Translate this page into:
A study of prevalence of autoantibodies in patients with lichen planus from Mumbai, India
2 Department of Clinical and Experimental Immunology, National Institute of Immunohematology, Indian Council of Medical Research, Mumbai, Maharashtra, India
Correspondence Address:
Vidya Kharkar
Professor. Skin OPD No. 117, Department of Dermatology, Seth GS Medical College and King Edward Memorial Hospital, Parel, Mumbai - 400 012, Maharashtra
India
| How to cite this article: Rambhia KD, Kharkar V, Pradhan V, Patwardhan M, Ghosh K, Khopkar US. A study of prevalence of autoantibodies in patients with lichen planus from Mumbai, India. Indian J Dermatol Venereol Leprol 2018;84:667-671 |
Abstract
Background: Lichen planus is a common chronically relapsing autoimmune skin condition with poorly understood etiology. Apart from cellular immunity, presence of various antibodies has been hypothesized. Various studies have found the presence of serum anti-nuclear antibody, anti-mitochondrial antibody, anti-desmoglein 1 and 3 antibodies, anti-keratinocyte antibody and anti-thyroglobulin antibody in patients of cutaneous and oral lichen planus.
Aim: To study the prevalence of autoantibodies and the clinical spectrum of disease in an Indian patient subpopulation with lichen planus.
Methods: A cross-sectional epidemiological study comprising 100 lichen planus patients was conducted in the dermatology outpatient department of Seth G.S Medical College and King Edward Memorial Hospital, Mumbai, Maharashtra, India. Serum concentrations of circulating anti-nuclear antibodies, anti-desmoglein 1 antibody, anti-desmoglein 3 antibody, anti-keratinocyte antibodies, anti-mitochondrial antibodies and anti-thyroglobulin antibodies were determined by indirect immunofluorescence. Pairs of groups were compared using “Student's t-test” for normally distributed continuous data. The “χ2-test” was used for the categorical variables as needed. Statistical significance was set at P < 0.05.
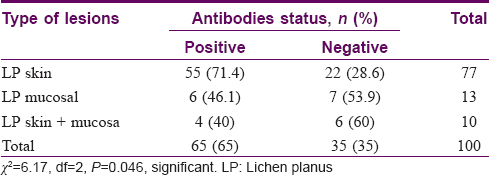
Results: It was found that 65 (65%) patients showed the presence of at least one of the six autoantibodies that we studied, while 35 (35%) tested negative for all six of them. Positivity of anti-keratinocyte antibody in 26 (26%), anti-nuclear antibody in 22 (22%), anti-desmoglein 1 antibody in 19 (19%), anti-desmoglein 3 antibody in 16 (16%), anti-mitochondrial antibody in 9 (9%) and anti-thyroglobulin antibody in 6 (6%) patients was detected. It was observed that 55 (71.4%) patients of cutaneous lichen planus, 6 (46.1%) patients of mucosal lichen planus and 4 (40%) patients of cutaneous and mucosal lichen planus overlap showed presence of at least one autoantibody.
Conclusion: This study provides the serological parameters of a population of lichen planus from western India. Presence of autoantibodies in lichen planus suggests the possible role of humoral immunity in lichen planus. Identifying antibodies linked to lichen planus may help in identifying suitable diagnostic tests and therapeutic targets. Well-controlled studies with larger sample size are the need of the hour to confirm the role of humoral immunity in lichen planus.
Limitations: Studies with a larger number of patients as well as controls should be undertaken to further evaluate the role of autoantibodies in lichen planus.
Introduction
Lichen planus is a common, pruritic, inflammatory disease of skin, mucous membranes and hair follicles. The natural history of lichen planus is highly variable and dependent on the site of involvement and the clinical pattern. Recurrences are frequently seen. The etiology of lichen planus is poorly understood and autoimmunity is proposed. Recent evidence suggests the role of humoral immunity in the pathogenesis of lichen planus. This study mainly focuses on the immunological profile of lichen planus patients from western India to understand the etiopathogenesis of lichen planus from an autoimmune point of view by detecting various autoantibodies such as anti-nuclear antibody, anti-desmoglein 1 antibody, anti-desmoglein 3 antibody, anti keratinocyte antibody, anti-mitochondrial antibodies and anti-thyroglobulin antibody
Methods
The aims of the study were: (1) to detect the presence of autoantibodies in lichen planus patients, (2) to investigate the association and the prevalence of auto-antibodies in lichen planus and (3) to study the clinical spectrum of lichen planus and correlate it with the antibodies.
Inclusion criteria
All cases of newly diagnosed lichen planus of either sex and any age group were enrolled in the study.
Exclusion criteria
Patients who were unwilling to give informed consent for the study were excluded.
Study design
A cross-sectional epidemiological study was conducted in the dermatology outpatient department of Seth G.S Medical College and King Edward Memorial Hospital, Mumbai, India during a period of 1 year. It was approved by the Institutional Ethics Committee and was conducted in accordance with the Declaration of Helsinki principles. Hundred patients with lichen planus were identified and diagnosed based on clinical features. The screened patients meeting the inclusion criteria were explained the details of the study and their written informed consent was taken. A detailed history was taken and physical examination was performed. Lichen planus was diagnosed clinically and was confirmed by histopathology wherever necessary.
Patients enrolled in the study were then classified clinically into three groups: those having cutaneous lichen planus (77%), the second group included patients with mucosal lichen planus (13%) and the third group included patients having both skin as well as mucosal lesions (10%). The skin and mucosal lesions were further classified according to the morphology of the lesions.
Four milliliters of blood was drawn in a sterile syringe with all aseptic precautions. Antibodies including anti-nuclear antibody, anti-desmoglein 1 antibody, anti-desmoglein 3 antibody, anti-keratinocyte antibody, anti-mitochondrial antibody and anti-thyroglobulin antibody were assayed in serum samples by indirect immunofluorescence according to commercially available testing kits from Medical and Biological Labs, Japan for desmoglein 1 antibody and desmoglein 3 antibody, Bio-Rad, USA for anti-nuclear antibody (Hep-2) and Immco, USA for anti-keratinocyte antibody, anti-mitochondrial antibody and anti-thyroglobulin antibody.
Statistical analysis
Pairs of groups were compared using “Student's t-test” for normally distributed continuous data. The “χ2-test” was used for the categorical variables as needed. Statistical significance was set at P < 0.05.
Results
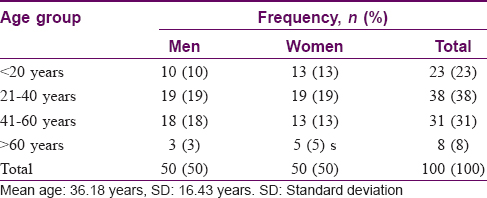
The mean age of the patients in the study population was found to be 36.18 years. The distribution of patients stratified by age at onset is shown in [Table - 1]. The mean duration of lichen planus in our study population was < 6 months in 45 (45%) cases, 6 months to 1 year in 27 (27%) patients and more than 1 year in 28 (28%) patients [Table - 2]. The most common presenting complaints of the patients were itching in 82 patients; 13 patients experienced pain; 1 patient had hair loss and 7 patients were completely asymptomatic. The risk factors were stress in 54 (54%) patients and metallic crown caps in 20 (20%) patients. Chronic diseases including hypertension, diabetes, hepatitis, HIV, malignancy were present in 13 (13%), addictions in 13 (13%), history of jaundice in 6 (6%), history of blood transfusion in 2 (2%) patients and family history of autoimmune disease was found in 8 (8%) patients. The initial site of involvement included lower extremity in 60 (60%) patients, upper extremity in 20 (20%), oral cavity in 12 (12%), trunk in 5 (5%) patients and genitals, scalp and nails in 1 (1%) each.

The number of patients with cutaneous lichen planus was 77, mucosal lichen planus was 13 and overlap of cutaneous and mucosal lichen planus was 10. Various morphological subtypes were seen in each type of lichen planus. Of the patients with cutaneous lichen planus, 58 had classical flat-topped lesions and 25 had hypertrophic lesions. Annular, follicular, perforating and erosive lesions were found in 1 patient each. The varied subtypes of mucosal lichen planus included reticular lesions in 13 patients, erosive lesions in 9 patients, plaque type lesions in 4 patients and mixed type (reticular and erosive) in 1 patient. Total number of patients with nail involvement was 11. Many patterns of nail involvement were seen: pitting in 4 patients, longitudinal ridges in 4 patients, 20 nail dystrophy in 1 patient and isolated nail dystrophy in 2 patients. Of the study population of 100 patients, 65 (65%) showed the presence of at least one of the six autoantibodies that we studied, while 35 (35%) tested negative for all six of them [Table - 3] and [Figure - 1]. The overall frequencies of autoantibodies were anti-keratinocyte antibody (26%), anti-nuclear antibody (22%), anti-desmoglein 1 antibody (19%), anti-desmoglein 3 antibody (16%), anti-mitochondrial antibody (9%) and anti-thyroglobulin antibody (6%) [Figure - 2].
 |
| Figure 1: Morphological type of lichen planus with antibody positivity. LP- Lichen planus |
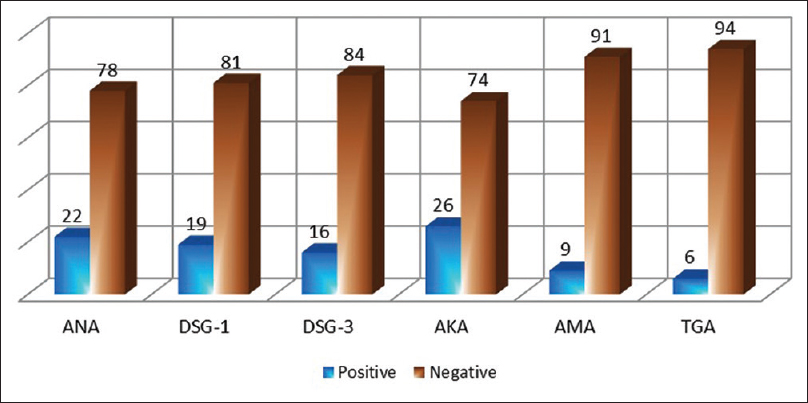 |
| Figure 2: Total antibody positivity in the study. ANA- anti-nuclear antibody, DSG 1, 3- anti-desmoglein 1 and 3 antibodies, AMA- anti-mitochondrial antibody, AKA- anti-keratinocyte antibody and TGA- anti-thyroglobulin antibody |
In cutaneous lichen planus, 55/77 patients (71.4%) had at least one autoantibody in their sera. In mucosal lichen planus, 6/13 patients (46.1%) showed presence of at least one autoantibody, whereas in cutaneous and mucosal lichen planus, 4/10 patients (40%) had an autoantibody in their sera [Table - 3]. The frequency of presence of serum autoantibodies was more in cutaneous lichen planus than in mucosal or cutaneous and mucosal lichen planus. The χ2-value was 6.17 and the P value = 0.046 (<0.05). Frequencies of presence of autoantibodies seen in Indian lichen planus patients were anti-keratinocyte antibody > anti-nuclear antibody > desmoglein 1 antibody > desmoglein 3 antibody > anti-mitochondrial antibody > anti-thyroglobulin antibody.
Discussion
The pathogenesis of lichen planus has not yet been fully elucidated. However, an autoimmune etiology with cellular immunity playing a role is postulated. The steps involved in the pathogenesis of lichen planus include recognition of the lichen planus-specific antigen by CD4+ T cells and NK cells, cytotoxic T cell activation and keratinocyte apoptosis.[1] Other mechanisms which may possibly be operative in the pathogenesis of lichen planus include activated fibrinogen cascade,[2] neoangiogenesis[3],[4] and nonantigen-mediated mechanisms, including activation of matrix metalloproteinases and mast cell degranulation.[5] Many studies hypothesizing the role of circulating antibodies have been described in lichen planus recently. In 1984, Olsen et al. demonstrated the presence of antibody to lichen planus-specific antigen which was present only in the stratum granulosum and spinosum.[6] Many similar studies performed with direct and indirect immunofluorescence in cutaneous and mucosal lichen planus using autologous, analogous or fetal skin later gave evidence of circulating autoantibodies against an lichen planus-specific antigen in granular layer.[7] Lichen planus specific antigen is specific for lichen planus and was found in about 80% of the patients with or without oral lesions.[6] In an Indian study, Rao and Shenoifound lichen planus-specific antigen in 88% of patients.[8] In the current study, the presence of serum autoantibodies in cutaneous lichen planus was seen in 55 (71.4%) patients. In mucosal lichen planus, it was seen in 6 (46.1%) and in overlap cutaneous and mucosal in 4 (40%) patients. Chang et al. had reported 60.9% frequency of autoantibodies among oral lichen planus patients which was comparatively higher than the present study.[9]
A study by Parodi et al. in 2007 described the prevalence of stratified epithelium-specific antinuclear antibodies directed to an antigen of 70 kd in patients with various forms of lichen planus.[10] Carizossa, Elorza, Carnacho found anti-nuclear antibodies in high proportion (40% using rat esophagus as substrate and 27.6% using monkey esophagus). These anti-nuclear antibodies exhibited a speckled pattern and were more frequently encountered in erosive lichen planus patients.[11] A study by Lodi et al. described presence of anti-nuclear antibodies in 43% patients of oral lichen planus, especially Hepatitis C virus-infected patients.[12] Carrozzo et al. discovered the presence of serum autoantibodies including anti-nuclear antibody, smooth muscle antibody, anti-mitochondrial antibody, gastric parietal cell antibody, anti-thyroid antibody in 41% of Hepatitis C virus antibody positive patients.[13] Chang et al. reported 28.1% anti-nuclear antibody positivity among oral lichen planus patients.[9] In the present study, the presence of anti-nuclear antibodies was found in 22% patients and was in concordance with previous reports in the literature.
Anti-basal cell antibodies directed to the cytoplasm or membrane of epitheliocytes were detected using rat esophagus as substrate by Lin et al., and they found that it was positive in 54% of oral lichen planus patients.[14] Anti-basal cell antibody positivity in oral lichen planus persisted and lasted for few months or years and a decrease in titers after treatment with topical triamcinolone was observed. Sun et al. had reported 50% positivity for anti-basal cell antibodies among erosive oral lichen planus patients and the disappearance of anti-nuclear antibody in erosive oral lichen planus patients after levamisole treatment.[15] Even though anti-keratinocyte antibodies have pathogenetic significance in lichen planus, they were positive in only about a quarter of the patients in our study. Hence, they do not seem be a sensitive-enough marker to have any diagnostic utility in this cohort.
Recently, Lukac et al. had demonstrated significantly higher concentrations of circulating autoantibodies to both desmoglein-1 and desmoglein-3 among erosive oral lichen planus patients and in patients with reticular oral lichen planus.[16] Kinjyo et al. described a case of oral lichen planus with antibodies to desmoglein 1 and 3.[17] Two more cases of erosive oral lichen planus with anti-desmoglein antibody positivity have been reported in literature.[18] In the present study, anti-desmoglein 1 antibody was positive in 19% patients and anti-desmoglein 3 antibody was positive in 16% patients. In contrast to the findings reported by Lukac et al., in the present study, nonerosive forms of lichen planus showed significantly higher titers of circulating autoantibodies to both desmoglein-1 and desmoglein-3 antibodies. Currently, it is difficult to infer whether the anti-desmoglein antibodies in lichen planus are of primary pathogenic significance or are a result of epitope spreading which is known to occur in autoimmune diseases. During the process of specific or nonspecific keratinocyte damage, antigenic material including desmogleins may be released producing autoantibodies.
Chang et al. had reported the frequency of anti-mitochondrial antibody as 1.6%; anti-thyroglobulin antibody positivity as 21.3% among oral lichen planus patients. The presence of anti-nuclear antibody, anti-gastric parietal cell antibody, anti-thyroglobulin antibody and anti-thyroid microsomal antibody were also reported in oral lichen planus.[16] Thus, the present study provides information of clinical details, demographic profile and serological parameters of a population of lichen planus from Mumbai, India.
Clinically persistent erosive lichen planus and pemphigus vulgaris are close differential diagnoses. The value of desmoglein antibody positivity in differentiating between these two common conditions needs to be further studied. Biopsy for histopathological examination and direct immunofluorescence are the other differentiating investigations that were not included in this study.
The study had several limitations. Follow-up investigations to evaluate the persistence of these autoantibodies were not done owing to cross-sectional design. Presence of any concomitant autoimmune disease esp. Hashimoto's thyroiditis, autoimmune hepatic disease, myasthenia gravis was not investigated. Inclusion of larger number of patients and controls would have further strengthened the observations of the current study and demonstrated the role of autoantibodies in the causation and pathogenesis of lichen planus.
Conclusion
Pathophysiology of lichen planus is still uncertain. Our study provides the serological parameters of a population of lichen planus from western India. Identifying antibodies linked to lichen planus may help in identifying suitable diagnostic tests and therapeutic targets. Well-controlled studies with larger sample size are the need of the hour to confirm the role of humoral immunity in lichen planus.
Acknowledgements
We acknowledge the participation of patients with lichen planus. We are grateful to Director, National Institute of Immunohematology, Indian Council of Medical Research, Mumbai, India, for funding this study. We are thankful to Dr Naresh Gill, Dr Amit Gulati and Dr Disha Rambhia for their assistance with the statistical analysis in this study.
Financial support and sponsorship
This study was funded by the Director, National Institute of Immunohematology, Indian Council of Medical Research, Mumbai, Maharashtra, India.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Pittelkow MR, Daud MS. Lichen planus. In: Wolff K, Goldsmith LA, Katz SI, editors. Fitzpatrick Dermatology in General Medicine. 7th ed. New York: McGraw Hill; 2008. p. 244-5.
[Google Scholar]
|
| 2. |
Nangia A, Kumar V, Logani KB. An immunopathological study of lichen planus. Indian J Dermatol Venereol Leprol 2000;66:76-8.
[Google Scholar]
|
| 3. |
Salem SA, Aly DG, Youssef NS. Immunohistochemical assessment of angiogenesis and vascular endothelial growth factor expression in cutaneous lichen planus: Relation to the degree of inflammation. Eur J Dermatol 2011;21:197-202.
[Google Scholar]
|
| 4. |
Mittal N, Shankari GM, Palaskar S. Role of angiogenesis in the pathogenesis of oral lichen planus. J Oral Maxillofac Pathol 2012;16:45-8.
[Google Scholar]
|
| 5. |
Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A, et al. Pathogenesis of oral lichen planus – A review. J Oral Pathol Med 2010;39:729-34.
[Google Scholar]
|
| 6. |
Olsen RG, Du Plessis DP, Schulz EJ, Camisa C. Indirect immunofluorescence microscopy of lichen planus. Br J Dermatol 1984;110:9-15.
[Google Scholar]
|
| 7. |
Olsen RG, DuPlessis D, Schulz EJ. Serum from lichen planus patients reacts with fetal skin tissues. Med Microbiol Immunol 1984;172:249-56.
[Google Scholar]
|
| 8. |
Rao R, Shenoi SD. Indirect immunofluorescence to demonstrate lichen planus specific antigen (LPSA) in lichen planus. Indian J Dermatol Venereol Leprol 2006;72:350-2.
[Google Scholar]
|
| 9. |
Chang JY, Chiang CP, Hsiao CK, Sun A. Significantly higher frequencies of presence of serum autoantibodies in Chinese patients with oral lichen planus. J Oral Pathol Med 2009;38:48-54.
[Google Scholar]
|
| 10. |
Parodi A, Cozzani E, Massone C, Rebora A, Priano L, Ghigliotti G, et al. Prevalence of stratified epithelium-specific antinuclear antibodies in 138 patients with lichen planus. J Am Acad Dermatol 2007;56:974-8.
[Google Scholar]
|
| 11. |
Carrizosa AM, Elorza FL, Camacho FM. Antinuclear antibodies in patients with lichen planus. Exp Dermatol 1997;6:54-6.
[Google Scholar]
|
| 12. |
Lodi G, Olsen I, Piattelli A, D'Amico E, Artese L, Porter SR, et al. Antibodies to epithelial components in oral lichen planus (OLP) associated with hepatitis C virus (HCV) infection. J Oral Pathol Med 1997;26:36-9.
[Google Scholar]
|
| 13. |
Carrozzo M, Gandolfo S, Lodi G, Carbone M, Garzino-Demo P, Carbonero C, et al. Oral lichen planus in patients infected or noninfected with hepatitis C virus: The role of autoimmunity. J Oral Pathol Med 1999;28:16-9.
[Google Scholar]
|
| 14. |
Lin SC, Sun A, Wu YC, Chiang CP. Presence of anti-basal cell antibodies in oral lichen planus. J Am Acad Dermatol 1992;26:943-7.
[Google Scholar]
|
| 15. |
Sun A, Chiang CP, Chiou PS, Wang JT, Liu BY, Wu YC, et al. Immunomodulation by levamisole in patients with recurrent aphthous ulcers or oral lichen planus. J Oral Pathol Med 1994;23:172-7.
[Google Scholar]
|
| 16. |
Lukac J, Brozović S, Vucicević-Boras V, Mravak-Stipetić M, Malenica B, Kusić Z, et al. Serum autoantibodies to desmogleins 1 and 3 in patients with oral lichen planus. Croat Med J 2006;47:53-8.
[Google Scholar]
|
| 17. |
Kinjyo C, Kaneko T, Korekawa A, Rokunohe A, Aizu T, Matsuzaki Y, et al. Oral lichen planus with antibodies to desmogleins 1 and 3. J Dermatol 2015;42:40-1.
[Google Scholar]
|
| 18. |
Muramatsu K, Nishie W, Natsuga K, Fujita Y, Iwata H, Yamada T, et al. Two cases of erosive oral lichen planus with autoantibodies to desmoglein 3. J Dermatol 2016;43:1350-3.
[Google Scholar]
|
Fulltext Views
5,171
PDF downloads
1,800





