Translate this page into:
A rare mixed variant: Atrophic pigmented dermatofibrosarcoma protuberans
Corresponding author: Dr. Lin Feng, Department of Dermatology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing, China. linfeng0310@126.com
-
Received: ,
Accepted: ,
How to cite this article: Zhong S, Feng L, Yu Y, Wang M. A rare mixed variant: Atrophic pigmented dermatofibrosarcoma protuberans. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_785_2024
Dear Editor,
Dermatofibrosarcoma protuberans (DFSP) is a low-grade malignant soft tissue tumour, clinically characterised by a localised, multinodular, reddish-blue, bulging plaque. We report a patient with rare mixed variant DFSP, presenting with a pigmented atrophic lesion. The unusual clinical presentation is a diagnostic challenge, which can be verified by histopathological and immunohistochemical analyses.
A 23-year-old female presented with a dark-brown plaque on her waist for 2 years. The lesion increased in size gradually without itchiness or pain. Systemic examination and family history were unremarkable. Physical examination revealed a round black-brown plaque about 1.5 cm in diameter on the waist, with a central dark colour and a sunken surface with infiltrating tactility [Figure 1]. Routine laboratory tests were normal. Histopathology showed mild epidermal hyperplasia with attenuation of dermal thickness. Abundant spindle cells infiltrated the mid to deep dermis and subcutaneous fat, forming a wavy pattern in the shallow layer and a storiform pattern in the deep layer. The spindle cells distributed the adipocytes into honeycomb pattern. Prominently pigmented dendritic cells were scattered among the tumour cells. The pigment showed positivity for Masson-Fontana and negativity for Prussian blue, confirmatory for melanin. [Figures 2a-2c]. Immunohistochemically, the tumour cells were diffusely positive for CD34 and vimentin. Immunofluorescence analyses revealed that the pigment cells were positive for Melan-A, S100, and S0X10 with a red signal, confirmatory for melanocytes. The Ki67 index was less than 5%. Translocation recombination of the COL1A1/ PDGFB gene was detected by fluorescence in situ hybridization (FISH) [Figures 3a-3d]. Based on the clinico-pathological correlation and molecular analysis, atrophic-pigmented DFSP was diagnosed. The patient underwent Mohs’ micrographic surgery after diagnosis. No recurrence or metastasis was noted during the 12 months of follow-up.
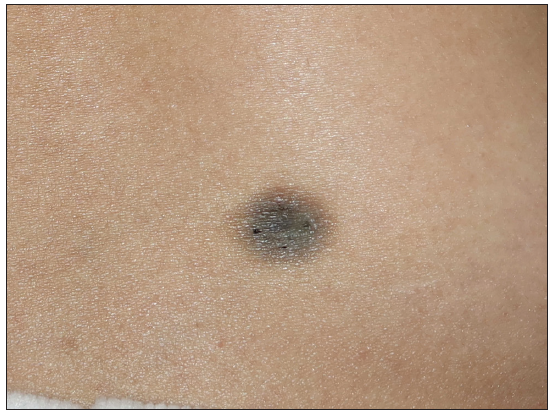
- A round black-brown plaque on the waist of a young woman, exhibiting a dark central colour and sunken surface with infiltrating tactility.
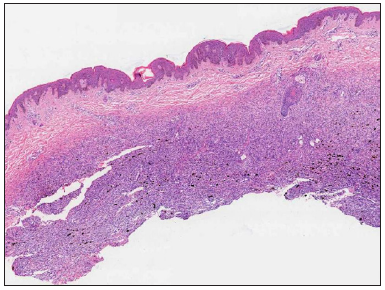
- Mild epidermal hyperplasia and attenuation of dermal thickness. Tumour mass in the mid to deep dermis and subcutaneous fat showing a diffuse infiltrative growth pattern with fatty replacement and scattered pigment (Haematoxylin and eosin, 20x).
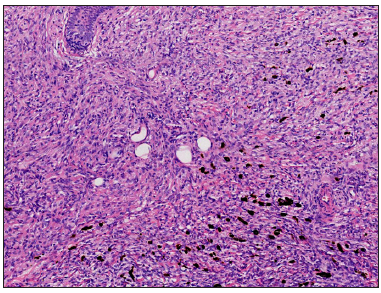
- Large quantities of spindle tumour cells are arranged in a storiform pattern, invading and dividing the adipocytes into a honeycomb pattern, partly surrounding small blood vessels and adnexa. Scattered pigment cells can be seen (Haematoxylin and eosin, 100x).
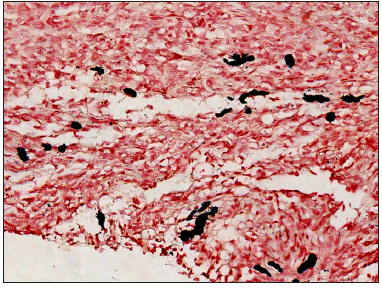
- Black granules seen are evidence that pigment is melanin (Masson-Fontana stain, 400x).

- Positivity for Melan-A with a red signal under fluorescence excitation, suggesting that the pigment cells were melanocytes (Melan-A stain labeled with Cy3, 400x).

- Diffuse positivity for CD34 in tumour cells (CD34 stain, 200x).

- Diffuse positivity for vimentin in tumour cells (vimentin stain, 200x).
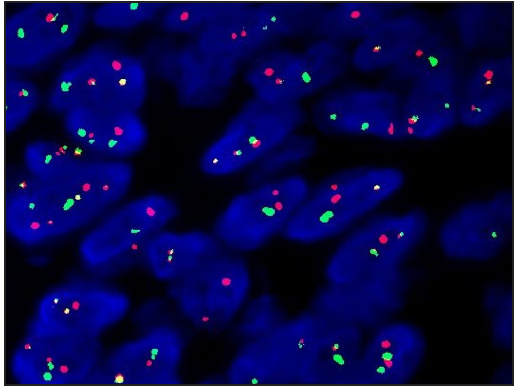
- Translocation recombination of COL1A1/ PDGFB gene was detected (FISH).
DFSP is a low-grade, malignant soft tissue tumour. The typical skin lesion is a localised, invasive, painless, multinodular, and reddish-blue bulging plaque, which can rupture. Pigmented DFSP, accounting for less than 5% of cases,1 has a red-brown or black-brown to blue-black coloured ecchymosis-like appearance clinically and is histologically characterised by dendritic cells containing melanin granules among the tumour cells. Atrophic DFSP, accounting for only 1.7% of the cases,2 is clinically characterised by an atrophic or sclerotic plaque while histology involves reduced dermal thickness by more than 50%. The superficial tumour cells are sparse, arranged in a wavy pattern parallel to the epidermis and lacking a distinctive storiform pattern. Mixed variant pigmentation with atrophy is extremely rare, which may resemble contusion, morphea, or melanocytic tumours clinically. Only nine cases have been reported worldwide since 2016.3,4
Fibroblasts are currently considered the tissue origin of DFSP.5 However, the emergence of pigmented DFSP has generated controversy about its origin. Some have suggested that the pigment cells are secondary to colonisation from melanocytes in the epidermis or hair follicles.5 Most believe that the presence of pigment cells represents neuroectodermal multidirectional differentiation positioning pigmented DFSP in the neuroectodermal tumour lineage.6 The immunohistochemical results in our case demonstrated neural crest origin of the pigment cells. The proposed origin of DFSP involves multipotential differentiation of primitive mesenchymal cells. Well-documented by histopathological and immunohistochemical approaches, this disease can be differentiated from other pigmented and spindle-cell tumours, such as pigmented neurofibroma, cellular or aneurysmal fibrous histiocytoma, medallion-like dermal dendrocyte hamartoma, cellular blue naevus, and melanoma [Table 1].
| Differential diagnosis | Histopathology | Immunohistochemistry or special stain |
|---|---|---|
| Pigmented neurofibroma |
loose distribution wavy nuclei salient mast cells |
S100﹢, CD34﹣ |
| Cellular or aneurysmal fibrous histiocytoma |
cytological polymorphism focal storiform pattern limited superficial infiltration of the subcutis |
CD68﹢, CD34﹣/ marginal focal expression Prussian blue﹢, Masson-Fontana-(aneurysmal variant) |
| Medallion-like dermal dendrocyte hamartoma |
vertical to the epidermis in the papillary layer parallel in the deeper layer |
CD34﹢, lack COL1A1-PDGFB fusion gene |
| Cellular blue nevus |
spindle cells and epithelioid cells dense pigment non-storiform arrangement |
S100﹢, HMB45﹢, CD34﹣ |
| Melanoma |
nested or scattered distribution atypia increased nuclear division |
S100﹢, HMB45﹢, PRAME﹢, CD34﹣ |
The local recurrence rate of DFSP ranges from 20% to 50%, while the metastasis rate is less than 0.3%.1 Although the recurrence rate of pigmented DFSP is reportedly lower than the classical variant, sporadic reports suggest classic DFSP recurrence as the pigmented variant with fibrosarcomatous changes, recurrent and metastatic pigmented DFSP with fibrosarcomatous changes, and death after multiple recurrences and multiple metastases.1 Clinical examinations are advised every 6 months for 5 years, thereafter in yearly intervals until the tenth year. Lymph node ultrasound, chest radiograph, abdominal ultrasound or CT scans, and ultrasound or magnetic resonance of the lesion are required for patients with suspicion of metastasis or recurrence and DFSP with fibrosarcomatous changes.7 Wide excision or Mohs’ micrographic surgery can reduce the recurrence rate. Postoperative radiotherapy and imatinib mesylate have been recommended. The distinctive clinical manifestations of this rare mixed variant pose a diagnostic challenge. Thus, increasing awareness and biopsy rate and ensuring biopsy depth are critical factors for its diagnosis.
Acknowledgement
The authors wish to thank Professor Lei Wang of Xijing Hospital, Fourth Military Medical University for his assistance on immunohistochemical staining.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Metastatic bednar tumor (pigmented dermatofibrosarcoma protuberans) with fibrosarcomatous change: A case report. J Orthop Sci. 2004;9:662-5.
- [CrossRef] [PubMed] [Google Scholar]
- Atrophic dermatofibrosarcoma protuberans: A clinicopathological study of 16 cases. Pathology. 2019;51:615-20.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of bednar tumors in the United states. J Am Acad Dermatol. 2016;75:1064-6.
- [CrossRef] [PubMed] [Google Scholar]
- Atrophic pigmented dermatofibrosarcoma protuberans: A case and short review. J Cosmet Dermatol. 2023;22:3192-4.
- [PubMed] [Google Scholar]
- Pigmented dermatofibrosarcoma protuberans (Bednar tumour): Melanocytic colonization or neuroectodermal differentiation? A clinicopathological and immunohistochemical study. Histopathology. 1988;13:631-43.
- [CrossRef] [PubMed] [Google Scholar]
- Bednár tumor associated with dermal melanocytosis: Melanocytic colonization or neuroectodermal multidirectional differentiation? J Cutan Pathol. 2003;30:147-51.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-8.
- [CrossRef] [PubMed] [Google Scholar]






