Translate this page into:
Activated charcoal and baking soda to reduce odor associated with extensive blistering disorders
2 Department of Community Medicine, PSG Hospitals, Peelamedu, Coimbatore, India
Correspondence Address:
C R Srinivas
Department of Dermatology, PSG Hospitals, Peelamedu, Coimbatore - 641 004, Tamil Nadu
India
| How to cite this article: Chakravarthi A, Srinivas C R, Mathew AC. Activated charcoal and baking soda to reduce odor associated with extensive blistering disorders. Indian J Dermatol Venereol Leprol 2008;74:122-124 |
Abstract
Background: Skin disease leading to extensive blistering and loss of skin is associated with a characteristic smell. Odor can cause physiologic disturbances such as increase in heart rate and respiratory rate. It can also cause nausea and vomiting and is disturbing to bystanders. Aims: To test odor reducing capability of activated charcoal. Methods: In this blinded experimental study we used putrefied amniotic membrane to produce odor and studied the effectiveness of activated charcoal and soda-bi-carbonate to reduce odor. Results: Statistical analysis with Kruskal Wall's Chi Square Test and Man Whitney U test showed significant reduction of odor using activated charcoal by itself or along with soda-bi-carbonate. Conclusion: We recommend the usage of activated charcoal with/without soda bicarbonate as an inexpensive practical measure to reduce foul odor associated with extensive skin loss.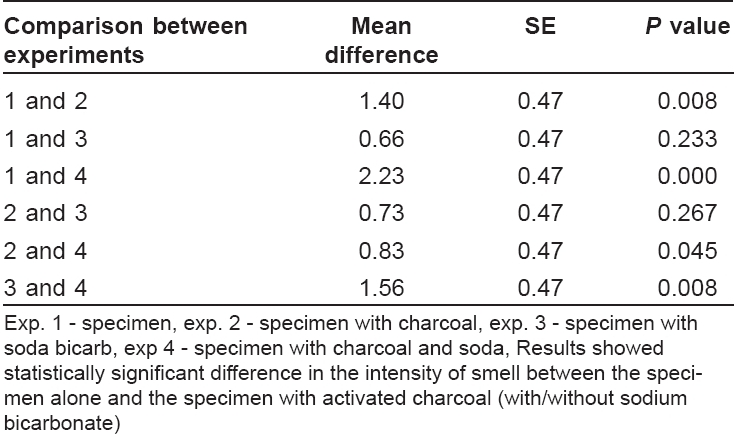


 |
| Figure 1: Plastic box with funnel attached |
 |
| Figure 1: Plastic box with funnel attached |
Introduction
Patients with extensive blistering diseases, such as toxic epidermal necrolysis (TEN) and pemphigus, develop a characteristic malodor. [1] Possibly, it results from the generation of thiol group of chemicals by the infecting bacteria. [2] Odor-producing chemicals are generally of small molecular size, containing 3-4 to 18-20 carbon atoms. Molecules with the same number of carbon atoms but different structural configurations have different odors. [3] The olfactory threshold for different odor-producing chemicals varies. [3] Methyl mercaptan (a chemical present in garlic and which is structurally similar to the odor-producing chemicals in a putrefying material) can be recognized at a concentration of 0.0000004 mg/L, whereas ethyl ether is recognized at a concentration of 5.83 mg/L. [3] Unpleasant odor can induce nausea, vomiting and increased heart rate. [4] The present study was undertaken to find out whether activated charcoal and sodium bicarbonate are effective in reducing the odor commonly associated with extensive vesiculo-bullous disorders.
Methods
During a study to standardize the use of amniotic membrane for covering extensive areas of skin loss associated with blistering disorders, we found that the amniotic membrane left as such at room temperature got putrefied and emitted malodor. The odor is due to chemicals containing thiol (SH) group. [2] We used putrefied amniotic membrane as the source material for malodor. A piece of amniotic membrane (2 x 2 cm 2 ) was placed in each of the four identical plastic bottles containing 2 mL of normal saline. The bottles were closed and the tissues kept inside were allowed to putrefy at room temperature for 2 days. Another four plastic containers (20 x 15 x 8 cm) were taken. Small holes were made on the lid through which a plastic funnel was introduced [Figure - 1]. The point of passage of the stem of the funnel through the lid was sealed with wax. Inside each plastic container two petri-dishes and a plastic bottle with putrefying amniotic membrane were placed. In the first container, the petri-dishes were left empty. In the 2 nd container, 15 gm of activated charcoal was kept in one petri-dish and the other was left empty. Charcoal was spread evenly over the petri-dish using a spatula. In the third container, instead of charcoal, same amount of sodium bicarbonate was kept in one of the petri-dishes. In the 4 th container, both activated charcoal and soda bicarbonate were kept. The lids of the containers were closed and the opening of the funnel was blocked with a piece of cotton. All the plastic containers were covered with thick paper to look identical. Fifteen volunteers with informed consent were asked to grade the intensity of odor on a 10-point scale as described; the containers were placed on a table. The volunteer was asked to sit on a chair, remove the cotton plug from the opening of the funnel and smell at the level of the opening of the funnel. This was repeated for all the four containers with an interval of 15 min before each recording. The order of the containers for the volunteers to assess the smell was randomized. The intensity of smell in each container was recorded on a 10-point scale.
Results
The data were analyzed using SPSS CPC (11.5 version). Statistical significance was assessed by Kruskal Walli′s Chi-squared test and the difference in intensity of smell between each sub-group was compared with Mann Whitney U -test. P < 0.05 was considered statistically significant.
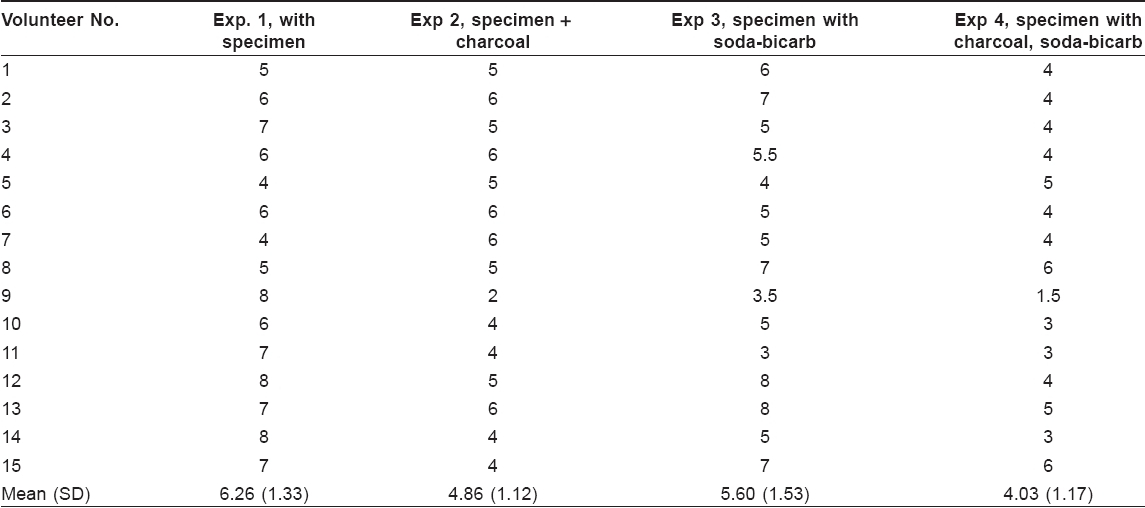
The individual reading as recorded by each volunteer has been shown in [Table - 1].
Mean and standard error (SE) are shown in [Table - 2].
Different sub-groups were compared with Mann Whitney U -test (MW-U), which showed the following results. Specimen alone versus specimen with charcoal (MW-U = 49.0, P = 0.008), and specimen alone versus specimen with charcoal and sodium bicarbonate (MW-U = 25.00, P = 0.000) showed significant difference. However, the difference between specimen alone and specimen with sodium bicarbonate alone was not significant (MW-U = 83.00, P = 0.233). There was significant difference between soda-bi-carb alone and soda-bi-carb with activated charcoal (MW-U=49.000, P =0.08).
Discussion
Bad odor can be masked to some extent by using fragrance, which acts by stimulating different groups of receptors. However, the fragrance does not block the receptors that recognize malodor. Hence, the appropriate way to reduce bad odor would be to deplete the chemicals responsible for generating the odor. Activated charcoal is known to adsorb certain chemicals, and sodium bicarbonate neutralizes volatile sulfur-containing compounds.
Odors can be classified based on the degree of pleasant feeling. [6] Pleasant odors are perceived in the left frontal brain. Malodorous stimuli evoke unpleasant emotions in the bilateral frontal lobes and extensive regions in the brain. [7] Studies indicate that reaction time in response to unpleasant odors were significantly shorter than for pleasant odors as evidenced during affective judgment and right nostril stimulation, indicating greater efficiency of right cerebral hemisphere in decoding unpleasant affects induced by malodors. [7] Oral malodor is caused by hydrogen sulfide, methyl mercaptan and dimethyl-sulfide as determined by gas chromatography. [5]
The study indicates that activated charcoal by itself can significantly reduce bad odor and becomes more efficient when used along with sodium bicarbonate. However, sodium bicarbonate by itself was not significantly effective in reducing the malodor. Activated charcoal is cheap and easy to use. Controlled trials should be conducted to assess the quantity of activated charcoal to be used for this purpose and the frequency at which it has to be changed. In conclusion, the use of activated charcoal may prove to be an inexpensive method to reduce the foul odor associated with severe skin disorders.[9]
| 1. |
Kanwar AJ, Ghosh S, Dhar S, Kaur S. Odor in pemphigus. Dermatology 1992;185:215.
[Google Scholar]
|
| 2. |
Bahl A, Bahl BS. Organosulfur compounds thioalcohols and thioethers. In: Advanced Organic Chemistry. 1 st ed. S Chand and Company: New Delhi; 2005. p. 420-30.
[Google Scholar]
|
| 3. |
Ganong WF. Smell and test review of medical physiology. 19 th ed. Prentics-Hall International Inc: 1999. p. 177-8.
[Google Scholar]
|
| 4. |
Swallow BL, Lindow SW, Masson EA, Hay DM. Women with nausea and vomiting in pregnancy demonstrate wore health and are adversely affected by odours. J Obstet Gynaecol 2005;25:544-9.
[Google Scholar]
|
| 5. |
Murata T, Rahardjo A, Fujiyama Y, Yamaga T. Development of a compact and simple gas chromatography for oral malodor measurement. J Periodontol 2006;77:1142-7.
[Google Scholar]
|
| 6. |
Fiedler N, Kipen HM. Controlled exposures to volatile organic compounds in sensitive groups. Ann N Y Acad Sci 2001;933:24-37.
[Google Scholar]
|
| 7. |
Bensafi M, Rouby C, Farget V, Bertrand B. Influence of affective and cognitive judgments on autonomic parameters during inhalation of pleasant and unpleasant odors in humans. Neurosci Lett 2002;319:162-6.
[Google Scholar]
|
| 8. |
Bensafi M, Rouby C, Farget V. Asymmetry of pleasant vs. unpleasant odor processing during affective judgment in humans. Neurosci Lett 2002;328:309-13.
[Google Scholar]
|
| 9. |
Kim YK, Watanuki S. Characteristics of electroencephalographic responses induced by a pleasant and an unpleasant odor. J Physiol Anthropol Appl Human Sci 2003;22:285-91.
[Google Scholar]
|
Fulltext Views
18,374
PDF downloads
2,254





