Translate this page into:
Adaptation and validation of the Bengali version of the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL)
Corresponding author: Dr. Indrashis Podder, Department of Dermatology, College of Medicine and Sagore Dutta Hospital, Kolkata 700058, West Bengal, India. ipodder88@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dhabal A, Mondal H, Mondal S, Baiardini I, Chakraborty SS, Chakraborty T, et al. Adaptation and validation of the Bengali version of the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL). Indian J Dermatol Venereol Leprol 2023;89:385-92.
Abstract
Background
Chronic urticaria exerts a profound impact on quality of life. Recent guidelines recommend its evaluation in all chronic urticaria patients. Currently, the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) is the only validated tool to assess chronic urticaria-specific quality of life.
Objective
To validate and adapt the CU-Q2oL to the Bengali language for its widespread use.
Methods
The CU-Q2oL questionnaire was translated into Bengali. Its internal consistency and reliability were tested by asking 42 chronic urticaria patients to complete this version. They completed the validated Bengali Dermatology Life Quality Index and Urticaria Control test questionnaires, and their scores were correlated with CU-Q2oL score to assess the validity of our Bengali version.
Results
The mean CU-Q2oL score of our patients (mean age 38.41 ± 13.4 years, male: female 29:13) was 48.8 ± 16.5. Domain 4 (sleep problems) was worst affected, followed by domain 1 (pruritus), while domain 2 (swelling) was least affected. We detected an excellent overall internal consistency (Cronbach’s alpha = 0.93) of our version and nearly complete agreement (intra-class correlation coefficient = 0.91) between the test-retest scores. We found a significant positive correlation between the overall CU-Q2oL and Dermatology Life Quality Index scores (rs = 0.53, P = 0.0002), thus implying the validity of our version. Additionally, we noted a significant negative correlation between the overall CU-Q2oL and Urticaria Control test scores (rs = -0.48, P = 0.0007), suggestive of a more severe impairment of quality of life with poorer disease control.
Limitations
Small sample size, observational design and bias in test-retest reliability analysis due to the use of rescue therapy in-between assessment sessions were important limitations of our study.
Conclusion
The Bengali version of CU-Q2oL questionnaire is a valid and reliable tool suitable for both clinical and research use in Bengali speaking chronic urticaria patients.
Keywords
Chronic urticaria quality of life questionnaire (CU-Q2oL)
Bengali
adaptation
validation
Plain Language Summary
Chronic urticaria is a skin condition characterised by transient itchy wheals occurring for at least six weeks. It has a profound impact on the patients’ quality of life due to its on-and-off course. While there are non-specific scales to assess the quality of life in skin disorders in general, the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) is the only validated tool available to specifically assess the quality of life in chronic urticaria patients. The questionnaire was originally developed in Italian and is now available in several other languages. However, its use among the Bengali population was restricted due to the lack of a Bengali version so far. Therefore, we attempted to translate the CU-Q2oL to Bengali language for more widespread use in the Bengali population. On applying our translated version on Bengali-speaking chronic urticaria patients, we found it was well-adapted by the patients. The results were also consistent and reliable when compared to those obtained through other similar scales. Sleep problems and itching were the primary causes of impairment of quality of life in our patients. Thus, we concluded that the Bengali version of the CU-Q2oL is a valid and reliable tool for assessing the quality of life in Bengali-speaking chronic urticaria patients.
Introduction
Chronic urticaria is a disabling skin condition characterised by transient pruritic wheals, with or without angioedema, persisting for at least six weeks.1 Usually it follows a relapsing and remitting course, having a global point prevalence of about 0.5-1%, and an average duration of 3-5 years in adults.2 We can broadly classify chronic urticaria as spontaneous (symptoms occur independently without any definite trigger) and inducible (symptoms occur in response to specific triggers or stimuli).1 Despite having a profound impact on the patients’ quality of life, comparable to triple coronary artery disease and higher than dermatoses such as psoriasis and atopic dermatitis, this aspect is often overlooked in assessing the severity of chronic urticaria.3 Recently, Goncalo et al. have reported the worsening impact of chronic urticaria on individual domains of the health-related quality of life, such as sleep; sexual function; daily life, work and sports activities; family-life and society-interactions, and patients’ performance at school and work.2 The impact on quality of life may interfere with its overall treatment outcome. Thus, quality of life should be assessed routinely in all chronic urticaria patients to evaluate disease burden, in addition to disease activity and control, to optimise therapeutic benefit.
Previously, quality of life was assessed by a speciality-specific questionnaire for all skin disorders called Dermatology Life Quality Index,4 until Baiardini et al. developed a specific questionnaire to assess the quality of life in chronic urticaria patients called the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL).5 The original questionnaire, in the Italian language, makes a detailed assessment of life quality in chronic urticaria patients, comprising 23 items including the physical, emotional, social and practical aspects that characterise this disease. Currently, it is the only validated instrument available to specifically measure the Health-related Quality of life (HRQoL) in chronic urticaria patients.
Over the years, this questionnaire has been translated into several languages including Spanish, German, Greek, Polish, Brazilian-Portuguese, Turkish, Persian, Hebrew and Arabic, and validated for its widespread use in different populations and international studies.6 However, a validated Bengali version is not available, despite Bengali is one of the most widely spoken languages in the world by about 250 million people, and is the 2nd most common language used in India by over 8% of its population.7 Apart from being a major language in India, it is also the official and national language of the Republic of Bangladesh.
The present study aims to (1) evaluate the internal consistency and reliability of the adapted CU-Q2oL questionnaire in the Bengali language, to increase its use among native chronic urticaria patients and (2) to assess its validity by correlating the overall scores obtained in the Bengali version of the CU-Q2oL questionnaire with validated Bengali Urticaria Control test and Dermatology Life Quality Index scores (generic quality of life questionnaire).
Materials and Methods
Study design and data collection
This study was divided into two parts. The first part involved the adaptation and translation of the CU-Q2oL questionnaire in Bengali using the standard methodology for translation and cross-cultural adaptation. For this purpose, we recruited two adult expert translators, two adult public health experts with previous experience in questionnaire adaptation and two independent dermatologists with more than five years of clinical experience. Among the two dermatologists, one acted as a translator, making three translators. All of them were native Bengali speakers with bilingual proficiency. They participated in this study voluntarily after signing written informed consent.
The second part involved a cross-sectional study conducted at the Dermatology out-patient department (OPD) of the College of Medicine and Sagore Dutta Hospital in Kolkata, India across three months (June to August 2021). All participants were adult (age > 18 years) chronic urticaria patients and were recruited after obtaining written informed consent. Acute urticaria (<6 weeks), urticarial vasculitis, angioedema as a solitary symptom, other skin diseases, psychiatric disorders, and any chronic disease with the potential to interfere with the quality of life formed our exclusion criteria. All patients were provided with the self-reportable CU-Q2oL, Dermatology Life Quality Index and Urticaria Control test questionnaires and asked to fill them by themselves, or via an interview with a professional psychologist (illiterate, old-age and/or visually impaired patients) [Day 0]. We obtained the scores along with the socio-demographic and disease parameters. Relevant laboratory investigations were performed when necessary. We advised the patients to return after 2-weeks to complete the CU-Q2oL questionnaire again (re-test) [Day 14]. The study was approved by the Institutional Review Board and conducted as per the WMA Declaration of Helsinki (updated in 2013).
We recruited 56 eligible chronic urticaria patients on day 0, while 42 patients participated in the re-test session on day 14. Thus, the final sample size of our study translated to 42 subjects.
Description of the CU-Q2oL, Dermatology Life Quality Index and Urticaria Control test questionnaires
The original Chronic Urticaria Quality of Life (CU-Q2oL) Questionnaire contains 10 statements and 13 questions with a total of 23 items in the Italian language. The items are categorised into six domains: pruritus (2 items), swelling (2 items), impact on life activities (6 items), sleep problems (5 items), limitations (3 items) and appearance (5 items). Each item is scored on a 5-point Likert scale (0-4) to indicate its intensities over the last 2 weeks. The total summed score is calculated and linearly transformed to a 0-100 score, with the maximum score of 100 indicating the worst HRQoL.5 Currently it is the only validated scale to measure specific quality of life in chronic urticaria (both inducible and spontaneous).
The Dermatology Life Quality Index is a generic HRQoL questionnaire for all dermatologic diseases to enable quick assessment in routine clinical practice. It comprises 10 questions spanning six domains-symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. Each question is scored on a Likert scale (0 for not at all to 3 for very much). The total score ranges from 0 to 30, with higher scores indicating poorer quality of life.4 The Dermatology Life Quality Index has been validated in multiple languages including the Bengali language.8
The Urticaria Control test is the only validated and reliable tool for assessing disease control in both chronic spontaneous and inducible urticaria, with a recall period of four weeks. It comprises four items, each being marked on a 0-4 scale. The total score is obtained by adding all the items, ranging from minimum 0 to maximum 16, with 16 points indicating complete disease control. The Urticaria Control test has been validated in multiple languages including Bengali.9
Translation of the CU-Q2oL questionnaire and further modifications
The translation process is presented in Figure 1. Apart from the original Italian version we also utilised the validated English version of the questionnaire.6 Three expert translators translated the English version (V1.0) with previous experience in adapting health-related questionnaires from English to Bengali. After translating the questionnaire individually, the translators came to a consensus and yielded a single Bengali version of the questionnaire (V1.1). Then this questionnaire was sent to another set of three translators who translated the questionnaire into English and came to a consensus to yield a single English version (V1.2). The conceptual equivalence between the original questionnaire (V1.0) and the back-translated version (V1.2) was checked and found to be of accepted equivalence. It was checked by two health care experts with previous experience with two Bengali questionnaire adaptations. The questionnaire was found to be conceptually equivalent and the version was marked as V1.3. This questionnaire was used to conduct a cognitive interview with 10 patients suffering from chronic urticaria. Their understanding of the question, and their suggestions on addition, and modification of the questionnaire were noted for further amendment of the questionnaire. We added two synonyms of words in parenthesis for a better understanding of the questionnaire. As it was only the addition of the synonyms, keeping other questions the same as previous, the version (V1.4) was not again sent for review to the translators. This version was final for the clinical test.

- Process of adaptation of the questionnaire from English to Bengali. Footnote for reviewers: The infographic was created by HM in Microsoft Word 2010® for this manuscript only
Based on the feedback of our cognitive interview session, we have made slight additional modifications. The original CU-Q2oL questionnaire contains 10 statements and 13 questions with a total of 23 items with five-point Likert-type response options. We have changed the 13 questions to statements for a uniform type of items keeping the response options the same. The logic behind converting the questions into statements is described below with an example. If a question is asked as “Do you have difficulty falling asleep?,” the answer may be-“yes, I have difficulty” or “No, I don’t have difficulty.” However, if the statement is “I have difficulty in falling asleep.,” the response may be ranging from “Not at all” to “very much.” Furthermore, the original questionnaire does not have any instruction section on how to fill the questionnaire up. Hence, we added a section of text on how to mark the responses on the questionnaire. This would help to make the questionnaire more suitable for self-administration. In addition to instructional text, a figure is also used to show how to mark the response. This would further ease the understanding of how to respond.
Assessment of the internal consistency, reliability and validity of the Bengali CU-Q2oL
We calculated the Cronbach alpha coefficient to assess the internal consistency of the Bengali CU-Q2oL. Based on existing literature, we considered a coefficient <0.6 as unacceptable, 0.6-0.65 undesirable, 0.65-0.7 minimally acceptable, 0.7-0.8 respectable, 0.8-0.9 excellent and >0.9 excessive.10
Test-retest reliability was assessed across 2 visits 14-day apart (D0 and D14) to evaluate the consistency of Bengali CU-Q2oL by calculating the intra-class correlation coefficient. Intra-class correlation coefficient values <0.4, 0.4-0.75 and >0.75 indicate poor, acceptable and strong reliability, respectively.10
We used the validated Bengali Dermatology Life Quality Index and Urticaria Control test questionnaires to determine the overall HRQoL and disease control of our patients. The scores obtained were statistically correlated with the score of Bengali CU-Q2oL (test-scale) by Spearman’s correlation to assess the validity of this scale, as construct validity of a test scale is indicated by the correlation with other standard scales. We have considered Spearman correlation coefficients of <0.3, 0.3-0.6 and >0.6 to indicate weak, moderate and strong correlations, respectively.10
Statistical analysis
Continuous variables were expressed as mean and standard deviation while categorical data were presented as percentages. Categorical data were statistically analysed by either the Binomial test or the Chi-square test, while Mann-Whitney U and Kruskal-Wallis test with the Bonferroni correction method (α = 0.05/6 = 0.0083) were used for continuous data as appropriate. The survey response obtained in the test and re-test phases were coded on Microsoft Excel 2010 (Microsoft Inc., USA) spreadsheet. We analysed data in IBM SPSS Statistics 20 (IBM, Chicago, USA) and GraphPad Prism 6.01 (GraphPad Software, CA, USA). Spearman correlation was used to obtain a correlation between the Bengali CU-Q2oL, Dermatology Life Quality Index and Urticaria Control test scores. Internal consistency was tested by Cronbach’s alpha and test-retest reliability was tested by intra-class correlation coefficient. For all the statistical tests, a P < 0.05 was considered statistically significant.
Results
Patient characteristics
The mean age of our patients (n = 42) was 38.41 ± 13.38 years (male: female 29:13). The mean duration of chronic urticaria was 8.53 ± 1.55 weeks. Ten (23.8%) and 3 (7.1%, 2- symptomatic dermographism,1- delayed pressure urticaria) patients had concomitant angioedema and physical urticaria respectively. All patients were receiving second-generation antihistamines as their treatment. Table 1 highlights the demographic details of our patients.
| Age (years) (Mean ± SD) | 38.41 ± 13.38 | ||
| Duration (weeks) (Mean ± SD) | 8.53 ± 1.55 | ||
| Parameters | Category | Number (%) | P |
| Gender | Male | 29 (69.1) | *0.02 |
| Female | 13 (31.0) | ||
| Marital status | Unmarried | 10 (23.9) | †<0.0001 |
| Married | 28 (66.7) | ||
| Divorced/widow/widower | 4 (9.5) | ||
| Education | Primary | 2 (4.8) | †<0.0001 |
| Secondary | 4 (9.5) | ||
| Higher Secondary | 13 (31.0) | ||
| Graduation and above | 23 (54.7) | ||
| Residence | Urban | 30 (71.4) | *0.008 |
| Rural | 12 (28.6) | ||
| Employment | Employed | 23 (54.8) | *0.64 |
| Unemployed/home-maker | 19 (45.2) |
SD: Standard deviation, *P value of Binomial test, †P value of Chi-square test
None of our patients complained of any difficulty in understanding or answering the Bengali CU-Q2oL questionnaire. The average time taken to complete the CU-Q2oL, Dermatology Life Quality Index and UCT questionnaires were 8-10 minutes, 5-6 minutes and 3-4 minutes respectively.
CU-Q2oL Questionnaire
The CU-Q2oL was adapted and translated to the Bengali language with forwarding and backward translation methods and checking the conceptual equivalence. An editable Bengali version such as Microsoft Word or Portable Document Format version can be obtained from the corresponding author. Domain wise distribution of question numbers is presented in Table 2.
| Domains | Theme | Item number |
|---|---|---|
| Domain 1 | Pruritus | 1,2 |
| Domain 2 | Swelling | 3,4 |
| Domain 3 | Impact on life activities | 5,6,8-10 |
| Domain 4 | Sleep problems | 7, 11-14 |
| Domain 5 | Limits | 17,20-22 |
| Domain 6 | Discomfort | 15,16,18,19,23 |
Quality of life scores
In our patients the total CU-Q2oL score ranged from 22.6 to 74.8, mean ± SD being 48.8 ± 16.5 (95% confidence interval 43.7-53.9) and median (IQR) being 50.4 (32.2-57.4) [Table 3, Figure 2]. Domain 4 (sleep problems) was most severely affected (56.48 ± 23.74), followed by domain 1 (pruritus) [55.91 ± 19.33], while domain 2 (swelling) [35.95 ± 17.39] was least affected [Figure 3]. Inter-domain analysis revealed a statistically significant difference between domain 1 and domains 2, 3 and 5; and domain 2 and domains 3,4 and 6 (all P ’s <0.05, K-W test with Bonferroni correction). Overall, females showed a higher score compared to males (49.77 ± 13.86 vs. 48.43 ± 17.81, P = 0.8). Interestingly, both age (rs = -0.4, P = 0.003) and disease duration (rs = -0.3, P = 0.02) showed a statistically significant negative correlation with total CU-Q2oL score.
| Scale | Domains | Male (n = 29) | Female (n = 13) | U, P | Overall (n = 42) |
|---|---|---|---|---|---|
| Bengali CU-Q2oL | Domain 1 | 57.59 ± 16.18 | 60.77 ± 13.2 | 173, 0.67 | 55.91 ± 19.33 |
| Domain 2 | 35.17 ± 18.05 | 37.69 ± 16.41 | 164.5, 0.49 | 35.95 ± 17.39 | |
| Domain 3 | 47.31 ± 20.81 | 47.08 ± 18.41 | 187, 0.97 | 47.24 ± 19.88 | |
| Domain 4 | 55.17 ± 25.44 | 59.38 ± 20.06 | 167, 0.57 | 56.48 ± 23.74 | |
| Domain 5 | 43.62 ± 22.48 | 42.31 ± 17.87 | 181, 0.84 | 43.21 ± 20.95 | |
| Domain 6 | 48.28 ± 20.42 | 49.23 ± 17.62 | 187, 0.97 | 48.57 ± 19.39 | |
| Overall | 48.43 ± 17.81 | 49.77 ± 13.86 | 180.5, 0.84 | 48.84 ± 16.53 | |
| Bengali DLQI | Overall | 11.45 ± 5.5 | 10.77 ± 5.4 | 173.5, 0.69 | 11.24 ± 5.41 |
| Bengali UCT | Overall | 10.1 ± 3.59 | 10.46 ± 3.4 | 177, 0.76 | 10.21 ± 3.5 |
CU-Q2oL: Chronic Urticaria Quality of Life Questionnaire, DLQI: Dermatology Life Quality Index, UCT: Urticaria Control Test, U: Mann-Whitney U value
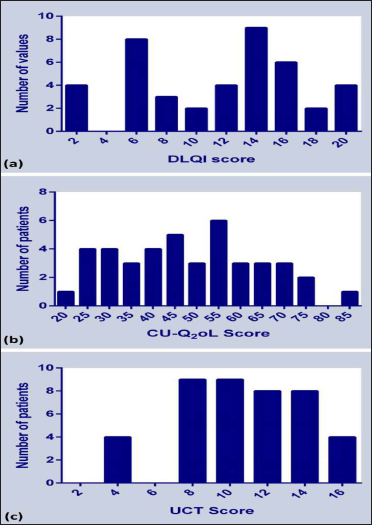
- Frequency distribution of overall score obtained from (a) Bengali DLQI, (b) Bengali CU-Q2oL and (c) Bengali UCT
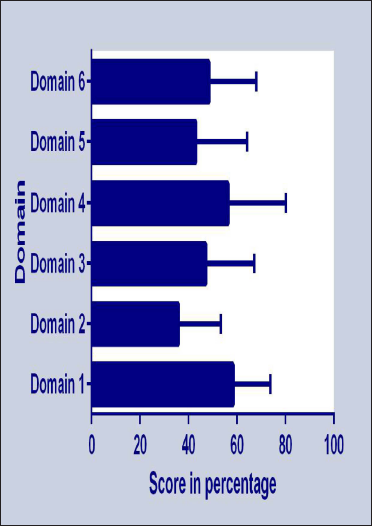
- Individual domain-wise scores (in percentage) of the Bengali CU-Q2oL questionnaire
The overall mean ± SD Dermatology Life Quality Index score of our subjects was 11.24 ± 5.41 (95% confidence interval 9.5-12.9; range 2-20), males showing a higher score than females, although statistically comparable (P = 0.7) [Table 3 and Figure 2]. Most patients (71.4%, 30/42) reported ‘very large’ effect on their quality of life, followed by ‘small effect’ (19%, 8/42) and a ‘moderate effect’ (9.5%, 4/42).
The overall Urticaria Control test mean ± SD score of our patients was 10.21 ± 3.5 (95% confidence interval 9.1-11.3; range 3-16), comparable across genders [Table 3 and Figure 2]. Interestingly, equal proportion of patients (50%, 21/42) had poorly controlled (Urticaria Control test < 12) and well-controlled urticaria (Urticaria Control test ≥ 12) each.
Internal consistency and reliability of the Bengali CU-Q2oL questionnaire
The internal consistency of the questionnaire and test-re-test reliability according to the domain is highlighted in Table 4. We noted an excellent overall internal consistency (Cronbach’s alpha = 0.93) of the questionnaire and nearly complete agreement (intra-class correlation coefficient = 0.91) between the test and re-test score. Hence, the adapted Bengali CU-Q2oL questionnaire has good psychometric properties for possible further application in Bengali-speaking chronic urticaria patients to assess disease burden.
| Bengali CU-Q2oL | Chronbach’s alpha | Test-retest ICC |
|---|---|---|
| Domain 1 | 0.15 | 0.86 |
| Domain 2 | 0.24 | 0.81 |
| Domain 3 | 0.76 | 0.88 |
| Domain 4 | 0.88 | 0.79 |
| Domain 5 | 0.82 | 0.89 |
| Domain 6 | 0.85 | 0.83 |
| Overall | 0.93 | 0.91 |
CU-Q2oL: Chronic Urticaria Quality of Life Questionnaire
All the tests were with 42 research participants. Chronbach’s alpha indicate internal consistency and Intraclass correlation coefficient (ICC) indicates test-retest reliability
Validity of the Bengali CU-Q2oL questionnaire
We found a significant positive correlation (rs = 0.53, P = 0.0002) between the overall scores of Bengali Dermatology Life Quality Index and Bengali CU-Q2oL scales [Figure 4a]. The positive correlation establishes the validity of the Bengali CU-Q2oL to determine the chronic urticaria-specific quality of life. In contrast, we found a significant negative correlation (rs = -0.48, P = 0.0007) between the Bengali Urticaria Control test (a higher score indicates well-controlled disease) and Bengali CU-Q2oL scores [Figure 4b]. We have also correlated the domain wise score of Bengali CU-Q2oL with total Dermatology Life Quality Index and Urticaria Control test scores. Except Domain 1 and 2, all other domains showed a statistically significant positive correlation with the total Bengali Dermatology Life Quality Index score and a negative correlation with the overall Bengali Urticaria Control test score [Table 5].
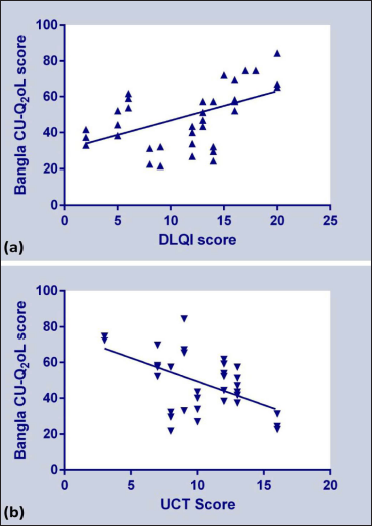
- Correlation of overall score obtained from: (a) Bengali DLQI and, Bengali CU-Q2oL questionnaires, and (b) Bengali UCT and Bengali CU-Q2oL questionnaires
| Domains | DLQI | UCT | ||
|---|---|---|---|---|
| Domains | rs | P | rs | P |
| Domain 1 | 0.22 | 0.08 | −0.14 | 0.18 |
| Domain 2 | 0.23 | 0.07 | −0.19 | 0.11 |
| Domain 3 | 0.56 | <0.0001 | −0.44 | 0.002 |
| Domain 4 | 0.64 | <0.0001 | −0.55 | <0.0001 |
| Domain 5 | 0.36 | 0.01 | −0.32 | 0.02 |
| Domain 6 | 0.27 | 0.04 | −0.29 | 0.03 |
| Overall | 0.53 | 0.0002 | −0.48 | 0.0007 |
CU-Q2oL: Chronic Urticaria Quality of Life Questionnaire,
DLQI: Dermatology Life Quality Index, UCT: Urticaria Control Test,
n: number, rs: Spearman correlation coefficient
Discussion
The recently updated EAACI/GA2LEN/ EuroGuiderm/ APAACI 2021 guideline recommends routine evaluation of Patient-Reported Outcomes including HRQoL in all chronic urticaria patients for better treatment outcome.11 Currently, CU-Q2oL is the only disease-specific instrument available to assess the quality of life in chronic urticaria patients and is preferred to generic questionnaires as the latter are less likely to identify minor but clinically important disease-centric changes.6 We need to adapt, translate and validate this questionnaire in multiple languages to broaden its use and acceptability.
Our Bengali version of the CU-Q2oL was self-administered by the patients with very few incomplete responses in the cognitive interviews, thus implying its easy comprehensibility. Similar to the original questionnaire designed by Baiardini et al. we retained all six domains with slight modifications5. The German12, Spanish,13 Polish,14 Turkish,15 Persian16 and Hebrew6 versions have also retained the original six domains, while the Arabic10 and Brazilian-Portuguese17 versions modified it into a 5-scale and 3-scale structure respectively.
In our patients, the most affected domain was ‘sleep problems’ (domain 4) followed by ‘Pruritus’ domain 1). In most other populations, ‘Itching/pruritus’ has been maximally impaired,6,12-15 while the Hebrew version recorded sleep and concentration sub-scale to be the second-highest affected factor.6 Our finding is consistent with several recent reports highlighting sleep disturbances in chronic urticaria patients.18 However, this aspect often remains overlooked in the present setting and we need more real-world studies to explore this association. Swelling (domain 2) was least affected in our patients, consistent with the Arabic version,10 while the Hebrew version revealed ‘medication side effects to be least affected sub-scale.6 This may be explained by the reluctance of our patients to consult a dermatologist unless there is a significant impairment in their daily activities.
Concerning internal consistency, our Bengali version of the CU-Q2oL depicted an excellent overall Cronbach alpha value of 0.93, much higher than the recommended threshold of 0.70. All our individual domains also recorded a score >0.70, except domains 1 (pruritus) and 2 (swelling). This may be attributed to the fewer number of questions in domains 1 and 2 (2 questions each), which may have contributed to lower scores.19 In terms of test-retest reliability, our version performed remarkably well with an overall intra-class correlation coefficient value of 0.91 and all individual domains having intra-class correlation coefficient values above 0.75. Our findings are comparable to or better than the corresponding results for the original questionnaire as well as its other translations.5,6,10,16,17,20
We noted a significant moderate positive correlation (rs = 0.53, P = 0.0002) between the overall Bengali CU-Q2oL score and speciality-specific quality of life (Bengali Dermatology Life Quality Index score), thereby establishing the validity of our version as an effective tool for chronic urticaria-specific quality of life assessment in this population. Notably, a comparatively stronger correlation with Dermatology Life Quality Index scores was found in the Israeli and Arabic studies.6,10 These studies also showed a positive correlation of Urticaria Activity Score with CU-Q2oL score, thereby demonstrating increased disease severity to be a predictor of high CU-Q2oL scores. However, we compared the Urticaria Control test score with the overall CU-Q2oL score and found a significant negative correlation (rs = -0.48, P = 0.0007) between them. Therefore, the poorer the disease control (indicated by lower Urticaria Control test scores), the higher the quality of life impairment by the Cu2QoL questionnaire (higher score). Interestingly, CU-Q2oL showed a higher negative correlation with the Urticaria Control test score, compared to the generic Dermatology Life Quality Index score, suggesting that it should be the preferred scale in future chronic urticaria studies.
Additionally, we found that except for domains 1 (pruritus) and 2 (swelling), all other domains of the Bengali CU-Q2oL showed a statistically significant positive correlation with Bengali Dermatology Life Quality Index and negative correlation with Urticaria Control test score, with domain 4 (sleep problems) showing maximum degree of correlation in both cases. The Arabic study showed similar domain-wise correlations of CU-Q2oL with total Dermatology Life Quality Index score.10
In our patients, the mean (SD) CU-Q2oL score was 48.8±16.5, higher than Israeli patients (41 ± 21.7),6 Arabic patients (39.6 ± 14.2)10 and much higher than Spanish13 and Turkish15 patients, who reported a mean score around 25. On the contrary, Persian patients showed a worse CU-Q2oL score (58.43 ± 16.83).16 Thus, a higher CU-Q2oL score in our study indicates worse life-quality of Bengali speaking chronic urticaria patients, compared to most other populations, and so we need more studies to explore the possible reasons. In our study, females reported a higher CU-Q2oL score, compared to males, consistent with most authors.6,12 This may be attributed to the higher sensitivity of women to chronic urticaria symptoms, especially when exposed areas of the body are involved. We found age and disease duration to be significantly associated with total CU-Q2oL score while most authors have not reported any significant association with age, duration or gender.16
Limitations
Our study was limited by its small sample size, and observational design, therefore lacking the ability to design responsiveness, e.g., before and after therapy, and lack of objective criteria such as laboratory investigations to assess the variations in the quality of life. Another important limitation was the use of rescue antihistamines in some patients in-between the test and re-test sessions, although ideally the test-retest reliability analysis should be conducted in patients without any variation in disease activity. We also failed to correlate individual domains of the CU-Q2oL questionnaire with individual domains of the Dermatology Life Quality Index scale, as their psychometric properties are different.
Conclusion
Our Bengali adaptation of the CU-Q2oL showed good psychometric properties, comparable to other language versions. It may be considered suitable for use among Bengali speaking patients with chronic urticaria in both research and clinical settings, to compare the corresponding scores with patients who have validated CU-Q2oL in their maternal language. These authors recommend further studies to evaluate the minimal important difference between the validated Bengali version of the CU-Q2oL questionnaire and explore other psychometric properties of this tool.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
- [CrossRef] [PubMed] [Google Scholar]
- The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226-36.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of the impactof chronic urticaria, psoriasis and atopicdermatitis on the quality of life. Br J Dermatol. 2005;152:289-95.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatology Life Quality Index (DLQI)-a simplepractical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-6.
- [CrossRef] [PubMed] [Google Scholar]
- A new tool to evaluate the impact of chronic urticaria on quality of life: Chronic urticaria quality of life questionnaire (CU-Q2oL) Allergy. 2005;60:1073-8.
- [CrossRef] [PubMed] [Google Scholar]
- Adaptation and validation of the Israeli version of the Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) Isr Med Assoc J. 2016;18:461-5.
- [PubMed] [Google Scholar]
- Office of the Registrar General & Census Commissioner, India. Available from: https://censusindia.gov.in/2011Census/Language_MTs.html [Last accessed on 20.10.2021, 21:25 hours]
- [Google Scholar]
- Validation of Bangla Dermatology Life Quality Index among patients with psoriasis. J Pak Assoc Dermatol. 2021;31:165-72.
- [Google Scholar]
- Development and validation of the Urticaria Control Test: A patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014;133:1365-72.
- [CrossRef] [PubMed] [Google Scholar]
- The Arabic urticaria activity score and Chronic Urticaria Quality of Life Questionnaire: Validation and correlations. Int J Dermatol. 2020;59:893-901.
- [CrossRef] [PubMed] [Google Scholar]
- The International EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 77:734-66.
- [CrossRef] [Google Scholar]
- The German version of the chronic urticaria quality-of-life questionnaire (CU-Q2oL): Factor analysis, validation and initial clinical findings. Allergy. 2009;64:927-36.
- [CrossRef] [PubMed] [Google Scholar]
- Adaptation and validationof the Spanish version of the chronic urticarial quality of life questionnaire (CU-Q2oL) J Investig Allergol Clin Immunol. 2008;18:426-32.
- [PubMed] [Google Scholar]
- Adaptation and initial results of the polish version of the GA2LEN chronicurticaria quality of life questionnaire (CU-Q2oL) J Dermatol Sci. 2011;62:36-41.
- [CrossRef] [PubMed] [Google Scholar]
- Turkish version of the chronic urticaria quality of life questionnaire: Cultural adaptation, assessment of reliability and validity. Acta Derm Venereol. 2012;92:419-25.
- [CrossRef] [PubMed] [Google Scholar]
- The Persian version of the Chronic Urticaria Quality of Life Questionnaire: Factor analysis, validation, and initial clinical findings. Iran J Allergy Asthma Immunol. 2014;13:278-85.
- [PubMed] [Google Scholar]
- Cross-cultural adaptation of the Brazilian-Portuguese version of the chronic urticaria quality-of-life questionnaire - CU-Q2oL. Allergy. 2011;66:1487-93.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep disturbance in patients with urticaria and atopic dermatitis: An underestimated burden. Acta Derm Venereol. 2020;100:adv00073.
- [CrossRef] [PubMed] [Google Scholar]
- Minimal clinical important difference (MCID) of the Thai Chronic Urticaria Quality of Life Questionnaire (CU-Q2oL) Asian Pac J Allergy Immunol. 2016;34:137-45.
- [CrossRef] [PubMed] [Google Scholar]






