a urine pregnancy test. The diagnosis of alopecia areata was based on clinical evaluation. Patients were randomized in a 1:1 ratio to receive either topical latanoprost 0.005% ophthalmic solution (group 1) or topical betamethasone diproproionate0.05% lotion (group 2). The randomization sequence was computer-generated and the assignments were placed in sealed opaque envelopes. Patients were instructed to apply either latanoprost 0.005% ophthalmic solution twice daily (group 1) to alopecia areata patches using a cotton tip applicator or betamethasone propionate 0.05% lotion using fingers (group 2) twice daily.
Clinical assessment and measurement of the area involved with alopecia areata was performed at the baseline and thereafter at 4-weeks intervals throughout the study period. A transparent sheet was placed on the alopecic patch and a bidimensional picture of each patch was traced onto the sheet and then onto a graph paper. The longest and the shortest diameters of the alopecia patch were taken for the calculation of the area with hair loss which was expressed in cm2. Photographic documentation was also done at baseline and throughout the study period at 4-weeks intervals. All the measurements were performed by the same assessor. Treatment was continued in all the patients for 16 weeks or till complete regrowth of hair whichever occurred earlier. Those patients who had complete hair regrowth at 16 weeks were further followed up for 8 weeks to assess for any relapse. Therapeutic success was evaluated on the basis of a modification of the hair regrowth score (a five-point semi-quantitative score) at the end of 16 weeks: 0 = no change or further loss, 1 = 1–24% regrowth, 2 = 25–49% regrowth, 3 = 50–74% regrowth, 4 = 75–99% regrowth and 5 = 100% regrowth.6 All adverse effects occurring during the study period as a result of the treatment were also recorded.
Study outcomes
The primary outcome of the study was the percentage reduction in the area involved with alopecia areata at 16 weeks. The secondary outcomes included the number of patients having a complete response to the treatment (i.e. a regrowth score of 5), the median regrowth score and the frequency of adverse effects (including erythema, skin atrophy, telangiectasia, dermatitis and others).
Sample size calculation
We planned a superiority trial and assumed an average percentage reduction of 50% in the control (betamethasone) group based on an approximation from a previous study.7 To detect a difference of 10% in the primary outcome (percentage reduction in the area involved with alopecia areata at 16 weeks) between the 2 groups—at a significance level of 5% and a power of 80%, a sample size of 17 participants per group was required. To allow for a 30% dropout, 25 participants were included in each group.
Statistical analysis
Statistical analysis was performed using the commercial statistical package for social sciences for MS-Windows (Version 22, SPSS Inc, Chicago, IL). Data are presented descriptively as a number with a percentage or median with interquartile range. The Shapiro-Wilk test was used to determine the normality of distribution. Chi-square test (or Fisher’s exact test) was used to analyze the differences between categorical variables including the proportion of study subjects with a complete response. Mann-Whitney U test was used for comparing the continuous variables including the percentage reduction in area (primary outcome) and the regrowth score. Both intention-to-treat and per-protocol analyses were performed. For the intention-to-treat analysis, the worst-case scenario was considered (the subjects lost to follow-up were considered to have no response to the study intervention). To find the trend associated with parameters observed over the period, repeated measure analysis of variance test was applied. All statistical tests were two-sided and were performed at a significance level of α = 0.05.
Results
Seventy eight patients with localized alopecia areata were assessed for eligibility and 50 patients ((median [interquartile range] age, 24 [14.5–34.3] years, 16 [32%] women) were finally included in the study [Figure 1]. The median (interquartile range) duration of disease was 3 (1–5.3) months while the median surface area involved with alopecia areata was 7.5 (3–13) cm2 [Table 1]. Nail changes were seen in 21 (42%) patients, the most common being leukonychia (11 [22%]), Beau’s lines (7 [14%]) and pitting (5 [10%]).

- Flow diagram (CONSORT figure) depicting the study protocol and the participant inclusion process
| Baseline characteristics | Topical latanoprost (n=25) | Topical betamethasone (n=25) | Total (n=50) | P |
|---|---|---|---|---|
| Age (years) | 22 (11-37) | 25 (16.5-33.5) | 24 (14.5-34.3) | 0.89 |
| Gender (male:female) | 17:8 | 17:8 | 34:16 | 1.00 |
| Age of onset of disease (years) | 22 (11-37) | 25 (16.5-33.5) | 24 (14.5-34.3) | 0.87 |
| Total area involved by AA (cm2) | 5 (2.1-15.9) | 9.9 (4.2-14.2) | 7.5 (3-13) | 0.19 |
| Duration of disease (months) | 3 (2-4.5) | 3 (1-6) | 3 (1-5.3) | 0.79 |
| Family history of alopecia | 9 (36.0) | 5 (20.0) | 14 (28.0) | 0.21 |
| Family history of atopy | 5 (20.0) | 4 (16.0) | 9 (18.0) | 0.71 |
| Personal history of atopy | 9 (36.0) | 5 (20.0) | 14 (28.0) | 0.21 |
| Elevated TSH | 3 (12.0) | 1 (4.0) | 4 (8.0) | 0.30 |
| Positive ANA | 2 (8.0) | 0 | 2 (4.0) | 0.15 |
All values are expressed as median (interquartile range) or n (%). TSH: Thyroid-stimulating hormone, ANA: Antinuclear antibody, AA: Alopecia areata
In the intention-to-treat analysis, there was a significant difference in the median (interquartile range) percentage reduction in the area involved with alopecia areata at the end of 16 weeks [Table 1] in the latanoprost as compared to the betamethasone group (11.1 [0–100] vs. 100 [13.6–100], P = 0.02) at the end of 16 weeks. A significantly lower proportion of patients in the latanoprost group had a complete response to treatment as compared to the betamethasone group [6 [24%] vs. 14 [56%], P = 0.02; Figures 2 and 3]. The median (interquartile range) regrowth score was significantly lower in the latanoprost group as compared to the betamethasone group at the end of 16 weeks (1 [0–4.5] vs. 5 [1–5], P = 0.02). Forty-four subjects (21 in group 1 and 23 in group 2) completed 16 weeks follow-up. Similar results were obtained on the per-protocol analysis [Table 2]. None of the subjects in either group had a relapse at the end of 24 weeks. Erythema was the only adverse effect observed in the latanoprost group while erythema, skin atrophy, telangiectasia, dermatitis and pustules were observed in the betamethasone group [Table 3]. There was no significant difference in the number of subjects reporting adverse effects in the latanoprost and betamethasone groups (4 [16%] vs. 4 [16%], P = 1.00). Topical betamethasone had a greater rate of decrease in the involved area signifying a more rapid response than latanoprost [Figure 4].
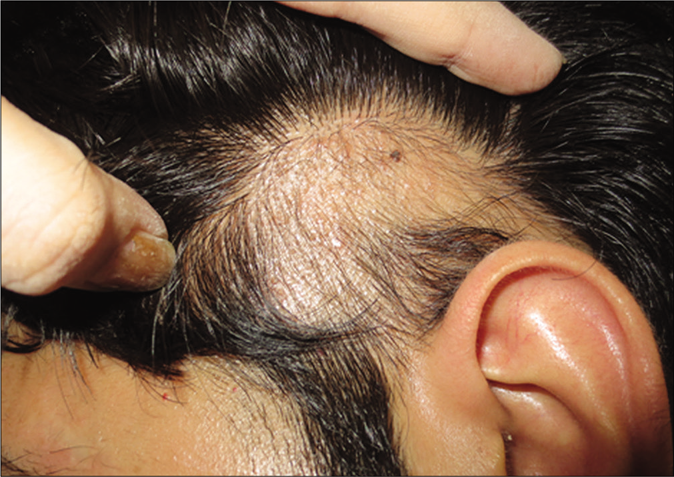
- Alopecia areata. A 27-year-old man with scalp alopecia areata treated with betamethasone lotion. Alopecia areata patch at baseline
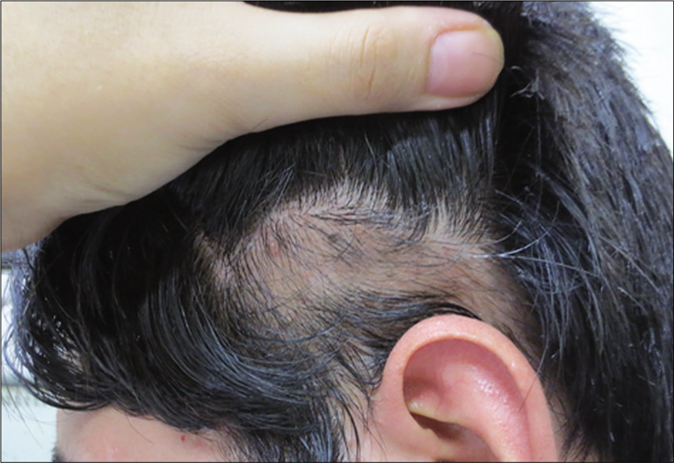
- Alopecia areata. A 27-year-old man with scalp alopecia areata treated with betamethasone lotion. After 4 weeks of therapy (initial response)
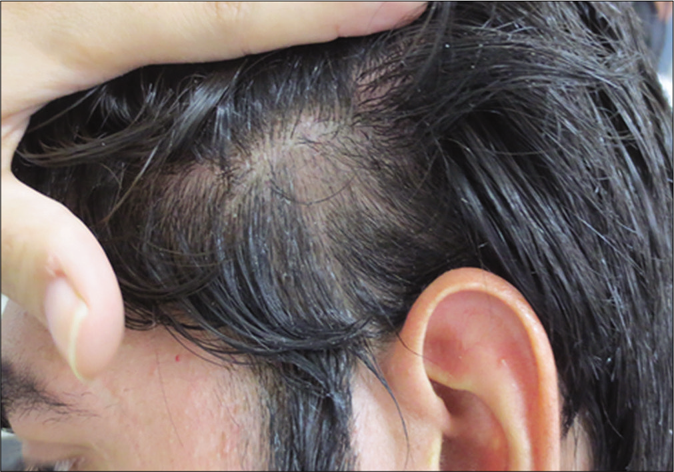
- Alopecia areata. A 27-year-old man with scalp alopecia areata treated with betamethasone lotion. After 8 weeks of treatment
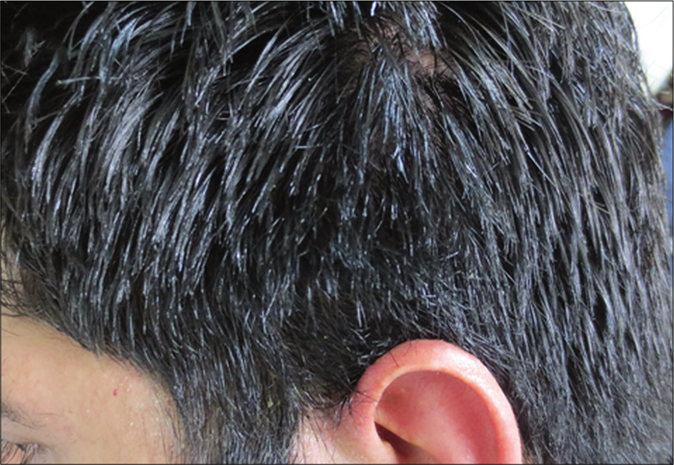
- Alopecia areata. A 27-year-old man with scalp alopecia areata treated with betamethasone lotion. Complete hair regrowth at 16 weeks
- Alopecia areata. A 35-year-old man with scalp alopecia areata treated with latanoprost solution. Alopecia areata patch at baseline

- Alopecia areata. A 35-year-old man with scalp alopecia areata treated with latanoprost solution. After 4 weeks of therapy (initial response)

- Alopecia areata. A 35-year-old man with scalp alopecia areata treated with latanoprost solution. After 8 weeks of treatment

- Alopecia areata. A 35-year-old man with scalp alopecia areata treated with latanoprost solution. Complete hair regrowth at 16 weeks
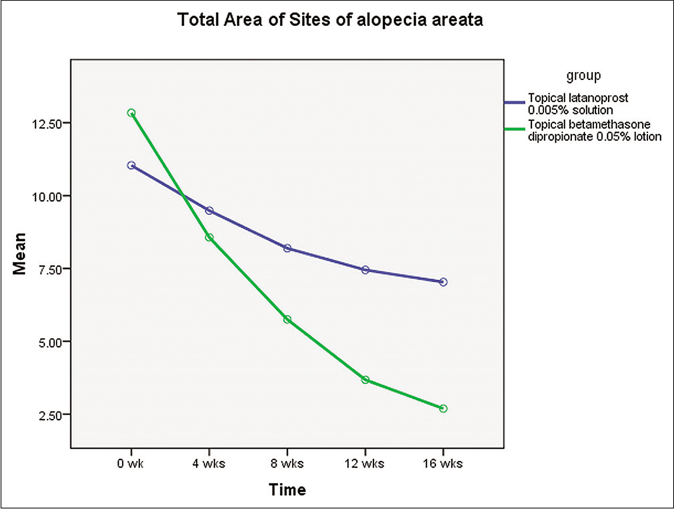
- Graph depicting the rate of decrease in the scalp area involved with alopecia areata during the treatment period
| Outcome | Topical latanoprost | Topical betamethasone | Estimated difference(95% CI) | P |
|---|---|---|---|---|
| Intention-to-treat analysis | n=25 | n=25 | ||
| Primary outcome | ||||
| Percentage reduction in an area with hair loss between baseline and 16 weeks | 11.1 (0-99.1) | 100 (13.6-100) | - | 0.02 |
| Other outcomes | ||||
| Complete response | 6 (24.0) | 14 (56.0) | 0.32 (0.05-0.53) | 0.02 |
| Hair RGS at 16 weeks | 1 (0-4.5) | 5 (1-5) | - | 0.02 |
| Per protocol analysis | n=21 | n=23 | ||
| Primary outcome | ||||
| Percentage reduction in an area with hair loss between baseline and 16 weeks | 71.7 (0-100) | 100 (50.8-100) | - | 0.03 |
| Other outcomes | ||||
| Complete response | 6 (28.6) | 14 (60.9) | 0.32 (0.03-0.55) | 0.03 |
| Hair RGS at 16 weeks | 3 (0-5) | 5 (3-5) | - | 0.03 |
All values are median (interquartile range) or n (%). RGS: Regrowth score, CI: Confidence interval
| Adverse effect | Topical latanoprost (n=25) | Topical betamethasone (n=25) | Total (n=50) | P |
|---|---|---|---|---|
| Any adverse effect* | 4 (16.0) | 4 (16.0) | 8 (16.0) | 1.00 |
| Erythema | 4 (16.0) | 1 (4.0) | 5 (10.0) | 0.13 |
| Skin atrophy | 0 | 3 (12.0) | 3 (6.0) | 0.09 |
| Telangiectasia | 0 | 2 (8.0) | 2 (4.0) | 0.17 |
| Dermatitis | 0 | 1 (4.0) | 1 (2.0) | 0.33 |
| Pustules | 0 | 2 (8.0) | 2 (4.0) | 0.17 |
Discussion
This study has shown that topical latanoprost (0.005% ophthalmic solution) is less effective than topical betamethasone dipropionate 0.05% lotion in the treatment of alopecia areata. This is the first study evaluating the efficacy of topical latanoprost on non-eyelash alopecia areata of scalp area and comparing topical latanoprost and topical steroids head-to-head in non-eyelash alopecia areata. All the earlier studies have evaluated the efficacy of topical latanoprost in eyelash alopecia areata.
Topical latanoprost was originally used for treating eyelash alopecia areata. Eyelash hair follicles express prostaglandin F2α receptors in the dermal papilla and outer root sheath. Latanoprost induces anagen phase in the telogen follicles by acting on these receptors. Besides, it prolongs the anagen phase of the hair cycle.
Alopecia areata is a common autoimmune hair disorder with a chronic relapsing course. The available treatment options are limited. There have been reports of eyelash hypertrichosis in patients of glaucoma treated with latanoprost. Subsequently, several studies have examined the use of latanoprost in the treatment of eyelash alopecia areata.4,8-17 However, none of the earlier trials have studied the effect of latanoprost in scalp alopecia areata. This is the first study evaluating the efficacy of topical latanoprost on non-eyelash (scalp) alopecia areata and comparing it head-to-head with a potent topical steroid, so as to assess it as monotherapy, as an alternative to topical corticosteroid.
This study has shown that topical latanoprost solution is less effective than topical betamethasone dipropionate lotion in the treatment of localized alopecia areata. The results of the current study are in accordance with other studies that have also reported lesser efficacy of latanoprost in promoting hair regrowth in patients with eyelash and eyebrow alopecia areata.18-20
In a recent study by Zaher et al., bimatoprost, a PGF2α analogue was shown to be more effective than topical mometasone furoate cream in the treatment of localized alopecia areata.21 A few factors could explain these results. First, the steroid used in the study by Zaher et al (mometasone furoate) is of lesser potency than the topical corticosteroid used in the present study (betamethasone propionate),21 hence explaining better results with betamethasone in our study. It has also been demonstrated that bimatoprost causes earlier and more extensive hypertrichosis compared to latanoprost.22 This higher efficacy could be attributed to the fact that bimatoprost does not need to be converted into an active metabolite for its pharmacological activity, unlike latanoprost. Moreover, Zaher et al. have commented on the percentage of hair regrowth only and not on complete hair regrowth. In contrast, in our study, one of the outcomes was complete hair regrowth which is a clinically more relevant outcome in the treatment of alopecia areata for an individual patient.
Latanoprost was well-tolerated in most patients with application site erythema as the only adverse effect. This is attributed to vasodilatation due to its action on PGF2α receptors on dermal vessels. Several other studies have confirmed no side effect or only erythematous reaction as an adverse effect at the latanoprost treated sites.8,21,23 This is in contrast to the steroid treatment which causes many adverse effects such as pustules, telangiectasia, acneiform facial eruptions and mild local atrophy.24
Strengths and Limitations
The main strength of our study is that it is a randomized study. It is the first study on the use of latanoprost in alopecia areata. Our study also has limitations, the major one being the lack of multiple assessors and assessor blinding. The other limitations are a short treatment duration and short post-treatment follow-up. We used the actual percentage reduction in the area with hair loss as the primary outcome. We did not assess the Severity of the Alopecia Tool (SALT) score which is a more widely used and validated measure of assessing hair loss in alopecia areata. Some factors might have contributed to the poor response of patients to latanoprost in our study. Insufficient penetration to the follicular bulb is the foremost consideration as the formulation used in the study was one that is meant for ophthalmic use, mainly in glaucoma. A higher strength of latanoprost and change in the vehicle might overcome this limitation by allowing the penetration of the drug up to the level of the dermal papilla and outer root sheath of the hair follicle where prostaglandin receptors are present. Moreover, prolongation of treatment duration to 6 months or more could have helped in improving the results by allowing a minimum time for the treatment to take effect. Other prostaglandin analogues such as bimatoprost and travoprost also need to be studied for their effectiveness in promoting hair regrowth.
Conclusion
Topical latanoprost represents a less efficacious therapeutic option for scalp alopecia areata than topical betamethasone. Topical latanoprost was also associated with a lesser number of adverse effects in this study. However, larger studies for a longer duration of treatment and follow-up are required to establish the true potential of this therapeutic modality in this chronic relapsing disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Alopecia areata: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:231-46.
- [CrossRef] [PubMed] [Google Scholar]
- Interventions for alopecia areata. Cochrane Database Syst Rev. 2008;16:CD004413.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-7.
- [CrossRef] [Google Scholar]
- Latanoprost and hyperpigmentation of eyelashes. Arch Ophthalmol. 1997;115:1206-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: A randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-7.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized comparison of topical betamethasone valerate foam, intralesional triamcinolone acetonide and tacrolimus ointment in management of localized alopecia areata. Int J Trichology. 2011;3:20-4.
- [CrossRef] [PubMed] [Google Scholar]
- Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J Eur Acad Dermatol Venereol. 2010;24:481-5.
- [CrossRef] [PubMed] [Google Scholar]
- Side-effects and risk profile of latanoprost 0.005% (Xalatan) Ophthalmologe. 2002;99:724-9.
- [CrossRef] [PubMed] [Google Scholar]
- Latanoprost and timolol combination therapy vs monotherapy: One-year randomized trial. Arch Ophthalmol. 2002;120:915-22.
- [CrossRef] [PubMed] [Google Scholar]
- One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-93.
- [CrossRef] [PubMed] [Google Scholar]
- Bimatoprost and travoprost: A review of recent studies of two new glaucoma drugs. Surv Ophthalmol. 2002;47(Suppl 1):S105-15.
- [CrossRef] [Google Scholar]
- Topical bimatoprost: A review of its use in open-angle glaucoma and ocular hypertension. Drugs Aging. 2002;19:231-48.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrichosis induced by latanoprost. J Am Acad Dermatol. 2001;44:721-3.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrichosis of vellus hairs of the malar region after unilateral treatment with bimatoprost. Am J Ophthalmol. 2004;137:756-7.
- [CrossRef] [Google Scholar]
- Eyelash formation secondary to latanoprost treatment in a patient with alopecia. Arch Ophthalmol. 2000;118:718-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired trichomegaly of the eyelashes and hypertrichosis induced by bimatoprost. J Eur Acad Dermatol Venereol. 2004;18:644-5.
- [CrossRef] [PubMed] [Google Scholar]
- Lack of efficacy of topical latanoprost and bimatoprost ophthalmic solutions in promoting eyelash growth in patients with alopecia areata. J Am Acad Dermatol. 2009;60:705-6.
- [CrossRef] [PubMed] [Google Scholar]
- Lack of efficacy of topical latanoprost in the treatment of eyebrow alopecia areata. J Am Acad Dermatol. 2005;53:1095-6.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of latanoprost in the treatment of alopecia areata of eyelashes and eyebrows. Eur J Dermatol. 2009;19:586-7.
- [CrossRef] [PubMed] [Google Scholar]
- Bimatoprost versus mometasone furoate in the treatment of scalp alopecia areata: A pilot study. Dermatology. 2015;230:308-13.
- [CrossRef] [PubMed] [Google Scholar]
- Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther. 2001;18:110-21.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol. 2012;66:794-800.
- [CrossRef] [PubMed] [Google Scholar]
- Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-8.
- [CrossRef] [PubMed] [Google Scholar]






