Translate this page into:
A study on various Neisseria gonorrhoeae phenotypes circulating in Tripura
2 Department of Microbiology, Agartala Government Medical College, Agartala, Tripura, India
3 Department of Human Physiology, Tripura University, Agartala, Tripura, India
Correspondence Address:
Tapan Majumdar
Department of Microbiology, Agartala Government Medical College, Kunjavan, Agartala, Tripura - 799 006
India
| How to cite this article: Mullick JB, Majumdar T, Bir R, Hore S, Sil SK. A study on various Neisseria gonorrhoeae phenotypes circulating in Tripura. Indian J Dermatol Venereol Leprol 2017;83:499-501 |
Sir,
Gonorrhea is one of the most common sexually transmitted infections in the developed world caused by the Gram-negative diplococcus Neisseria gonorrhoeae. Once an easily treatable disease, it has become a therapeutic problem owing to coinfections, asymptomatic infections, misdiagnosis and antibiotic misuse leading to multidrug resistance. There was lack of epidemiologic data from Tripura on the frequency of disease and drug resistance patterns, which encouraged us to conduct this study which would help to identify the circulating drug resistant phenotypes and thereby determine the drugs effective in the local population.
The study was conducted at Agartala Government Medical College, Agartala, Tripura, between July 2013 and June 2015 after receiving clearance from the Institutional Ethical Committee. Two hundred and seventy five subjects suspected to have clinically compatible features of sexually transmitted infections in the reproductive age group of 18–49 years attending the dermatology or the obstetrics and gynecology clinics were included in the study. Direct Gram-stain of urethral/cervical smear was performed followed by culturing on chocolate agar (HiMedia Laboratories, Mumbai, India). Identification was done as per standard protocol. Chlamydia trachomatis screening was done using polymerase chain reaction.[1] For diagnosis of human immunodeficiency virus infection, National Acquired Immune Deficiency Syndrome Control Organization guideline of strategy III was followed. Antimicrobial susceptibility testing was performed against penicillin G, tetracycline, ciprofloxacin, nalidixic acid, azithromycin, spectinomycin and ceftriaxone through E-test and Kirby–Bauer disc diffusion methods. Modified Hodge test was carried out among the resistant phenotypes.[2] Briefly, plain chocolate agar was lawn cultured with β-lactam sensitive Staphylococcus aureus strain (ATCC ® 25923™). Single penicillin disc (10 U) was placed in the center and test strain was streaked radially out from the disk. Similarly, three more streaks [Figure - 1] of second test strain, positive control (β-lactamase producing strain of N. gonorrhoeae ATCC ® 31426™) and negative control (β-lactam sensitive strain of S. aureus ATCC ® 29213™) were made at 90°, 180° and 270° respective to the first strain. Liquid acidometric test was done as per standard protocol.[3] Briefly, benzylpenicillin solution (HiMedia Laboratories, Mumbai, India) containing 0.1% aqueous cresol red (HiMedia Laboratories, Mumbai, India) (pH 8.0) was aliquoted at 50 μl/well in a 96-well microtiter plate. To each well, a loop full of test culture was dispersed. A change in color from cherry red to bright yellow (pH 7.2) [Figure - 2] within 30 minutes indicated positive β-lactamase production.
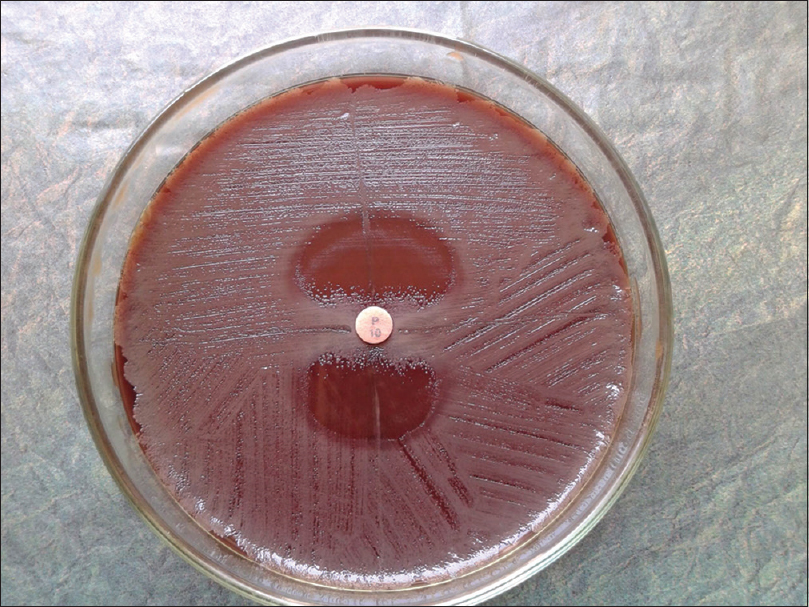 |
| Figure 1: Penicillinase production confirmation through Hodge test. Disruption of inhibition zone of β-lactam sensitive S. aureus at 0° and 180° positions by β-lactamase producing strains. At 90° and 270° positions are β-lactam sensitive isolates |
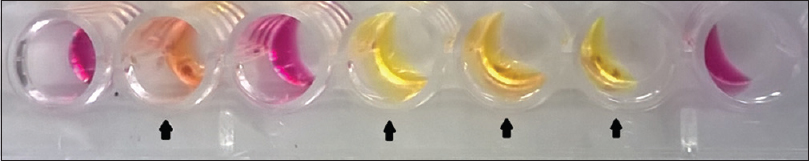 |
Figure 2: β-lactamase production through liquid culture method. Bright yellow/ orange coloration in wells ( ) due to β-lactamase production by N. gonorrhoeae, cherry red coloration due to lack of β-lactamase production ) due to β-lactamase production by N. gonorrhoeae, cherry red coloration due to lack of β-lactamase production |
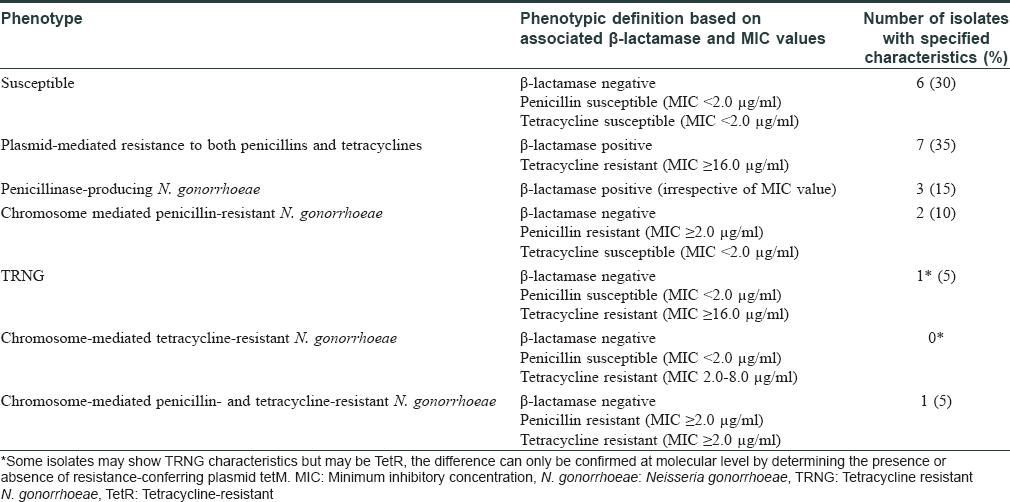
Among the study subjects of 275 cases, 29 (10.5%) were positive on direct microscopy of Gram-stained slides for intra- and extra-cellular Gram-negative diplococci. However, isolation of N. gonorrhoeae on culture could be done in 20/29 (69%) cases. The time lapse between acquisition of infection to the time of visit to the clinic ranged from 4 to 60 days with a median value of 10 days. The duration from exposure to onset of symptoms ranged between 2 and 14 days [Figure - 3]. Of the 29 Gram-stain positive subjects, six were coinfected with C. trachomatis and one was human immunodeficiency virus seropositive. Susceptibility testing towards the standard drugs showed the highest sensitivity for ceftriaxone 20/20 (100%) and spectinomycin 19/20 (95%) while least sensitivity was for fluoroquinolones 5/20 (25%) [Table - 1]. A single isolate showed multidrug resistance with resistance towards spectinomycin, fluoroquinolones, tetracyclines and azithromycin. The predominant drug resistant phenotype found was plasmid-mediated resistance to both penicillins and tetracyclines 7/20 (35%) followed by penicillinase-producing N. gonorrhoeae (15%) and chromosome-mediated penicillin-resistant N. gonorrhoeae 2/20 (10%) [Table - 2].
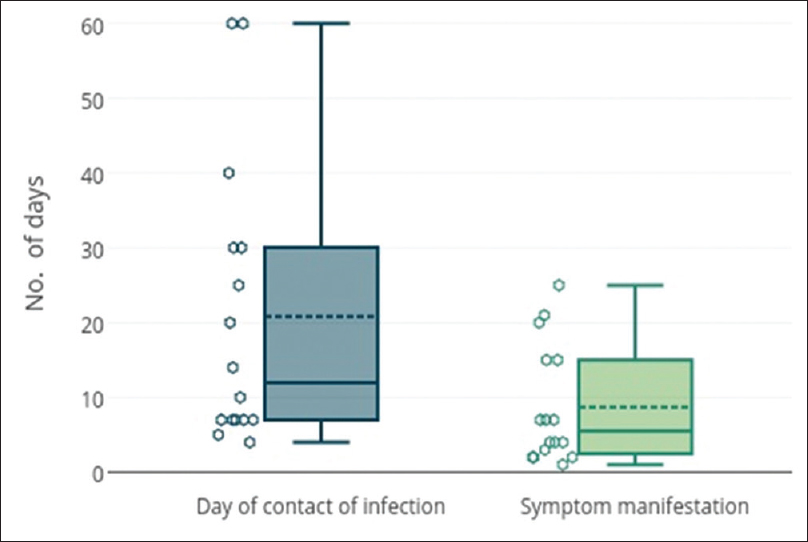 |
| Figure 3: Box and whisker plot of days lapsed since contact of gonococcal infection to visit to clinic and days passed between contact and manifestation of symptomsN. |
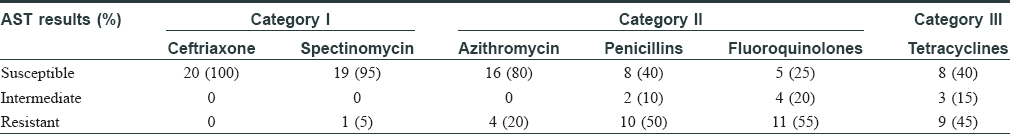
Gonorrhoea accounted for 10.5% of all sexually transmitted infections in our study, which is similar to other studies from India.[4],[5] As per revised category of drugs proposed for treatment of gonorrhoea, 1/20 (5%) cases were found to be resistant to Category I drugs; 17/20 (85%) were resistant to one or more drugs of Category II; and 9/20 (45%) were found to be resistant to Category III drugs [Table - 1]. Among the Category II drugs, in spite of increased reports of resistance, azithromycin has been proposed for more frequent use.[6] Our study found azithromycin resistance rate to be 4/20 (20%), which is higher than the 5% failure rate criteria.[7] Whether such high resistance still qualifies it as a candidate for treatment requires a larger prevalence study.
On the basis of minimum inhibitory concentration breakpoints and simple tests for β-lactamase production, it is possible to determine the mechanism of resistance without going into molecular studies.[8] However, simple tests like β-lactamase production are not appropriate for determining the mechanism of resistance for tetracyclines and for these drugs, confirmation requires molecular testing. Our study showed that resistance was plasmid-mediated in most cases, most commonly conferring resistance to both penicillins and tetracyclines through β-lactamase producing as well as tetracycline determinant (tetM) conjugative plasmids. This was followed by β-lactamase-producing plasmids conferring resistance to penicillins. The third most abundant penicillin-resistant phenotype was resistant to penicillins through chromosomal-mediated resistance. Limitations of the study were the small sample size and low participation by female subjects due to lack of awareness and stigma attached with sexually transmitted infections. Secondly, we were unable to study the effect of HIV on gonorrhoea as our study included only a single subject harboring this coinfection. To estimate the effect of gonorrhea on human immunodeficiency virus coinfection in the community, a large-scale prevalence study is essential.
In conclusion, frequency of gonococcal infection among symptomatic subjects was similar to studies from other parts of India. There was good sensitivity to the drugs recommended currently by the Centers for Disease Control and Prevention. Most common mechanism of drug resistance was found to be plasmid mediated conferring resistance to both penicillins and tetracyclines.
Acknowledgment
We would like to thank the Department of Biotechnology under Ministry of Science and Technology, India, for funding the study. In Agartala Government Medical Collegewe thank Dr. Syamal Kanti Chakraborty, associate professor, department of dermatology, Dr. Jayanta Ray, associate professor, department of obstetrics and gynaecology for their clinical expertise and Mr. Sanjib Debnath, department of microbiologyfor his technical help. We also thank Dr. KVR Reddy, Deputy Director (Sr. Grade) and Head, division of molecular immunology and microbiology, National Institute for Research in Reproductive Health, Mumbai, for his scientific advice and guidance. We thank HiMedia Laboratories, Mumbai, India, for providing with strains of S. aureus (ATCC ® 25923™, ATCC ® 29213™) and N. gonorrhoeae (ATCC ® 31426™, ATCC ® 49226™).
Financial support and sponsorship
The study was supported by the Department of Biotechnologyunder Ministry of Science and Technology (DBT Sanction Order No.: BT/241NE/TBP/2011, Dated: April 30, 2012).
Conflicts of interest
There are no conflicts of interest.
| 1. |
Mania-Pramanik J, Potdar S, Kerkar S. Diagnosis of Chlamydia trachomatis infection. J Clin Lab Anal 2006;20:8-14.
[Google Scholar]
|
| 2. |
Pasteran F, Mendez T, Guerriero L, Rapoport M, Corso A. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J Clin Microbiol 2009;47:1631-9.
[Google Scholar]
|
| 3. |
Anago E, Ayi-Fanou L, Akpovi CD, Hounkpe WB, Agassounon-Djikpo Tchibozo M, Bankole HS, et al. Antibiotic resistance and genotype of beta-lactamase producing Escherichia coli in nosocomial infections in Cotonou, Benin. Ann Clin Microbiol Antimicrob 2015;14:5.
[Google Scholar]
|
| 4. |
Banerjee S, Halder S, Halder A. Trend of sexually transmitted infections in HIV seropositive and seronegative males: A comparative study at a tertiary care hospital of North East India. Indian J Dermatol 2011;56:239-41.
[Google Scholar]
|
| 5. |
Narayanan B. A retrospective study of the pattern of sexually transmitted diseases during a ten-year period. Indian J Dermatol Venereol Leprol 2005;71:333-7.
[Google Scholar]
|
| 6. |
Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther 2009;7:821-34.
[Google Scholar]
|
| 7. |
van de Laar MJ, van Duynhoven YT, Dessens M, van Santen M, van Klingeren B. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in The Netherlands, 1977-95. Genitourin Med 1997;73:510-7.
[Google Scholar]
|
| 8. |
Perilla MJ, Ajello G, Bopp C, Elliott J, Facklam R, Knapp JS, et al. Centers for Disease Control and Prevention, Atlanta, Georgia, USA. Manual for the Laboratory Identification and Antimicrobial Susceptibility Testing of Bacterial Pathogens of Public Health Importance in the Developing World. Vol. VI. Geneva: World Health Organization; 2003. p. 8. Available from: http://www.apps.who.int/medicinedocs/documents/s16330e/s16330e.pdf. [Last accessed on 2016 Sep 22].
[Google Scholar]
|
Fulltext Views
3,460
PDF downloads
2,281





