Translate this page into:
A retrospective case series of 12 patients with chronic reactive arthritis with emphasis on treatment outcome with biologics
Corresponding author: Dr. Neena Khanna, Department of Dermatology and Venereology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029, India. neena_aiims@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Gupta V, Mohta P, Sharma VK, Khanna N. A retrospective case series of 12 patients with chronic reactive arthritis with emphasis on treatment outcome with biologics. Indian J Dermatol Venereol Leprol 2021;87:227-34.
Abstract
Background:
Patients with reactive arthritis frequently present to dermatologists. However, there is paucity of information regarding its clinical aspects and management in dermatological literature.
Objective:
To review the clinical features and management of patients with chronic reactive arthritis admitted to the dermatology department of a teaching hospital.
Methods:
This was a retrospective analysis of patients with reactive arthritis admitted to the Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India from January 2016 to February 2018.
Results:
There were 12 males (disease duration 9–180 months). Biologics were used in 9 (75%) patients on 16 different occasions, the most frequent being infliximab (n = 10 times), followed by adalimumab (n = 3), etanercept, secukinumab and itolizumab (n = 1 each), in combination with other systemic agents. Response rate with treatment regimens including biologics (69% responders, 31% partial responders) was statistically significantly better than those without biologics (27% responders, 46% partial responders, 27% nonresponders; P = 0.036), using a composite measure assessing improvement in skin and joint symptoms. Biologics were discontinued on 50% of the occasions, after a median of 3.5 months (range 1.5–7.5 months) because of satisfactory response (n = 4), therapeutic fatigue (n = 3) or adverse event (n = 1). After biologic discontinuation, the response was sustained for a median of 5 months (range 3–6 months) before disease exacerbation. The number of treatment switches increased with the follow-up duration (median three switches per patient, range 1–8). The median follow-up duration was 10.5 months (range 4–76 months).
Conclusion:
Biologics produce rapid improvement in skin and joint symptoms in chronic reactive arthritis, but the response is not long-lasting. Patients with chronic reactive arthritis have a waxing and waning course despite regular treatment.
Limitations:
The limitations are retrospective design, small sample size and lack of a validated outcome measure.
Keywords
Biologics
dermatological manifestations
reactive arthritis
treatment outcome
Introduction
Reactive arthritis, also known as Reiter’s disease, is characterized by the triad of arthritis, urethritis and conjunctivitis, and typically follows an episode of enteric or genitourinary infection.1 Mucocutaneous involvement is frequent in patients with reactive arthritis, and patients often present to dermatologists. However, most of the data on clinical aspects and management of reactive arthritis is available in the rheumatology literature.2,3 We conducted this study to review the clinical profile and management of patients with reactive arthritis admitted to our department.
Methods
This was a retrospective analysis of patients admitted with the diagnosis of reactive arthritis for initial evaluation or disease exacerbation in the Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India, from January 2016 to February 2018. The diagnosis of reactive arthritis was based on the typical mucocutaneous lesions and peripheral arthritis. Cases where diagnosis of psoriasis with psoriatic arthritis could not be definitively ruled out were not included. Demographic, clinical and treatment details of patients with reactive arthritis were reviewed. Treatment response was categorized separately for skin and joint symptoms as patient global assessment on a 6-point Likert scale (worsening, <25% improvement, 25–49% improvement, 50–74% improvement, 75–90% improvement and > 90% improvement). Global response was classified using a composite measure as follows: responders, ≥75% improvement in skin and ≥50% improvement in joint disease; nonresponders, <25% response in either skin or joint symptoms; and others as partial responders. As per the standard of care in our department, patients were assessed every 4–8 weeks if on conventional systemic agents and before every dose if on biologic treatment. Treatment was changed after 3 months in nonresponders. Serological markers of inflammation (erythrocyte sedimentation rate, C-reactive protein) were also assessed when available.
Results
Baseline patient characteristics
Data of 12 patients with reactive arthritis was available for review and is summarized in Table 1. All patients were males, and presented with skin lesions: rupioid plaques, with 4 (33%) patients having additional peripheral pustulation [Figure 1]. Keratoderma blenorrhagicum [Figure 2] and circinate balanitis [Figure 3] were seen in 5 (42%) and 3 (25%) patients, respectively. Symmetric polyarthritis was the most common pattern of joint involvement, seen in 8 (67%) patients. Seven (58%) patients were bed-ridden due to joint pains or deformities. Enzyme-linked immunosorbent assay for HIV-1 and -2 was negative in all the patients. Urine and stool cultures were negative. Urine polymerase chain reaction was positive for Chlamydia trachomatis and Ureaplasma urealyticum in one patient, Ureaplasma urealyticum in one patient and Mycoplasma genitalium in another patient. Only one patient demonstrated the “complete” triad, while four patients had “incomplete” Reiter’s syndrome.
| Parameters | Values |
|---|---|
| Age (years), mean±SD (range) | 25.5±6.9 (14-40) |
| Duration (months), mean±SD (range) | |
| Skin symptoms | 40.6±31.6 (3-120) |
| Joint symptoms | 55.9±51.1 (9-180) |
| Affected body surface area | |
| Mean±SD (range) | 20.9±24.1 (5-90) |
| Median | 15 |
| Sites of cutaneous involvement, n (%) | 12/12 (100) |
| Lower limbs | 12 |
| Trunk | 9 |
| Upper limbs | 6 |
| Palms and soles | 5 |
| Face | 2 |
| Genital mucosa | 3 |
| Nails, n (%) | 10/12 (83) |
| Nail plate thickening | 10 |
| Subungual hyperkeratosis | 10 |
| Distal onycholysis | 1 |
| Pattern of arthritis, n (%) | 12/12 (100) |
| Symmetric polyarthritis | 8 |
| Asymmetric polyarthritis | 1 |
| Asymmetric oligoarthritis | 3 |
| Affected joints, n (%) | 12/12 (100) |
| Knees | 11 |
| Sacro-iliac joints | 8 |
| Elbows | 8 |
| Wrists | 8 |
| Ankles | 7 |
| Interphalangeal joints | 6 |
| Shoulders | 4 |
| Joint deformities, n (%) | 4/12 (33) |
| Knees | 2 |
| Hips | 1 |
| Elbows | 1 |
| Small joints of hands | 1 |
| Enthesitis, n (%) | 2 (17) |
| Plantar fasciitis | 2 |
| Achilles tendinitis | 1 |
| Abnormal ocular examination, n (%) | 2/12 (17) |
| Conjunctivitis | 1 |
| Healed uveitis | 1 |
| Comorbidities, n (%) | 6/12 (50) |
| Osteopenia | 3 |
| Osteoporosis | 2 |
| Secondary renal amyloidosis | 1 |
| Anemia, Hb <12 g/dL, n (%) | 12/12 (100) |
| Normocytic hypochromic | 7 |
| Microcytic hypochromic | 5 |
| Raised inflammatory markers, n (%) | |
| ESR | 7/7 |
| C-reactive protein | 4/4 |
| HLA-B27, n (%) | 4/7 (57) |
| Radiographic changes, n (%) | 10/12 (83) |
| Reduced joint space | 7 |
| Juxta-articular osteopenia | 4 |
| Periarticular erosions | 4 |
| History of preceding infection, n (%) | 1/12 (8) |
| Enteric infection | 1 |
| Genitourinary infection | 0 |
SD: Standard deviation, ESR: Erythrocyte sedimentation rate
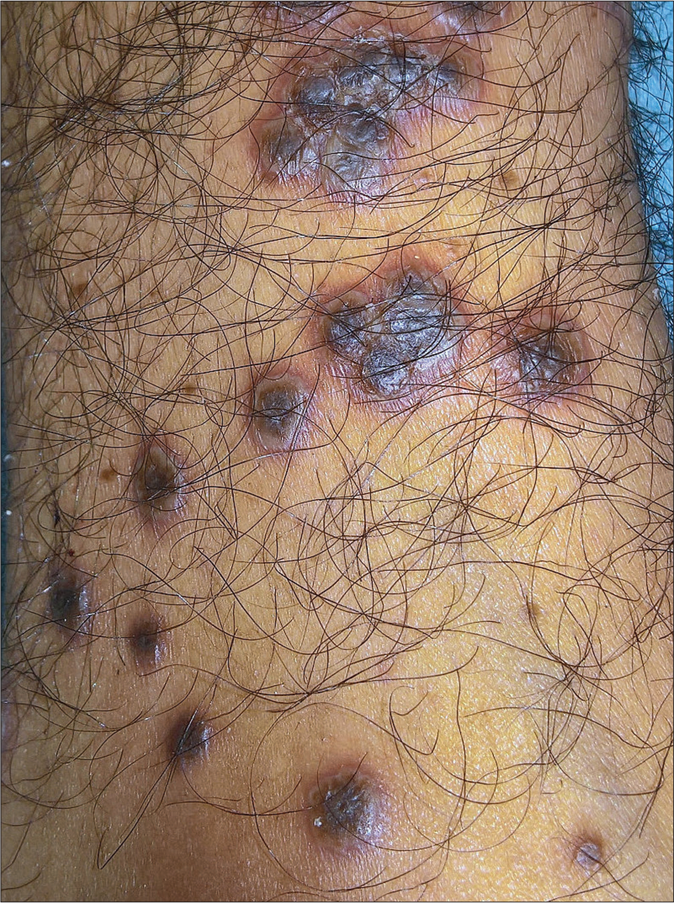
- Rupioid lesions: papules and plaques with central keratotic wet scales, surrounding pustulation and erythema on the leg
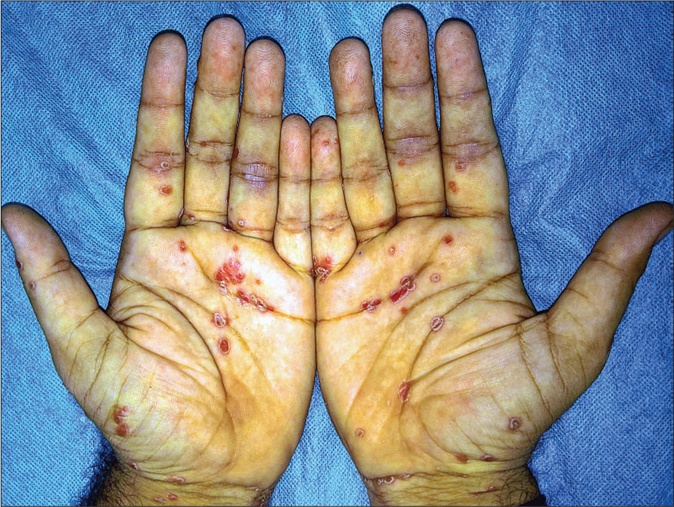
- Keratoderma blennorrhagicum: erythematous scaly keratotic papules and plaques present symmetrically on palms
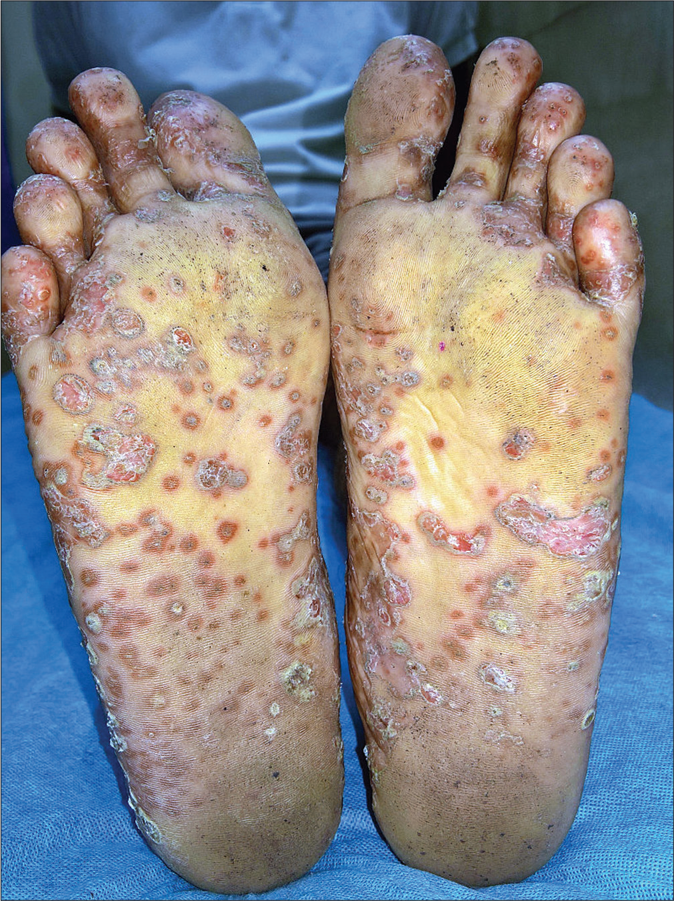
- Keratoderma blennorrhagicum: erythematous scaly keratotic papules and plaques present symmetrically on soles
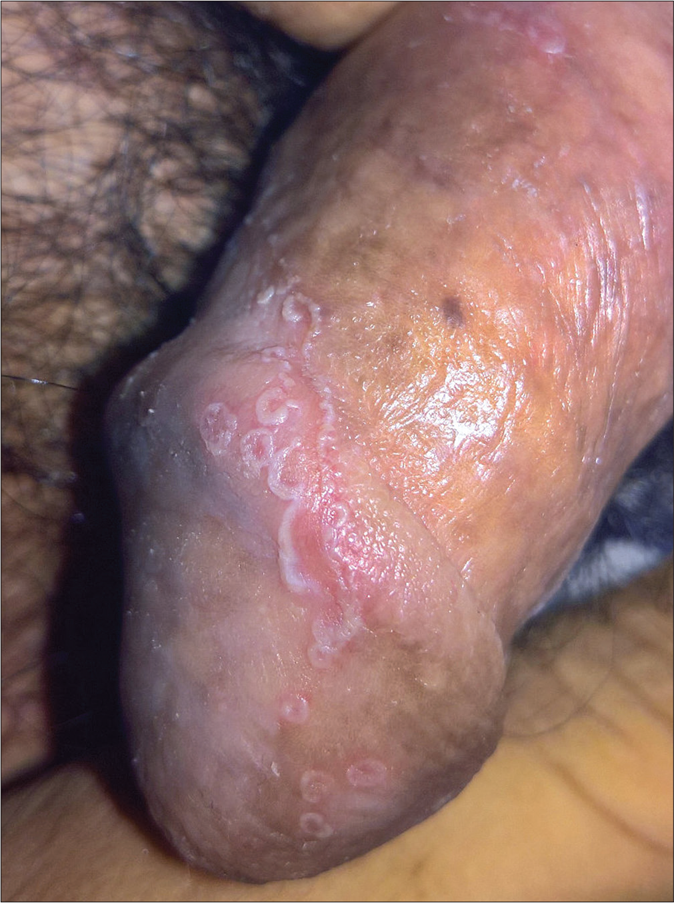
- Circinate balanitis: erythematous lesion with serpiginous borders studded with pustules on the glans penis
Treatment outcome
All patients were initially treated with weekly methotrexate in combination with other agents: systemic corticosteroids [n = 5; dexamethasone (300 mg) monthly pulses in two patients, oral betamethasone (100 mg) weekly pulses in one patient, weekly oral betamethasone (10 mg) minipulse in one patient and a short course of daily oral prednisolone in one patient], biologics (n = 5) and sulfasalazine (n = 4). In addition, doxycycline (100 mg twice daily) was given for 3 months to three patients. All patients also used potent topical corticosteroids/salicylic acid combination for skin lesions.
Treatment details are summarized in Table 2. Eight (67%) patients were classified as responders to the initial treatment. Of these, four experienced a partial loss of response in joint symptoms (patients 1, 2 and 8) or overall disease worsening (patient 3), necessitating a change in treatment. Two (patients 4 and 11) responded partially, while two (patients 7 and 10) were nonresponders who were switched to another treatment after 3 months. Overall, 6 (50%) patients needed a change in their initial treatment following primary (n = 2) or secondary (n = 4) nonresponse. The number of treatment switches per patient increased with the follow-up duration: once for three patients (median follow-up 8 months, range 5–9), thrice for one patient (20 months follow-up), four times for one patient (54 months follow-up) and eight times for one patient (76 months follow-up). The subsequent treatment options included up-dosing methotrexate (n = 2), adding/ switching biologics (n = 11), adding weekly dexamethasone pulses (n = 2), radiosynovectomy of joints (n = 2) and/or doxycycline (n = 1) to the ongoing treatment.
| Patient/ age/ sex |
Disease duration, months |
Past treatment |
Initial treatment |
Response |
Time to switch treatment (months), reason |
Subsequent treatment** |
Adverse effects |
Current status |
Follow-up duration, months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS/ months |
DMARD/ mg |
Biologic/ doses |
Others |
CS/ months |
DMARD/mg |
Biologic/ doses |
Others |
||||||||
| 1/25/ | 120 | NSAID, | mDP/10 | MTX/15 | - | NSAID, | Responder | 10, partial loss of | wDP/2, | MTX/20-25*, | INF/6 | RS, | MTX-induced | S: >50%, J: | 76 |
| male | IM/CS, | TRA | S: >75%, J: | response | IA | HCQS/400, | ETN/8 | morphine | transaminitis, | >50% | |||||
| PRED | >75% | SSZ/3000* | ADA/8 SEC/5 INF/5* |
lower respiratory tract infection | Planned for knee and hip joint replacement surgery | ||||||||||
| 2/26/ | 48 | NSAID, | wOBP/26 | MTX/15 | - | Doxy, | Responder | 20, partial loss of | - | MTX/20*, | INF/6 | RS | CS-induced | S: >25%, J: | 54 |
| male | MTX, IA/ CS | NSAID, TRA | S: >75%, J: >75% |
response | SSZ/4500, | ADA/2* | cataract | >25% | |||||||
| 3/14/ | 24 | NSAID, | - | MTX/15* | INF/4 | NSAID | Responder | 9, disease | wDP/3 | SSZ/3000* | ITO/5 | Doxy, | - | S: >75%, J: | 20 |
| male | MTX, SSZ | S: >90%, J: >90% |
worsening | INF/4* | morphine | >75% | |||||||||
| 4/30/ | 24 | NSAID, | OMP/9 | MTX/15,* | - | NSAID, | Partial | - | - | - | - | - | S: >50%, J: | 14 | |
| male | MTX, PRED | SSZ/2000* | TRA | responder S: >75%, J: >25% |
>25%; partial loss of response after 10 months | ||||||||||
| 5/24/ | 36 | NSAID, | - | MTX/15* | INF/6 | NSAID, | Responder | - | - | - | - | - | MTX-induced | S: >75%, J: | 12 |
| male | IM/CS, PRED | TRA | S: >75%, J: >50% |
transaminitis | >50% | ||||||||||
| 6/24/ male | 30 | NSAID, IM/CS | IA | MTX/15* | INF/8* | NSAID, TRA | Responder S: >75%, J: >75% |
- | - | - | - | - | - | S: >75%, J: >50%; partial loss of response at 9 months |
12 |
| 7/29/ | 60 | NSAID, | - | MTX/15,* | - | NSAID, | Nonresponder | 3, primary | - | INF/5* | - | S: >75%, J: | 9 | ||
| male | IM/CS | SSZ/3000* | TRA | S:>25%, J:<25% | nonresponse | >75% | |||||||||
| 8/40/ | 180 | NSAID, | mDP/6 | MTX/15* | - | Doxy, | Responder | 6, partial loss of | - | - | ADA/2* | - | CS-induced | S: >50%, J: | 8 |
| male | MTX, PRED, IM/ CS | NSAID, TRA | S: >75%, J: >75% |
response | cataract, T2DM | >50% | |||||||||
| 9/19/ male | 48 | NA | PRED/4 | MTX/15* | INF/6* | NSAID | Responder S: >75%, J: >75% |
- | - | - | - | - | T2DM | S: >75%, J: >75% |
7 |
| 10/20/ | 60 | NSAID, | - | MTX/15, | - | Doxy, | Nonresponder | 3, primary | - | MTX/25* | - | - | - | S: >25%, J: | 5 |
| male | MTX, PRED | SSZ/3000* | NSAID. TRA | S:>25%, J:<25% | nonresponse | <25% Planned for biological therapy |
|||||||||
| 11/30/ male | 9 | NSAID, IM/CS | - | MTX/15,* SSZ/3000* | - | NSAID | Partial responder S: >50%, J: >25% |
- | - | - | - | - | S: >50%, J: >25% |
5 | |
| 12/23/ male |
15 | NSAID, MTX |
- | MTX/15* |
INF/4* |
NSAID, TRA |
Responder S: >75%, J: >50% |
- | - | - | - | - | MTX-induced transaminitis |
S: >75%, J: >50% |
4 |
Overall, the skin lesions and arthritis improved to a similar extent in 6 (50%) patients, while skin lesions responded better in the remaining 6 patients. Four (patients 1, 2, 3 and 8) experienced a loss of response in both skin and joint disease; the response in joint disease was lost by a median of 2 months earlier than skin disease in three patients. One patient each lost improvement in only skin lesions (patient 4) and joint disease (patient 6). At the last follow-up visit, there were 6 (50%) responders, 5 (42%) partial responders and (8%) nonresponder (who is planned for biologic treatment). Erythrocyte sedimentation rate values normalized in one of five patients and decreased in the remaining four patients. C-reactive protein levels decreased in all four patients. The median follow-up duration was 10.5 months (range 4–76 months). Six (50%) patients had at least 12 months follow-up assessment. The median number of hospital admissions per patient was 1.5 (range 1–11), and median time of hospital stay per admission was 15 days (range 5–62 days).
Biologics in the treatment of chronic reactive arthritis
Overall, biologics were used in 9 (75%) patients on 16 different occasions (five times as initial treatment, 11 times as alternate treatment). The concomitant treatment with biologics included methotrexate (n = 16 times), sulfasalazine (n = 8), short course of oral prednisolone (n = 1) and weekly 1-day dexamethasone pulse (n = 1). Of the 11 times biologics were used after failure of initial treatment, seven were switches from another biologic (patients 1–3) while four were after nonresponse to conventional systemic therapy (patients 1, 2, 7 and 8). The most commonly used biologic was infliximab (n = 10 times), followed by adalimumab (n = 3), etanercept, secukinumab and itolizumab (n = 1 each). Dose and schedule of all the biologics were as per the regulatory agency-approved protocol. Biologics led to good response rapidly (within 2 weeks) on all the five occasions when used as initial therapy [Figure 4] but when used as alternate treatment, good response was seen on 55% (n = 6/11) and partial response on 45% (n = 5/11) of the times (P = 0.118). Overall, treatment regimens including biologics produced global response 69% (n = 11/16) of the times used and partial response 31% (n = 5/16) of the times, whereas those without biologics resulted in good response 27% (n = 3/11), partial response 46% (n = 5/11) and no response 27% (n = 3/11) times. This difference in the response rate of treatment regimens with and without biologics was statistically significant (P = 0.036). Treatment fatigue while continuing biologics occurred on 31% (n = 5/16) occasions after median months (range 2–9 months); only for joint symptoms on four occasions, and for both skin and joint disease one time. Biologics were discontinued on 8/16 (50%) occasions after a median of 3.5 months (range 1.5–7.5 months): four times because of good global response, three times because of therapeutic fatigue (loss of efficacy while continuing treatment) and once because of adverse event (lower respiratory tract infection after four doses of adalimumab). The global response was sustained on all the four occasions after stopping biologics (but continuing other systemic therapies): it lasted for median 5 months (range 3–5 months) followed by disease exacerbation on three occasions, while one (patient 5) continued to have good response 6 months after stopping infliximab at last follow-up. All the three disease exacerbation events were re-treated with biologics (good response, n = 2; partial response, n = 1). The treatment response with individual biologics is summarized in Table 3.
| Biologic* | Global response category | Duration of sustained global response after biologic discontinuation (median, range) | Time to therapeutic fatigue while on biologic treatment (median, range) |
|---|---|---|---|
| INF (n=10) | Responder (n=9) Partial responder (n=1) |
5 months (n=1)# | 2 months, range 2-9 months (n=3)^ |
| ADA (n=3) | Partial responder (n=3) | - | 3 months (n=1)^ |
| ETN (n=1) | Responder | 3 months (n=1) | - |
| SEC (n=1) | Responder | 5 months (n=1) | - |
| ITO (n=1) | Partial responder | - | 2 months (n=1)^^ |
| Total (n=16) | Responder (n=11) Partial responder (n=5) |
5 months, range 3-5 months (n=3) | 2 months, range 2-9 months (n=5) |
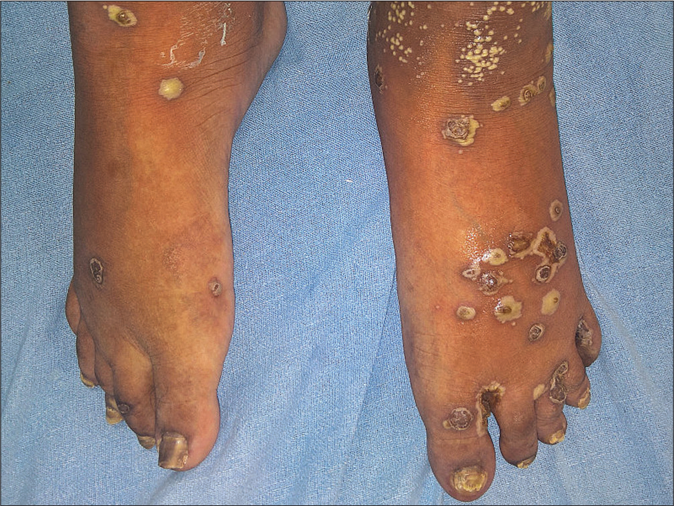
- Hyperpigmented keratotic papules and plaques with surrounding pustular lesions on bilateral feet at baseline
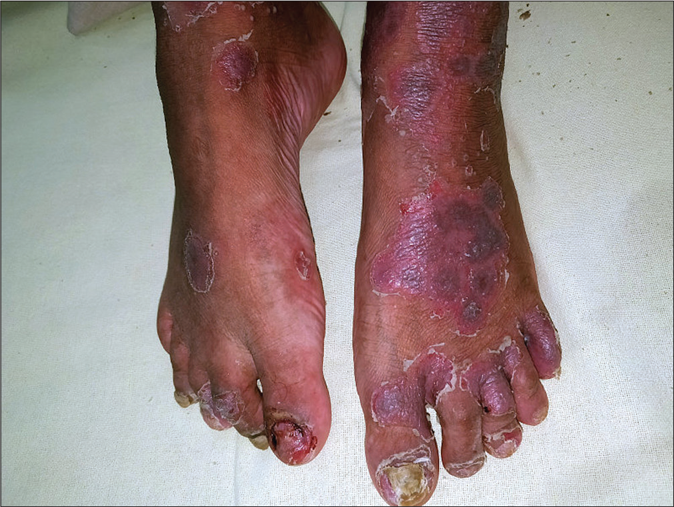
- Significant improvement in the skin lesions 2 weeks after one infliximab dose
Treatment-related adverse effects
Treatment-related adverse effects were seen in 6 (50%) patients. These included methotrexate-induced transaminitis (n = 3), lower respiratory tract infection in a patient receiving adalimumab (n = 1), corticosteroid-induced diabetes mellitus (n = 2) and corticosteroid-induced cataract (n = 2). Transaminitis resolved after temporary withdrawal of methotrexate and did not recur after re-starting methotrexate at lower doses in all the three patients. The lower respiratory tract infection was successfully treated with intravenous antibiotics and temporary discontinuation of adalimumab.
Discussion
There is no consensus on the diagnosis of reactive arthritis, and different diagnostic criteria emphasizing on arthritis and preceding enteric or genitourinary infection have been proposed. Notably, mucocutaneous features which are a frequent finding in reactive arthritis do not find a mention in these criteria.2,4,5 The classic triad of arthritis, urethritis and conjunctivitis (“complete” Reiter’s syndrome) has been shown to be 98% specific for reactive arthritis, but had a sensitivity of only 50%.2 However, it has been suggested that these criteria are too restrictive, and that this label can be applied even to patients with arthritis without urethritis or conjunctivitis, if they have other consistent clinical features.6 All our patients had inflammatory peripheral arthritis along with the characteristic rupioid plaques with moist scales, and no further investigations such as synovial tissue analysis or skin biopsy were considered necessary to confirm the diagnosis.
There are no treatment guidelines for reactive arthritis, and this is reflected in the wide variety of treatments used in our patients. Response with the combination of methotrexate and sulfasalazine produced an unsatisfactory response in our cohort. Though pulsed corticosteroids improved both skin and joint symptoms, they were discontinued in view of secondary nonresponse [time to therapeutic fatigue was 6–10 months with monthly pulses (n = 2), and 2–26 months (n = 3, median 3 months) with weekly pulses]. Good results with monthly dexamethasone pulse therapy have been reported in reactive arthritis earlier;7,8 however, concerns have been raised regarding the use of corticosteroids due to bacterial persistence in reactive arthritis.9 Indeed, antibiotics have been tried in an attempt to eradicate the underlying infection, but with inconsistent results. A recent meta-analysis did not find any significant effect on remission or other response parameters, but concluded that antibiotic use in the treatment of reactive arthritis requires further evaluation given the large heterogeneity in the included studies.10
Owing to lack of satisfactory response with conventional systemic agents, majority of our patients received biologics and experienced a dramatic but short-term benefit. There are small open-label case-series with encouraging preliminary results with tumor necrosis factor-alpha blockers in reactive arthritis.11,12 An open-label study reported nine responders out of 10 patients with reactive arthritis or undifferentiated arthritis treated with etanercept, who completed the 6-month study period.11 In another retrospective study, rapid improvement was noted with antitumor necrosis factor agents in nine of 10 patients with reactive arthritis refractory to conventional systemic agents. The authors reported relapse in three patients after discontinuation of biologics after a median of 6 months (range 3–6 months), a finding consistent with our results.12 However, unlike our series, most of the patients in this study had relatively short disease duration and therefore spontaneous resolution cannot be completely excluded. Though biologic use was associated with a good response in our patients, therapeutic fatigue and short-lasting response should be noted as limitations of this costly treatment. We observed a better response with treatment regimens with biologics than those without them. Further, no statistically significant difference was seen when biologics were used as initial treatment or as next-line treatment. However, one should be cautious in interpreting these results because of the small sample size and substantial heterogeneity in the treatment protocols. Generally, the response of skin disease was noted to be better and more sustained than joint disease. Further, the choice of therapeutic modality did not seem to influence the long-term outcome as all our patients continued to have a chronic waxing and waning course. This is in contrast from an earlier study which reported 75% of patients to be in remission after 2 years of disease onset.13 However, another follow-up study of 48 patients with reactive arthritis found only 22% patients to be in remission at 6 years.14 Prospective randomized trials using validated outcome measures on larger sample size are needed to study the relative efficacy of biologics and conventional systemic therapies in the treatment of reactive arthritis.
Limitations
The limitations of our study include its retrospective nature and the small sample size. A validated outcome measure for reactive arthritis such as the Disease Activity Index for Reactive Arthritis could not be used because of some missing data.15 However, we used a patient global assessment scale, a widely used patient-reported outcome measure, for evaluating the treatment response. Further, we also evaluated the improvement in skin lesions, an aspect not included in Disease Activity Index for Reactive Arthritis. As this study analyzed the data of patients admitted in the dermatology department of a tertiary care hospital, it is likely that only patients with prominent skin manifestations and severe disease were included.
Conclusion
Biologics are effective in the treatment of skin as well as joint symptoms in chronic reactive arthritis; however, the response is short-lived. These patients experience intermittent exacerbations despite regular treatment, necessitating frequent treatment switches. The results of our study provide an insight into the disease course of patients with chronic reactive arthritis, and treatment outcome with biologics.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Incomplete Reiter's syndrome: Clinical comparisons with classical triad. Ann Rheum Dis. 1979;38(Suppl 1):73-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reiter's syndrome, Evaluation of preliminary criteria for definite disease. Arthritis Rheum. 1981;24:844-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reiter's disease in Northern India. A clinical and immunogenetic study. Rheumatol Int. 1983;3:101-4.
- [CrossRef] [PubMed] [Google Scholar]
- Third International Workshop on Reactive Arthritis 23-26, September 1995, Berlin, Germany, Report and abstracts. Ann Rheum Dis. 1996;55:564-84.
- [CrossRef] [PubMed] [Google Scholar]
- Report on the Fourth International Workshop on Reactive Arthritis. Arthritis Rheum. 2000;43:720-34.
- [CrossRef] [Google Scholar]
- Reactive arthritis in India: A dermatologists' perspective. J Cutan Med Surg. 2013;17:180-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pluse therapy with dexamethasone in Reiter's disease. Indian J Dermatol Venereol Leprol. 1982;48:358-61.
- [Google Scholar]
- Pulse therapy as a cure for autoimmune diseases. Indian J Dermatol Venereol Leprol. 2003;69:323-8.
- [Google Scholar]
- Reactive arthritis: Clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21-44.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotics for treatment of reactive arthritis: A systematic review and metaanalysis. J Rheumatol. 2013;40:916-28.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: An open-label trial. Arthritis Rheum. 2005;53:613-7.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of anti-tumor necrosis factor? therapy in ten patients with recent-onset refractory reactive arthritis. Arthritis Rheum. 2011;63:1274-80.
- [CrossRef] [PubMed] [Google Scholar]
- Reiter's syndrome, Diagnosis and clinical features. Rheum Dis Clin North Am. 1998;24:677-95, vii
- [CrossRef] [Google Scholar]
- A follow-up study of 48 patients with Reiter's syndrome. Am J Med. 1979;67:808-10.
- [CrossRef] [Google Scholar]
- Development of a disease activity index for the assessment of reactive arthritis (DAREA) Rheumatology (Oxford). 2000;39:148-55.
- [CrossRef] [PubMed] [Google Scholar]






