Translate this page into:
Aetiology, pathogenesis and management of neuropathic itch: A narrative review with recent updates
Corresponding author: Dr. Indrashis Podder, Department of Dermatology, College of Medicine & Sagore Dutta Hospital, Kolkata, West Bengal, India. ipodder88@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Poddar S, Mondal H, Podder I. Aetiology, pathogenesis and management of neuropathic itch: A narrative review with recent updates. Indian J Dermatol Venereol Leprol. 2024;90:5-18. doi: 10.25259/IJDVL_846_2022
Abstract
Neuropathic itch is a relatively common yet under-reported cause of systemic pruritus. It is a debilitating condition often associated with pain, which impairs the patient’s quality of life. Although much literature exists about renal and hepatic pruritus, there is a dearth of information and awareness about neuropathic itch. The pathogenesis of neuropathic itch is complex and can result from an insult at any point along the itch pathway, ranging from the peripheral receptors and nerves until the brain. There are several causes of neuropathic itch, many of which do not produce any skin lesions and are thus, often missed. A detailed history and clinical examination are necessary for the diagnosis, while laboratory and radiologic investigations may be needed in select cases. Several therapeutic strategies currently exist involving both non-pharmacological and pharmacological measures, the latter including topical, systemic, and invasive options. Further research is ongoing to clarify its pathogenesis and to design newer targeted therapies with minimal adverse effects. This narrative review highlights the current understanding of this condition, focusing on its causes, pathogenesis, diagnosis, and management, along with newer investigational drugs.
Keywords
Systemic
pruritus
neuropathic
itch
Introduction
Pruritus or itch is a distinct sensory modality prompting a desire to scratch the area of perception. This unpleasant sensation is very common and transmitted by the peripheral and central nervous systems to be subsequently encoded by distinct brain neurons.1 The same pathway also regulates other important sensations like pain, so often pain and pruritus coexist.1 Frequently, the itch becomes chronic and severely worsens the patient’s quality of life, occasionally culminating in depression, anxiety and even suicidal ideation.2
Damage to the itch pathway at any level, starting from peripheral receptors in the skin to the effector areas in the brain, may result in pruritus. Pathophysiologically, the itch can be categorised as dermatological, systemic, psychologic and neuropathic. Amongst them, neuropathic itch is an emerging field of interest and remains poorly understood. It occurs primarily due to insults to the peripheral and/or central nervous system. Clinically, neuropathic itch is confusing as the source of itching is often different from where it is perceived. Itching may occur in the absence of any pruritogenic stimuli or systemic disorder, or as an inappropriate or exaggerated response to minor external stimuli, termed allokinesis and hyperkinesis, respectively.1 Another related condition, which frequently coexists with neuropathic itch, is neuropathic pain, as seen in herpes zoster. Although neuropathic pain has become a focus for drug development, the same cannot be said about neuropathic itch, which lacks any approved therapy till date.2 In neuropathic itch, there is a complex interplay between excitatory and inhibitory interneurons, and several inflammatory mediators are responsible, in addition to histamine, the canonical itch mediator. Thus, these patients often fail to improve with conventional therapies like systemic corticosteroids or antihistamines, and the underlying cause needs to be addressed to obtain relief.
So, it is vital to understand the detailed neuroanatomy, pathophysiology and aetiology of neuropathic itch to provide optimum treatment to these patients. Several off-label treatment options currently exist for neuropathic itch; however, information is scattered and not easily accessible to treating physicians. Other important unmet needs in neuropathic itch include a lack of standardised case definitions to diagnose and differentiate the sub-forms of neuropathic itch and lack of validated questionnaires to track symptoms. This review highlights the current understanding about the pathophysiology of neuropathic itch, its varied etiologies and clinical presentations, and recent updates regarding its diagnosis and management. These authors hope that the present review will benefit treating physicians in providing optimum care to patients suffering from neuropathic itch and help improve their quality of life.
Literature Search Methods
We conducted a comprehensive literature search across multiple databases (PubMed, EMBASE, MEDLINE and Google scholar) using the keywords (alone and in combination), which included MeSH items as well as non-MeSH terms such as “neuropathic”, “neurologic”, and “itch”, “pruritus”. We included all types of articles, while those not in the English language formed our exclusion criteria. The references of relevant articles were further scanned for more articles.
Pathophysiology and Classification of Itch
Peripheral itch originates when cutaneous nerve terminals are stimulated by damage to the skin, pruritogens, or light tactile stimulation.3 Based on the peripheral stimulus, the itch may be classified as a chemical or mechanical itch. Chemical itch occurs due to the release of pruritogens by damaged or inflamed skin, which can be further subdivided into histamine-dependent and histamine-independent varieties3. In contrast, the mechanical itch may be induced by gentle tactile stimuli such as insects crawling on the skin and may be considered dysesthesia in patients suffering from chronic itch. Interestingly, the pathomechanisms of mechanical itch apparently differ from the chemical itch and possibly involve central sensitization.4 A schematic diagram of the chemical and mechanical itch pathway is presented in Figure 1.
Based on aetiology, the International Forum for the Study of Itch has identified 6 categories- dermatological, systemic, neurological, psychogenic, mixed and “other” [Figure 2].5 Neurological pruritus can be further subdivided into two types—neuropathic itch (caused by neuronal or glial damage), and neurogenic itch (triggered by endogenous opioids or other neuro-stimulatory substances that stimulate itch neurons, in the absence of neuronal or glial damage, as seen in renal or hepatic pruritus).6 Based on duration, the itch may be called acute (up to 6 weeks) or chronic (persists for 6 or more weeks). The recent International Forum for the Study of Itch classification also categorises itch clinically into three groups, based on the presence or absence of primary or secondary skin lesions - Group I (itching in the presence of inflammatory, infectious, autoimmune skin diseases, adverse drug reactions, cutaneous lymphoma, and pregnancy dermatoses), Group II (itching without skin lesions/non-diseased skin referring to the systemic causes of pruritus such as endocrine, metabolic, psychiatric, neoplastic and neuropathic disorders) and Group III (itching with chronic scratch lesions, for example, prurigo nodularis and lichen simplex chronicus, clinically characterised by the presence of excoriated papules, nodules, lichenification and crusting). Notably, in chronic pruritic conditions, multiple causes may coexist.7

- Schematic diagram to show the different itch and pain transmission pathways.
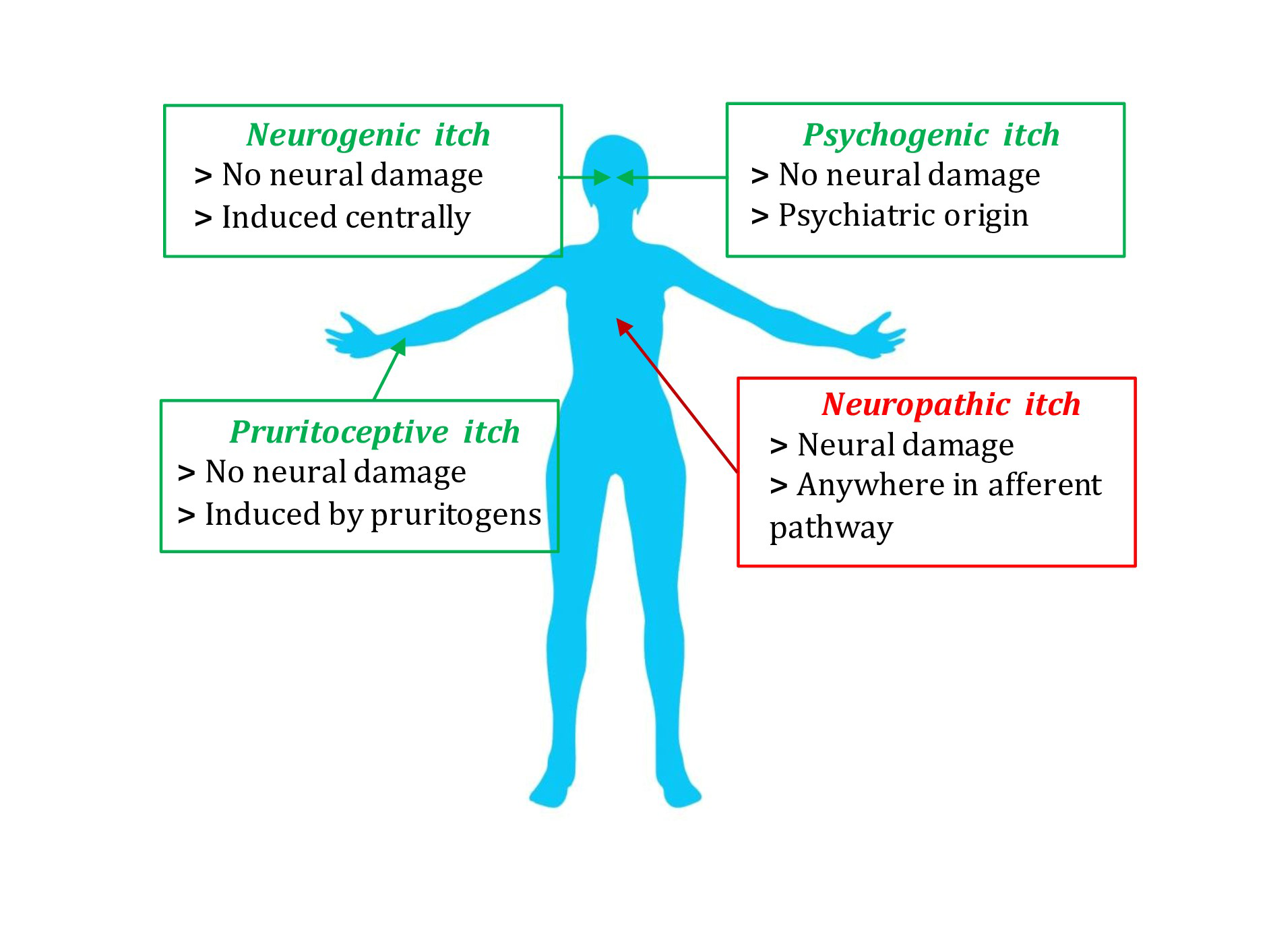
- Etiological classification of chronic pruritus.
The present article focuses on various pruritic conditions associated with damage or insult to the nervous system, that is, neuropathic pruritus. The insult to the nervous system can occur at any level, extending from the receptors and afferent pathway of an itch to the level of the brain. The next section briefly elaborates on the pathophysiology of neuropathic itch in connection with its pathway from the skin to the brain.
Itch Receptors and Afferent Pathway
Nerve injury or stimulation of specific itch receptors (pruriceptors) release several pro-inflammatory mediators such as interleukin-31, interleukin-33, and lysophosphatidic acid that may stimulate the free nerve endings and induce itch.8 The understanding of the sensory nerves carrying the itch sensation to the spinal cord is evolving. Animal and human experiments indicate that itch stimuli are carried by unmyelinated C-fibres. The role of these C-fibres is further corroborated by the generation of evoked itch response following in vivo administration of exogenous histamine in experimental animals or humans.9 Additionally, when capsaicin (an ingredient in chilli that induces burning pain) is applied repeatedly to C-fibres, it desensitises the neuron, which subsequently reduces histamine-induced itch, thus highlighting the close interlink between itch and pain pathways.10 It is also known that itch signals may be transmitted by more than one subtype of C-fibres.10 Chloroquine, an antimalarial drug, has been reported to induce itch in experimental animals by interacting with MAS-related GPR A3 (a G-protein coupled receptor) receptor on C-fibres.11 Interestingly, MAS-related GPR A3 also expresses receptors for histamine (H1) and capsaicin (transient receptor potential, vanilloid 1).3
Role in generating itch
Axonal damage by any systemic disease like diabetes mellitus, vitamin deficiencies, plasma-cell dyscrasias and toxin exposure may interrupt the transmission of itch signals towards the spinal cord and result in small-fibre polyneuropathy.10 Sjogren’s syndrome, an autoimmune disorder, may cause ganglionopathy and plexopathies that may eventually cause neuropathic itch. Shingles may cause postherpetic neuralgic pain that may be associated with postherpetic itch.
Spinal Cord
The sensory afferent unmyelinated C-nerve fibres transduce an itch signal from the skin to the spinal cord via the dorsal root ganglion. Within the spinal cord, these neurons synapse with interneurons and then ascend via the ascending pathways.
For the transduction of chemical itch, afferent neurons synapse at lamina I and II in the spinal cord gray matter. Here, neurons expressing gastrin-releasing peptide receptors are the primary stimulatory neurons, which are present in both the dorsal root ganglion and spinal cord. Ablation of these neurons has resulted in reduced itching in experimental animals without affecting the pain sensation. Thus, these gastrin-releasing peptide receptor expressing neurons may be selectively involved in itch processing.12 Additionally, another set of neurons expressing natriuretic polypeptide b receptor and secreting natriuretic polypeptide b also take part in itch processing.8 Some neurons express both gastrin-releasing peptide and natriuretic polypeptide b simultaneously. Furthermore, somatostatin also influences itch transmission in the spinal cord. Secreted somatostatin binds to its receptor–somatostatin receptor subtype 2A, and disinhibits gastrin-releasing peptide-secreting neurons by reducing the secretion of dynorphin neurons.13
Experimental animals lacking atonal-related transcription factor Bhlhb5 exhibit scratching behaviour and increased response to common pruritogens.14 These animals also demonstrate loss of interneuron that expresses galanin and neuronal nitric oxide synthase. This suggests that Bhlhb5 is crucial for the survival of galanin or neuronal nitric oxide synthase expressing interneurons, which play an important role in gating the chemical itch. Further experiments showed that spinal neurons expressing Bhlhb5 co-express dynorphin and act via κ-opioid signalling. Neurons expressing dynorphin and galanin coexist and play an important role in itch suppression.
Inhibitory neurons at the spinal cord level secrete gamma-aminobutyric acid and/or glycine. Activation of gamma-aminobutyric acidergic or glycinergic neurons, either by drugs or chemo-genetic methods, significantly reduces itching.
Role in generating itch
Spinal cord injury, especially to the lamina involved in the transmission of itch signal, may cause contralateral neuropathic itch, as the crossing of the spinothalamic tract occurs at the same level. Brown-Sequard syndrome, syringomyelia and intramedullary cavernous haemangioma can cause itching in the contralateral half of the body.15 Additionally, the ectopic firing of neurons induced by hemosiderin-laden phagocytes in intramedullary cavernous haemangioma may cause severe intractable itch.
Ascending Pathways in the Spinal Cord
Transmission of itch signals from the level of the spinal cord to the brain is probably mediated by the same tract responsible for pain transmission. The spinothalamic, spino-parabrachial and trigeminal-ophthalmic tracts are activated during the transmission of itch signals.16 However, variation in the type of itch stimuli alters the set of effector neurons. Neurons expressing neurokinin 1 receptor are possibly responsible for transmitting the chemical itch signal, as mechanical itch remains unaffected when neurokinin 1 receptor expressing neurons are ablated in experimental animals.17 Thus, chemical and mechanical itch are carried by a different set of ascending tracts in the spinal cord. Furthermore, histamine-dependent and independent chemical itch use separate ascending fibres. The fibres that reach the parabrachial nucleus receive input from gastrin-releasing peptide receptor expressing neurons.
Role in generating itch
Compression of the tract carrying itch signals at the level of the spinal cord and cranial nerve may cause neuropathic itch. Some common causes of compression at the spinal cord level include traumatic and other causes of radiculopathy like neoplastic infiltration, granulomas, diabetic truncal radiculopathy, osteoarthritis and acute swelling of nerve by viral infection. Any damage to the trigeminal nerve (a cranial nerve) caused by stroke, tumour, abscess, multiple sclerosis or Sjogren syndrome may present as a trigeminal trophic syndrome. In such patients, itching commonly involves the nasal ala, cheek, and frontal scalp, consistent with the innervation of this nerve. Similar to the trigeminal nerve, somatosensory axons of the facial nerve, glossopharyngeal, and vagus nerves can also cause neuropathic itch. Involvement of the somatosensory part of the facial nerve may cause itching in the outer ear canal and pre and postauricular areas. Glossopharyngeal pruritus may occur in the throat or behind the angle of the jaw. Vagus nerve (e.g., larynx, pharynx, part of the pinna, ear canal) involvement usually causes mucosal and throat itching, often associated with chronic cough. Multiple sclerosis may present with acute, symmetrically distributed, segmental onset of itch, which may be explained by the involvement of the spinothalamic tract while passing through the cervical intramedullary canal.
Brain
The brain is the highest centre for generating an itch sensation. Two areas play the most important role—the thalamus and para brachial nucleus. The ascending fibres project into these two important relay centres.
The thalamus acts as the relay centre for the majority of sensory information. The spinothalamic tracts project primarily on the ventrobasal and posterior thalamus. The para brachial nucleus also serves as an important relay centre for itch, as demonstrated by the strong activation of the para brachial nucleus during histamine and chloroquine-evoked itch.3 The glutamate transporter (Vglut2) is crucial for itch processing. The para brachial nucleus sends projections to the amygdala, and its involvement in itch processing is corroborated by reduced pruritus on infusing the amygdala with an inhibitory gamma-aminobutyric acid agonist. The involvement of the amygdala, along with the periaqueductal gray matter, is responsible for the emotional component of itch.18
Human brain imaging with positron emission tomography scans and functional magnetic resonance imaging has revealed the involvement of other brain areas in itch processing, in addition to the thalamus, such as both primary somatosensory cortex (S1) and secondary somatosensory cortex (S2), prefrontal cortex, insular cortex, parietal cortex, anterior cingulate cortex and premotor and motor cortex.19 The activation of the premotor and motor area results in the planning and execution of scratching to get rid of itching. The ventral tegmental area is associated with the pleasure obtained by scratching the itchy area. Both human and animal studies suggest an important role for dopaminergic neurons of the ventral tegmental area in the itch-scratch cycle.20 Activation of the insular cortex and prefrontal cortex (particularly the cingulate cortex) is possibly responsible for increased stress, anxiety and mood disorders in chronic pruritic disorders, which subsequently worsens the quality of life of these patients.21 Interestingly, human studies suggest that brain areas activated during histamine-dependent and independent pruritus are different. Although the exact neural mechanism for itch remains unclear, it is now believed that chronic itch, including psychogenic itch, pruritus ani or vulvar pruritus, may occur due to central sensitization and loss of descending control. Such patients develop an altered pruriceptive function (“pruriplastic pruritus”) and may respond better to centrally targeted therapies rather than peripheral or symptomatic treatment options.22 Recent researchers have suggested that neural and synaptic plasticity may be responsible for the close association between chronic pain and chronic itch.23
Role in generating itch
Any brain disorder affecting the itch processing centres may produce a neuropathic itch. Ischemic damage to the cerebellum may cause histamine-induced itch, while stroke involving the thalamus and adjacent areas or injury to the internal capsule may cause itching on the contralateral side. Damage to the prefrontal cortex and frontal lobes may cause itching in the face, chest and upper extremities. Although ischaemic and traumatic brain injury constitutes the most common causes of neuropathic itch arising from the brain, occasionally, brain tumours can also produce itching. Tumours involving the fourth ventricle may present with itching on the nostrils. Additionally, along with local tissue damage due to mass, there may be associated allergic reactions to tumour-specific antigens. Creutzfeldt-Jakob disease, a prion disease, may reduce diffusion in periaqueductal gray matter and cause neuropathic itch. Patients undergoing dialysis for chronic kidney diseases frequently complain of itching, and such patients often demonstrate reduced activity of some brain regions, including S1, superior parietal lobe, and insula, suggesting that local neuronal damage may be triggering the itch. Furthermore, an imbalance in endogenous opioids in the limbic system may also contribute to chronic kidney diseases-induced itch. Primary biliary cholangitis is another systemic disease involving the brain that may cause neuropathic itch.
Causes of Neuropathic Itch
The causes of neuropathic itch may be broadly classified based on the location of pathology in the itch pathway- peripheral nervous system, spinal cord, or brain [Table 1]. Some of the important causes are briefly discussed below, and Figure 3 illustrates some of them:
| Causes indicative of peripheral nervous system involvement | Causes indicative of spinal pathology | Causes indicative of brain involvement |
|---|---|---|
| Herpes zoster | Syringomyelia | Poststroke neuropathic itch |
| Small fibre neuropathy | Brown Sequard syndrome | Brain tumour/abscess/aneurysm |
| Prurigo nodularis | Transverse myelitis | Creutzfeldt-Jacob disease |
| Notalgia paraesthetica | Cavernous hemangioma | Trigeminal trophic syndrome |
| Brachioradial pruritus | Neuromyelitis optica | Uremic pruritus |
| Neuropathic anogenital pruritus | ||
| Sensitive skin | ||
| Scalp dysesthesia | ||
| Scar and keloidal itch | ||
| Postburn pruritus | ||
| Nerve entrapment neuropathy | ||
| Ganglionopathy | ||
| NaV1.7 mutation |
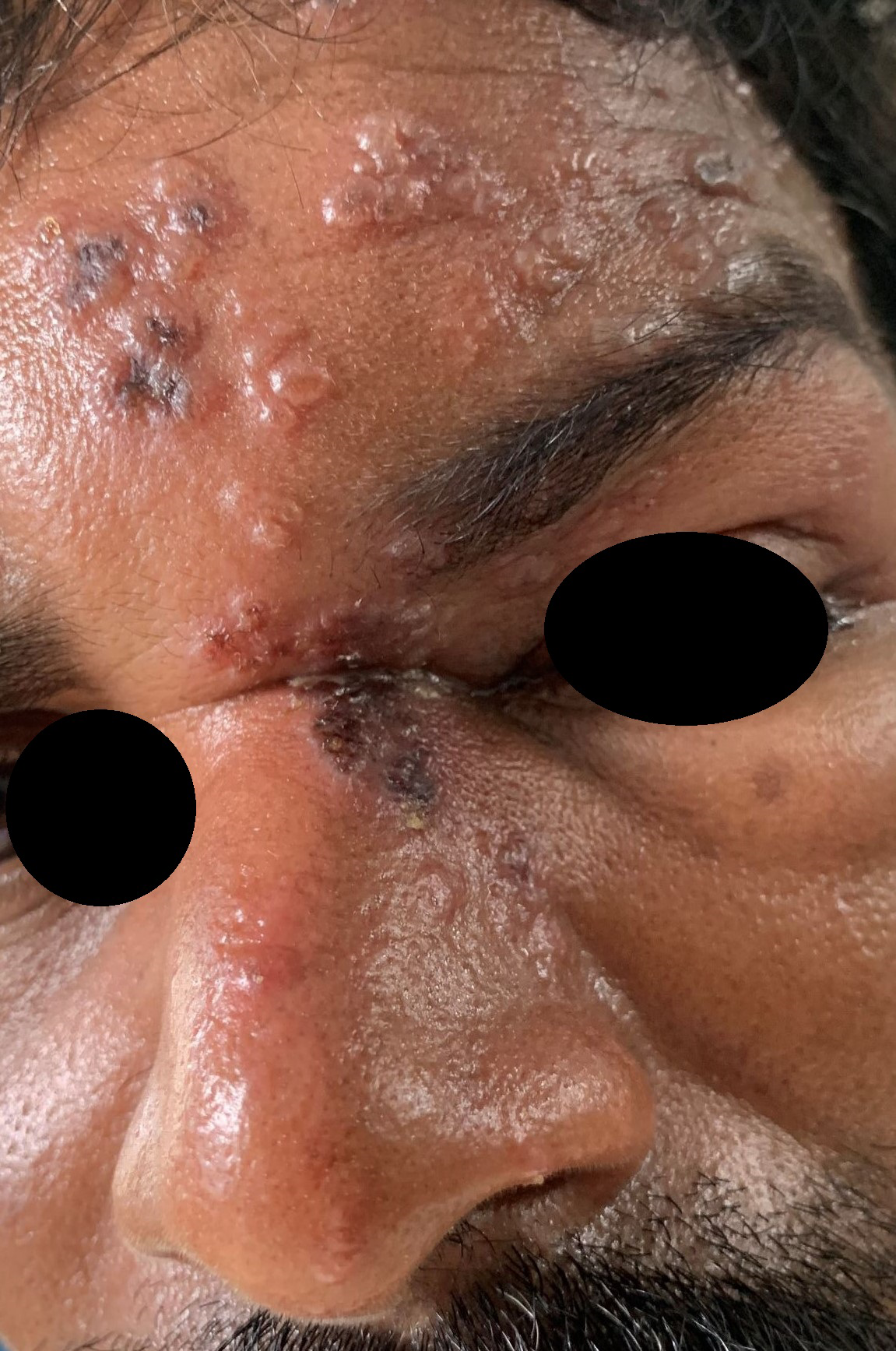
- Herpes zoster.
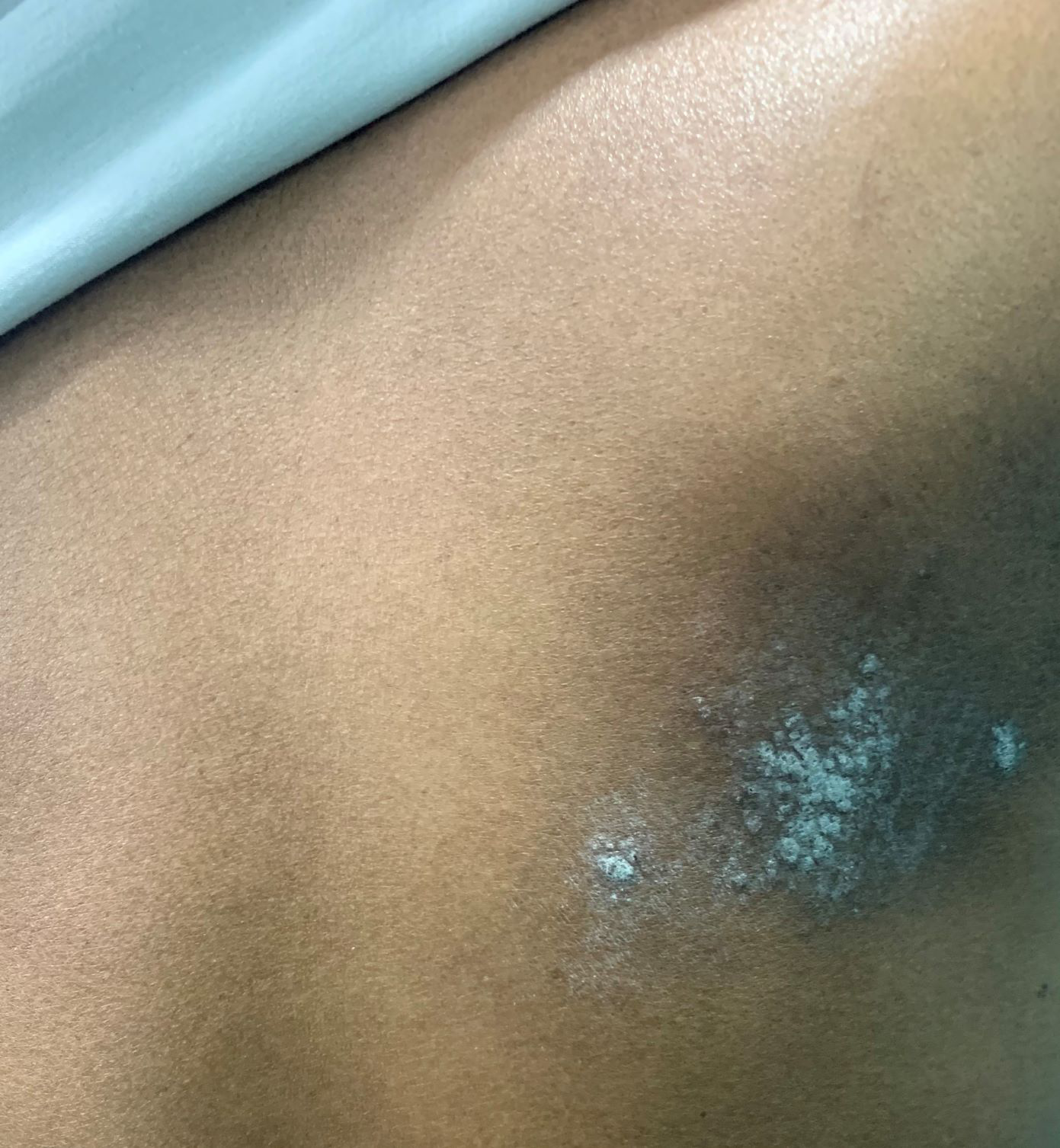
- Notalgia paresthetica (right infrascapular region).
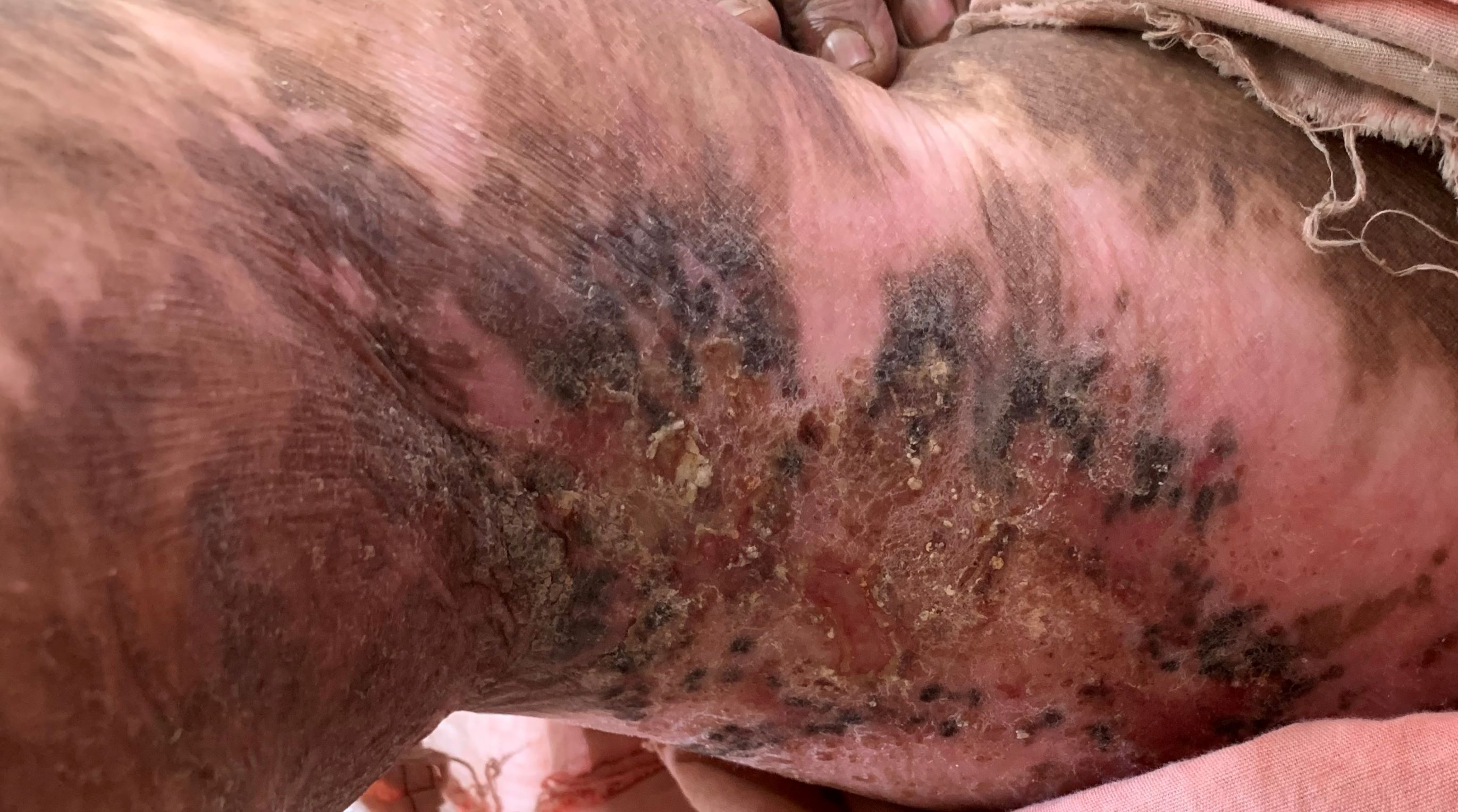
- Postburn scar with pruritus.
Causes indicating peripheral nervous system involvement
Herpes zoster
Postherpetic itch is not commonly discussed as a complication of herpes zoster as it is often masked by its predominant counterpart- neuropathic pain. However, itching has been reported in about 28–46% of cases in the acute stage and in 32–68% of cases in the chronic phase.24 The prevalence of itching is greater when the face is affected.25 While acute itch is neurogenic due to the release of histamine, it is neuropathic in the postherpetic phase due to damage to peripheral nerves. Histological studies have revealed a marked reduction of cutaneous innervation density in the affected dermatome.26 Varicella zoster virus causes demyelination of cutaneous nerve fibres, resulting in itching. This leads to ectopic discharges and over-excitation of primary neurons, causing increased itch transmission. The pruritus can range from mild to severe, sometimes disabling the patient.
Small fibre neuropathy
Small fibre neuropathy occurs when the peripheral C and Aδ fibres are predominantly or entirely affected. Pain and pruritus are well-recognised symptoms. In a study on 41 patients, burning (77.5%), pain (72.5%), heat sensations (70.2%), pruritus (68.3%) and numbness (67.5%) were the most frequent symptoms.27 Symptoms pertaining to autonomic and enteric dysfunction like syncope, dryness of eyes and mouth, diarrhea, and erectile dysfunction may also coexist. This disorder should be suspected when chronic itch presents in a length-dependent glove and stocking fashion on the extremities. Small fibre neuropathy can be idiopathic or occur in association with systemic diseases like diabetes mellitus, sarcoidosis, human immunodeficiency virus, vitamin B12 deficiency, paraneoplastic syndromes and paraproteinemia. Diagnosis requires a corroborative clinical picture, normal sural nerve conduction test and/or altered quantitative sensory testing results.28 A skin biopsy showing decreased intraepidermal nerve fibre density is the confirmatory test.
Prurigo nodularis
It is a chronic, inflammatory, idiopathic condition characterised clinically by intensely pruritic, excoriated, discrete hyperpigmented nodules predominantly affecting the extensors of the extremities followed by the trunk. The aetiology is complex, with frequent systemic associations like hyperthyroidism, malignancies, and hepatic and renal dysfunction. Studies have shown reduced intraepidermal nerve fibre density responsible for pruritus in both lesional and non-lesional skin, indicating that prurigo nodularis is a small fibre neuropathy. Increased levels of pruritogens such as substance P and calcitonin gene-related peptides have been demonstrated in the nerve fibre terminals. However, it remains ambiguous whether these pathophysiological changes are a result of an underlying small fibre neuropathy or chronic pruritus.29
Notalgia paraesthetica
It is an under-recognised sensory neuropathy characterised by unilateral infrascapular pruritus with accompanying variable pain, paraesthesia, allodynia and hyperalgesia, most commonly involving the T2–T6 dermatomes. Notably, no primary skin lesions are observed in notalgia paraesthetica. Pigmented lichenified patches and hyperkeratotic plaques appear in the affected area secondary to scratching. The pathogenesis is still unclear, but the impingement of nerves when they exit the spinal column or traverse through dorsal back muscles is the possible cause. One study suggested that increased lesional dermal sensory cutaneous innervation causes pruritus.30 The epidermal nerve fibre density is either decreased or remains normal. In a study by Savk and Savk on 43 patients, 28 (65.1%) had radiographic findings of degenerative vertebral changes or herniated discs in dermatomes corresponding to the symptoms.31 Hence, spinal imaging should be done to rule out any neurological or musculoskeletal symptoms.
Brachioradial pruritus
It is a localised neuropathic dysesthesia characterised by itching, burning and tingling sensation on the proximal dorsolateral forearm. Excoriation, papules, nodules, scars, and crusts occur secondary to scratching. It is common in white, middle-aged females and is exacerbated by ultraviolet radiation. The predisposing factor is impingement of the cervical nerve root at the level of C5–C8, indicating cervical spine disease. In a study including 111 patients with brachioradial pruritus, radiological imaging revealed cervical spine abnormalities in 103 (93%) patients.32 However, in some cases, it may extend to the upper arm, shoulder, neck, or upper trunk, hinting at the involvement of other nerves as well. The pathophysiology is poorly understood, and more studies are required. The ’ice pack sign’ is a disease-specific test where patients report remission of pruritus on applying an ice pack on the affected area. Radiological imaging is currently reserved for patients who present with concomitant neurological symptoms.
Neuropathic anogenital pruritus
Anogenital pruritus refers to an itch that is localised to the anus, perianal area, and genital skin (pruritus scroti in males and pruritus vulva in females). While acute pruritus may occur due to infections and contact dermatitis, chronic pruritus indicates systemic diseases, papulosquamous disorders, malignancies, psychogenic causes, and neuropathies. In the absence of any primary skin rash, a neuropathic cause should be ruled out before labelling it as idiopathic. A nerve conduction study done on 20 patients with anogenital pruritus revealed lumbar radiculopathy in 16 (80%) patients. Paravertebral injection of triamcinolone acetonide and lignocaine significantly reduced the mean pruritus score in such patients.33
Sensitive skin
It was first described in 1987 under the terminology’ cosmetic intolerance syndrome’. It is now defined as the onset of erythema, pain, burning and pruritus due to physical, environmental, psychological, or hormonal factors without visible clinical manifestations.34 The condition is believed to have no immunological or allergic origin and rather occurs due to reduced ceramide levels and increased transepidermal water loss. It leads to increased permeation of substances which can release cytokines. Current theories stress increased sensorineural impulses, which correspond to unpleasant sensations felt by the patient.35
Scalp dysesthesia
It is a cutaneous dysesthesia syndrome characterised by pruritus, burning, and pain of the scalp without objective findings. The pathogenesis is not yet known. In a study by Thornsberry and English on 15 women, 14 (93.3%) patients revealed abnormal cervical spine images.36 The most common finding was degenerative disc disease at the level of C5–C6. Lordosis, kyphosis, osteophytic spurring, anterolisthesis and nerve root impingement were the other abnormal findings. Chronic muscle tension of the pericranial muscles and scalp aponeurosis secondary to cervical spine disease possibly attributes to clinical dysesthesia.36
Scar and keloid itch
Keloids and hypertrophic scars are pathological scars that represent an excessive tissue response to dermal injury. They are characterised by the overproduction of collagen. Keloids extend beyond the borders of the original wound, whereas hypertrophic scars remain confined to the wound site. A fraction of the patients present with pain and itching in these lesions. Itch is present at the borders, while pain is localised to the center. Abnormal sensory testing indicates a small fibre neuropathy in these lesions.37 Another study demonstrated decreased intra-epidermal nerve fibre density in the lesional skin. It was hypothesised that chronic itch leads to self-regulated hypoplasia of the nerve fibres to modulate the persistent sensory inputs.38
Postburn itch
Postburn pruritus occurs during the process of wound healing in almost all patients. Interestingly postburn pruritus tends to be refractory to conventional antihistamines and rather responds to neuroleptic agents. It is postulated that there is peripheral sensitization due to increased excitability of the afferent fibres after burn injury. This is coupled with decreased afferent inhibition (gating) centrally due to the loss of inhibitory central nervous system neurons and interneuron activity. The C-fibres have a large innervation territory, and proximal inflammation within their nerve roots contributes to enlarged itch boundaries.39
Nerve entrapment neuropathy
localised pain and pruritus are the presenting features of entrapment neuropathies like meralgia paresthetica, cheiralgia paresthetica, pudendal neuralgia and suprascapular entrapment syndromes.
Meralgia paresthetica is due to the entrapment of the lateral femoral cutaneous nerve leading to burning, tingling, and itching on the anterolateral thigh. Wartenberg syndrome or cheiralgia paresthetica indicates compression of the superficial branch of the radial nerve. Pudendal neuralgia is another chronic disabling neuropathy in the distribution of pudendal nerve secondary to pelvic surgery, childbirth, trauma and chronic constipation. Suprascapular entrapment neuropathy is seeing an increasing prevalence owing to the replacement of briefcases with backpacks. It occurs due to compression of the suprascapular nerve on the back secondary to a cyst or repetitive stretch injuries. Occasionally, these neuropathies may present with concomitant pruritus.
Ganglionopathies
Sensory ganglionopathies occur in patients with an autoimmune disease (Sjogren syndrome) or a paraneoplastic disorder (small-cell lung cancer). Other causes are brachial and lumbosacral plexopathies. They are a subgroup of neuropathies that present as asymmetric, non-length dependent sensory impairment, occasional pruritus and early ataxia. It occurs due to primary degeneration of the dorsal root ganglion in the spinal cord and the trigeminal ganglion sensory neurons in the skull due to the abnormal capillary blood supply. Prompt diagnosis, workup, and disease-specific treatment should be initiated in these patients.
NaV1.7 mutation
NaV1.7 is a voltage-gated sodium channel expressed in the dorsal root ganglion and in sympathetic ganglion neurons. It modulates cell excitability and ion channel function. Mutation in the sodium voltage-gated channel ɑ-subunit 11 and sodium voltage-gated channel ɑ-subunit 9 genes coding for this channel can cause sensory dysfunction. Patients having a gain of function mutation of the sodium voltage-gated channel ɑ-subunit 11 gene exhibit congenital insensitivity to pain, pruritus, self-inflicted injuries, muscle weakness and delayed wound healing.40 A novel syndrome associated with gain of function mutation in the sodium voltage-gated channel ɑ-subunit 9 gene has also been recognised in three patients of the same family having a paroxysmal itch. Skin biopsy in these patients revealed decreased intraepidermal nerve fibre density in two patients consistent with small fibre neuropathy. Pregabalin successfully reduced the itch intensity and frequency in these patients.41
Causes indicating spinal cord pathology
Spinal cord diseases such as syringomyelia, abscess, tumours like cavernous haemangioma and meningioma, transverse myelitis, neuromyelitis optica and trauma can trigger localised pruritus corresponding to the level of injury. A case of chronic hemi corporal itch after trauma in brown sequard syndrome has been reported.42 One case has described paroxysmal itching related to syringomyelia with Chiari malformation in a 16-year-old female.43 Pruritus of 6 months duration localised to the neck, shoulder, and arm in an otherwise healthy 19-month-old child was found to be the sole manifestation of an intramedullary spinal tumour.44 Patients with dermatomal localisation or paroxysmal nature of itch should be referred to a neurologist for examination and imaging studies. Compression of the spinal cord, gliosis and deafferentation of itchy skin underlie the cause of itching. It has also been suggested that hemosiderin-laden phagocytes in the rim of cavernous haemangiomas may cause ectopic firing of adjacent neurons, making them highly pruritic.45
Causes indicating brain pathology
Poststroke neuropathic itch
Poststroke pruritus was first described following an infarct of the internal capsule.46 It is an under-recognised type of central itch resulting from brain lesions affecting the neural afferent pathways, control centres and modulating regions of itch.47 The onset may vary from days to weeks after the stroke episode. A case report described itch occurring 3 weeks post Wallenberg syndrome in a 56-year-old woman who responded to gabapentin and topical moisturisers.48 It has also been reported after contralateral thalamic stroke and cerebral infarction in the middle cerebral artery.49
Brain tumours, abscesses and aneurysms
Localised chronic pruritus has been rarely described in association with brain tumours. It is postulated to occur due to damage or activation of the prefrontal cortex and premotor areas by the tumour. Activation of the ipsilateral premotor areas invokes the desire to itch/ scratch.50 In a study done on 24 patients with brain tumours, 13 (54.1%) patients complained of pruritus. The most noteworthy was intractable nasal pruritus in six patients, which represented the advanced disease stage invading the floor of the fourth ventricle. Patients who had generalised pruritus were believed to have an allergic response to tumour-associated antigens. The itching subsided after the removal of the tumours.51 In a case report, two children suffering from neurofibromatosis type 1 had localised pruritus as the initial presenting symptom of a brain stem glioma.52
Creutzfeldt-Jakob disease
It is a fatal degenerative condition occurring due to the accumulation of abnormal prion proteins in the central nervous system. Pruritus is a well-recognised feature in familial cases expressing glutamine to lysine change at codon 200 genetic mutation and is usually absent in the sporadic variants. There is a lack of response to conventional antihistamines pointing towards the central origin of itch. In an magnetic resonance imaging study of the brain, bilateral reduction of diffusion was noted in periaqueductal gray matter, the area responsible for modulating itch stimulus. Hence, damage to this area leads to dysregulated itch in patients.53
Trigeminal trophic syndrome
Trigeminal nerves, best known for causing neuropathic pain, can also cause a chronic itch syndrome. The trigeminal trophic syndrome is a rare entity occurring due to an insult to the trigeminal nerve characterised by a triad of anaesthesia, paresthesia, and recurrent, persistent facial ulceration. Patients complain of rubbing and scratching the affected area and can have linear, crescentic ulcers with peripheral hyperpigmentation and atrophic scarring. The ipsilateral nasal ala and the adjacent cheek and upper lip, supplied by the V2 and V3 nerves, are characteristically involved with the sparing of the nasal tip. The ulcers were initially thought to occur due to loss of neuronal trophic factors in the deafferent skin; however, later, it was established that it occurs secondary to self-manipulation and chronic scratching of the painless area. Both central and peripheral causes, such as infections, brain stem infarcts and trigeminal neuralgia, and their therapeutic procedures, as well as meningiomas and gliomas can lead to trigeminal trophic syndrome.54
Uremic pruritus
Although classically a type of neurogenic pruritus, this condition has been mentioned here as recent studies indicate the role of structural brain dysfunction. The exact pathophysiology is still cryptic, with possible factors being systemic inflammation, imbalance in endogenous opioids, altered serum parathyroid hormone, calcium and phosphorus levels, and a neuropathic process. A study in end-stage renal disease patients on hemodialysis indicated that gray matter density in areas involved in itch modulation was altered, suggestive of neuropathic origin.55
Diagnosis and Approach to Neuropathic Pruritus
As depicted in Figure 2, the causes of chronic itch can be broadly categorised under four heads—dermatological, systemic, neuropathic, and psychogenic. Among them, neuropathic pruritus is an emerging area of interest and often remains underdiagnosed and untreated. An algorithmic approach involving history taking, systemic and local examination, relevant diagnostic tests and functional assessment would help us in its correct diagnosis.
History taking
An elaborate medical history is crucial for diagnosing neuropathic pruritus and ruling out other causes of chronic pruritus. The duration, location, onset, nature, severity, course, provoking and relieving factors for the itch should be enquired about in detail. History of any pre-existing dermatological, psychological, or systemic disorder should be assessed.
Neuropathic itch is generally localised, and primary lesions are absent (International Forum for the Study of Itch group II).7 Generalised itch in the absence of any identifiable dermatological cause also indicates systemic causes like renal, liver, endocrinological dysfunction, lymphomas, myeloproliferative or psychological disorders. All these causes need to be ruled out to diagnose neuropathic pruritus. A past or present history of substance abuse and relevant medical history of systemic conditions like thyroid, liver and renal dysfunction must be taken. Any known and suspected allergies in patients and family members should be noted. Additionally, the patient’s skincare routine, bathing practices, job profile, hobbies, personal stress and medication history should be enquired about. Dysesthesias such as stinging, tingling or electroshocks are typical for neuropathic itch. The location of itch at onset may point towards the involved somatosensory system, but generalisation can occur subsequently. For example, small fibre neuropathy manifests initially distally at the feet and advances proximally with the disease course, whereas neuropathic itch in postherpetic neuralgia remains localised to the affected dermatome.56 If the patient suggests relief on ice pack application, as in brachioradial pruritus or treatment with neuroleptics, it also suggests a neuropathic origin.
Clinical examination
A detailed skin, hair and mucosal examination should be conducted after history taking to rule out any identifiable dermatological cause. In neuropathic itch, primary skin lesions are generally absent. Skin lesions secondary to itching, like prurigo nodularis, lichenification and excoriations, may be present. It is important to distinguish dermatoses from secondary skin lesions. The distribution of the scratch lesions will point towards the affected somatosensory system, and the severity of the lesions will indicate the disease activity.57 The examination is incomplete without palpation of lymph nodes, especially in these patients without primary skin lesions, to rule out underlying lymphomas. A neurological examination must include testing for allokinesis and hyperkinesis. Allokinesis can be tested by stimulating the affected skin area with cotton wool or a brush. To test for hyperkinesis, skin can be subjected to pinprick testing. Patients experience a severe itch perception after stimulation of the skin in the affected site. These areas can exceed the borders of visually detectable skin changes. It acts as a guide for topical therapy. The presence of these phenomena indicates neuronal sensitization and contributes to the chronicity of itch.58 Autonomic testing is recommended for diagnosing small fibre peripheral neuropathy. The quantitative sudomotor axon reflex test measures the postganglionic sweat output resulting from axon reflex stimulation using acetylcholine electrophoresis.
A Neuropathic pruritus score has been suggested for the objective identification of neuropathic itch. It is based on the assessment of five parameters—the presence of twinges, absence of burning, worsening of the itch with activity, not worsening with stress and relief of itch with cold. When two out of five criteria are met, the ability to distinguish between neuropathic and non-neuropathic itch is 76% sensitive and 77% specific.59 For individuals with small fibre peripheral neuropathy, the validated Massachusetts General Hospital patient-reported ’small-fibre symptom survey’ captures ’skin that itches for no reason’.60
Investigations
Once a diagnosis of neuropathic pruritus has been suspected by history and clinical examination, relevant investigations need to be performed, when necessary, to confirm the diagnosis. However, in many cases, a typical history and clinical examination are sufficient for the diagnosis, like in postherpetic neuralgic itch.
Skin biopsy
The assessment of intraepidermal nerve fibre density is the gold standard for diagnosing small fibre neuropathy. The skin biopsy needs to be taken from an area of healthy skin 10 cm proximal to the lateral malleolus and should be immediately fixed with special fixatives like periodate-lysine-paraformaldehyde. They are immunolabelled with antibodies against a pan-axonal marker, PGP9.5. This helps in counting the small nerve endings with a light microscope. Nerve fibres that cross the basal membrane from the dermis side are counted and divided by the length of the dermo-epidermal junction. Fragments of nerve fibres in the epidermis and branching are not considered for the intraepidermal nerve fibre density.28
Laboratory tests
Baseline initial evaluation must include a complete blood count with differential count, ESR and platelet count. Liver, renal, thyroid function and glycemic status should be evaluated to exclude systemic diseases as the cause of chronic pruritus. Disease-specific tests for suspected neurological causes should be performed. For instance, a cerebrospinal fluid analysis should be done if a brain tumour is suspected. An exploratory workup to establish the cause of small fibre neuropathy must be carried out. Human immunodeficiency virus, hepatitis B and C serology, Serum vitamin B12 and folate levels, and genetic testing for heritable conditions should be considered.
Radiological examination
Magnetic resonance imaging and computerised tomography imaging should be done to identify tumours, abscesses and aneurysms in the central and peripheral nervous systems. Magnetic resonance imaging is also used to diagnose degenerative diseases of the spine, disc prolapse, herniation and nerve root compression. All these conditions have the propensity to induce neuropathic itch.
Functional assessment
Quantitative sensory testing can be done in C and Aδ fibres as they are primarily involved in itch transmission. It is feasible to infer a potential gain or loss of function of certain nerve fibre populations by measuring the pain and responsiveness to suprathreshold stimuli using a validated test that uses thermal and mechanical standardised stimuli. It also provides information on neural sensitization symptoms.61 However, it is a time-consuming method and requires specialised personnel. Electromyography and nerve conduction studies may be done while assessing small fibre neuropathy, neurogenic anogenital pruritus and brachioradial pruritus. Microneurography and evoked potentials are other methods that allow the assessment of selective nerve fibres, but are mostly used in research-based settings.62
Treatment
Despite being a relatively common cause of chronic pruritus, neuropathic itch is often missed and remains underdiagnosed. As a result, no specific guidelines exist currently for the management of neuropathic pruritus. It remains a challenging condition to manage, and patients should be under multi-departmental care involving dermatologists and neurologists. The treatment plan must be formulated based on underlying etiology, existing comorbidities, risk of possible drug interactions and patient preferences. The focus should be given to improving the quality of life of the patient. Both pharmacological and non-pharmacological strategies are often helpful in combating this chronic pruritus. Figure 4 highlights the drugs along with their sites of action on the itch pathway in a schematic diagram.
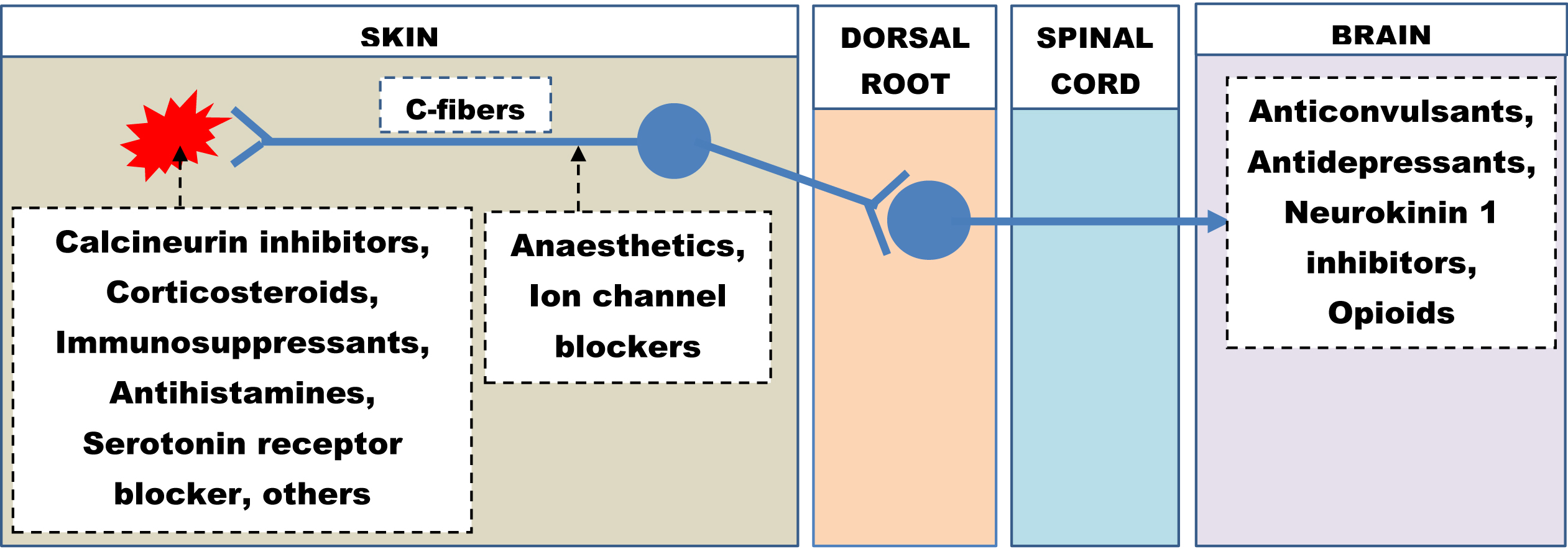
- Therapeutic agents and their sites of action on the itch pathway.
Pharmacological therapies
The efficacy of these therapies is largely guided by the clinician’s expertise and case reports. No large-scale clinical trials and validated data are available.
Topical therapy
Topical therapies are often helpful in neuropathic itch due to their localised nature. They offer the advantage of delivering high drug concentrations at the affected site with lesser adverse effects. Their penetration can be enhanced with the use of occlusive dressing following application. A summary of the available topical medicines, along with their indications and side effects, is mentioned in Table 2.
Class of drug
Medication
Mechanism of action
Indication
Adverse effects
Anaesthetics
Pramoxine 1% Lignocaine 2.5–5% Prilocaine 2.5% 5–10% Ketamine + amitriptyline + 5% lignocaine
Blocking sodium channels on unmyelinated C-fibres, thus interrupting the transmission of itch impulse
Prurigo nodularis, postburn pruritus, postherpetic itch, brachioradial pruritus, notalgia paraesthetica
Temporarily reduces the sensitivity of the skin.
Ion channel blockers
Capsaicin cream (0.025–0.1%)
The repeated application causes degeneration of C-fibres; however first few applications may cause burning and increase inflammation and itch.
Prurigo nodularis, uraemic itch, postherpetic neuralgia, brachioradial pruritus, notalgia paresthetica, small fibre neuropathy
Initial burning or pain sensation; is contraindicated in situations where nerve fibres are growing (children, healing stage).63 to be avoided near the eyes as it may denervate the cornea.
Calcineurin inhibitors
Tacrolimus 0.03 and 0.1% Pimecrolimus 1%
Reduces inflammation and subsequently the pruritus
Anogenital pruritus, prurigo nodularis, uremic pruritus, meralgia paraesthetica,
Brief burning sensation Food and Drugs Administration boxed warning for increased risk of malignancy.
Topical glucocorticosteroids
Class 1–class VII, depending on the site and severity of pruritus
It does not affect the primary mechanism of neuropathic itch but reduces inflammation and relieves itching
Usually, the 1st line topical medication used by general physicians or patients (OTC)
Pigmentary disturbance, striae, acneiform eruptions, telangiectasia, etc.
Others
Menthol 2%
Act as soothing agents and provide symptomatic relief.
Brachioradial pruritus
Gabapentin 10–12%
Neuropathic itch
Acetylsalicylic acid
Postherpetic neuralgia, notalgia paresthetica
Systemic therapy
They should be given in sufficient doses for therapeutic efficacy in cases not responding to topical therapies alone. A different class of medication can be added if the maximum therapeutic dose has been achieved, bearing in mind possible drug interactions. Table 3 summarises the oral therapies available for treating neuropathic pruritus.
Class of drugs
Medications
Mechanism of action
Dose
Indication
Adverse effects
Anticonvulsants
Gabapentin Pregabalin
Interfere with the functioning of alpha2 delta calcium channels and itch transmission at the supraspinal level.63
Up to 3600 mg/day Up to 450 mg/day
Prurigo nodularis, Postherpetic itch, Brachioradial pruritus, Notalgia paraesthetica, Scalp dysesthesia, Trigeminal trophic syndrome, sodium channel mutation
Sedation, Dizziness, Increased appetite, Weight gain, Constipation, Lower leg swelling
Carbamazepine, oxcarbazepine
Systemic sodium channel blockers reduce the ectopic firing of neurons.
200–800 mg/day (carbamaxzepine)
These drugs are especially effective in trigeminal neuralgia
Dizziness, Drowsiness, Nausea, Vomiting, Blurred vision, Constipation, Dryness in mouth
Antidepressants
Doxepin
Reduce depression and stress, which can coexist with neuropathic pruritus and aggravate symptoms
10–100 mg once at night
Neuropathic itch
Anticholinergic side effects
Amitriptyline
25–75 mg once at night
Postherpetic neuralgia, Brachioradial pruritus, Trigeminal trophic syndrome, Notalgia paresthetica
Hypotension
Neurokinin 1 inhibitors
Aprepitant Serlopitant Tradipitant
Inhibit NK1, an important mediator in the itch pathway.
80 mg daily
Prurigo nodularis, Brachioradial pruritus
Gastrointestinal symptoms
Opioid
Nalbuphine
µ-receptor antagonist and a k-receptor agonist. The activation of k-opioid receptors accounts for its utility as a treatment agent for pruritus64
120 mg
Prurigo nodularis
Gastrointestinal symptoms
Immunosuppressants
Thalidomide
Do not affect the primary cause. Reduce inflammation and improve neuropathic itch. Indicated in inflammatory conditions with neuropathic itch.
100–200 mg daily
Prurigo modularis
Sedation, Peripheral neuropathy, Teratogenicity
Methotrexate
15 mg weekly
Prurigo nodularis
Hepatotoxicity, Gastrointestinal symptoms, Decreased haematopoiesis
Cyclosporine
2.5–5 mg/kg/day
Prurigo nodularis
Hypertension Renal toxicity Hypertrichosis Dyslipidaemia
Antihistamines
Both 2nd and 1st generation antihistamines are used as 1st line by general physicians, although largely ineffective in neuropathic pruritus.
Largely ineffective in neuropathic pruritus (NP), as histamine is not the principal mediator of NP. Sedating antihistamines might be secondarily beneficial by improving sleep, reducing nocturnal scratching, and soothing scratch-induced skin inflammation.65
The dose depends on the particular antihistamines being used
All causes of neuropathic pruritus
Anticholinergic side effects like dry eyes, dry mouth, urinary retention and cardiac adverse effects, especially with sedating 1st generation antihistamines. Cautious use is warranted in patients >65 years.
5-hydroxytryptamine)/serotonin receptor blocker
Ondansetron
Serotonin is a known stimulator of the itch, transmitting C-fibres. This drug blocks serotonin and reduces the neuropathic itch. High dose (up to 24 mg) also reduces activation of the interoceptive and sensorimotor brain regions.66
8 mg daily
Has shown the best results in intrathecal morphine-induced pruritus and postburn pruritus.A recent randmised controlled study has demonstrated a better effect than antihistamine in reducing the severity of postburn pruritus.67
Nausea, vomiting, diarrhoea, stiff or twitching muscles, loss of coordination.
Invasive therapies
Invasive therapeutic strategies should be tried on those patients who are not responding adequately to topical and systemic therapies. Several procedures like intercostal nerve blocks/neurolysis, paravertebral neurolysis, epidural steroid injections, and dorsal root ganglion-radiofrequency ablation have effectively reduced postherpetic neuralgia, so their use may be extended to those suffering from additional pruritus.68
Botulinum toxin A has been used in patients of notalgia paraesthetica and brachioradial pruritus.69 It can act via the reduction of cholinergic transmission or substance P release along itch pathways. A series of injections of 1–5 U of toxin is given in the affected area at several points, 1 cm apart.
In refractory cases, targeted nerve and ganglion blocks such as stellate ganglion blocks have improved herpetic itch70 and notalgia paraesthetica. Peripheral nerve stimulation has also been reported to benefit notalgia paresthetica.71 Transcutaneous electrical nerve stimulation is another modality that has been found useful in recalcitrant postburn pruritus, brachioradial pruritus and notalgia paresthetica.72 Paravertebral injection of triamcinolone acetonide and lignocaine has been found to relieve itch in refractory neurogenic anogenital pruritus.33 In cases of refractory neuropathic pruritus due to compression of spinal nerve or roots, decompression surgery may be tried as the last retort. Leprosy neuropathy has also been reported to be relieved by decompression surgeries.73
Newer therapies and strategies
Currently, a lot of work is ongoing to explore the pathogenesis of neuropathic pruritus and thereby design more effective therapeutic strategies. Various newer technologies are being tried to obtain a more detailed understanding of this condition, such as live cell imaging, wireless optogenetic implants to map central pathways, and omics technologies to identify newer cells, circuits, and mediators crucial to its pathogenesis.74 Newer receptors such as brain natriuretic peptides 1 and 2, gastrin-releasing peptide receptor and somatostatin receptors, signalling pathways such as Janus kinase pathway and novel cytokine mediators for example, interleukin-31, interleukin-4, interleukin-13, interleukin-33, thymic stromal lymphopoietin are being linked to the pathogenesis of neuropathic pruritus. These new findings would eventually be helpful in devising newer and more effective targeted treatment strategies like Janus kinase inhibitors, antagonists or blockers of the newer receptors or mediators.
A number of more recent therapeutic modalities are presently undergoing clinical trials in various stages, including κ-opioid receptor agonists (ICI-199,441), µ-opioid receptor antagonists (naltrexone), protease-activated receptor antagonists (PAC-14028, BIIB074), endothelin A receptor antagonists (zibotentan), cytokine receptor antagonists (BMS-981164), channel blockers (sodium, transient receptor potential), tralokinumab, Janus kinase inhibitors (INCB039110 and INCB018424), and tropomyosin receptor kinase inhibitor (CT372).2 Interestingly, a potential therapy targeting NaV1.7 has demonstrated a reduction in pain and itch in animal models.75 Recently, an oral cannabinoid preparation (2.43 mg tetrahydrocannabinol/cannabidiol 2.75 mg) has been used successfully for treating refractory neuropathic itch induced by amyotrophic lateral sclerosis.76
Non-pharmacological strategies
General measures
This includes avoidance of triggers that could increase pruritus. For instance, the application of skin irritants should be avoided. Patients should be encouraged to wear loose, cotton clothes, reduce weight and use cosmetics containing soothing agents like menthol or polidocanol. Trimming of fingernails and/or wearing gloves during sleep may be advised to reduce scratching-induced skin damage.
Psychosocial support
The emotional and cognitive components of neuropathic itch must be jointly addressed for the best results. This can help in breaking the existing itch-scratch cycle to improve patients’ quality of life and minimise skin damage.
Support should be provided in the form of habit reversal training and relaxation training. Habit reversal training involves awareness training wherein the patient practices a competing response to replace dysfunctional behaviour. Relaxation training, such as meditation, induces progressive muscle relaxation and reduces aggression. Other techniques which have been reported to be beneficial include cognitive behavioural therapy, autogenetic training and occlusive therapy (where itchy patches are occluded to neutralise visual clues of itching).2,77 Additionally, tight occlusion of the pruritic area may alleviate itching by augmenting the inhibitory signals in the dorsal horn of the spinal cord and also improve the penetration of topical therapies.2 Biofield therapy with healing touch was found to be effective in a patient with intractable neuropathic itch.78
Physical therapy
This is particularly helpful in patients who have itch due to nerve compression or impingement. Strengthening and stretching exercises were found to help some patients of notalgia paresthetica.79
Limitation
The major limitation of this review was the inability to conduct a Preferred Reporting Items for Systematic Reviews and Meta-Analyses guided systematic review as there is a dearth of good quality, controlled trials in neuropathic pruritus.
Conclusion
Neuropathic itch is an extremely debilitating and obscure cause of systemic pruritus, as skin lesions are absent in most cases. Although hepatic and renal pruritus are well-studied causes of systemic pruritus, there is a dearth of organised information concerning neuropathic itch. Thus, most cases of neuropathic itch remain underdiagnosed and underreported. Furthermore, its pathophysiology is poorly elucidated, and therapy is often based on clinical experience and anecdotal reports. The causes of neuropathic itch may be attributed to the peripheral nervous system, spinal cord pathologies or brain pathologies such as tumours and other space-occupying lesions. A high index of clinical suspicion is necessary for diagnosis, while radiological and functional investigations may be helpful in selected cases. Antihistamines form the first line of treatment to provide symptomatic relief, while commonly prescribed definitive treatments include topical anaesthetic agents, topical capsaicin, topical glucocorticoids, systemic anticonvulsants and anti-psychotics (antidepressants, anxiolytics). Several emerging therapies based on newer receptors and signalling pathways have also been discussed to highlight the current understanding of neuropathic itch. However, appropriate psychosocial counselling forms the backbone of management, while physical therapies (strengthening and stretching exercises) are needed for refractory patients.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Peripheral and central mechanisms of itch. Neuron. 2018;98:482-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018;17:709-20.
- [CrossRef] [PubMed] [Google Scholar]
- Central circuit mechanisms of itch. Nat Commun. 2020;11:3052.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- New insights into the mechanisms behind mechanical itch. Exp Dermatol. 2020;29:680-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291-4.
- [CrossRef] [PubMed] [Google Scholar]
- Itch processing in the skin. Front Med (Lausanne). 2019;6:167.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol. 2017;595:3651-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neural processing of itch. Neuroscience. 2013;250:697-714.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circuit dissection of the role of somatostatin in itch and pain. Nat Neurosci. 2018;21:707-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Spinal cord disorders. In: Marx JA, Hockberger RS, Walls RM, eds. Rosen’s emergency medicine: Concepts and clinical practice (7 th ed). Philadelphia, USA: Mosby/Elsevier; 2010. p. :1389-97.
- [Google Scholar]
- The challenge of basic itch research. Acta Derm Venereol. 2020;100:adv00023.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cerebral networks linked to itch-related sensations induced by histamine and capsaicin. Acta Derm Venereol. 2015;95:645-52.
- [CrossRef] [PubMed] [Google Scholar]
- Central processing of itch in the midbrain reward center. Neuron. 2019;102:858-72.e5.
- [CrossRef] [PubMed] [Google Scholar]
- Circuit mechanisms of itch in the brain. J Invest Dermatol. 2022;142:23-30.
- [CrossRef] [PubMed] [Google Scholar]
- Pruriplastic itch-a novel pathogenic concept in chronic pruritus. Front Med (Lausanne). 2021;7:615118.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2017;18:113.
- [CrossRef] [PubMed] [Google Scholar]
- Herpes zoster itch: preliminary epidemiologic data. J Pain. 2003;4:338-43.
- [CrossRef] [PubMed] [Google Scholar]
- The neuropathic itch: Don’t scratch your head too hard! Clin Case Rep. 2021;9:914-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Increased density of cutaneous nerve fibres in the affected dermatomes after herpes zoster therapy. Acta Derm Venereol. 2014;94:168-72.
- [CrossRef] [PubMed] [Google Scholar]
- Pruritus: An underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-32.
- [CrossRef] [PubMed] [Google Scholar]
- European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903-12.
- [CrossRef] [PubMed] [Google Scholar]
- Association between prurigo nodularis and etiologies of peripheral neuropathy: Suggesting a role for neural dysregulation in pathogenesis. Medicines (Basel). 2020;7:4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Symptoms of notalgia paresthetica may be explained by increased dermal innervation. J Invest Dermatol. 1991;97:555-61.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of spinal pathology in notalgia paresthetica. J Am Acad Dermatol. 2005;52:1085-87.
- [CrossRef] [PubMed] [Google Scholar]
- Brachioradial pruritus: Mayo clinic experience over the past decade. Br J Dermatol. 2013;169:1007-15.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic scrotal pruritus: anogenital pruritus is a symptom of lumbosacral radiculopathy. J Am Acad Dermatol. 2005;52:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitive skin: An overview. Indian J Dermatol Venereol Leprol. 2013;79:9-16.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitive skin: Review of an ascending concept. An Bras Dermatol. 2017;92:521-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Scalp dysesthesia related to cervical spine disease. JAMA Dermatol. 2013;149:200-203.
- [CrossRef] [PubMed] [Google Scholar]
- Pruritus, pain, and small nerve fiber function in keloids: A controlled study. J Am Acad Dermatol. 2004;51:1002-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous innervation and itch in keloids. Acta Derm Venereol. 2012;92:529-531.
- [CrossRef] [PubMed] [Google Scholar]
- Post-burn pruritus. Int J Mol Sci. 2020;21:3880.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gain-of-function mutation in SCN11A causes itch and affects neurogenic inflammation and muscle function in Scn11a+/L799P mice. PLoS One. 2020;15:e0237101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Paroxysmal itch caused by gain-of-function Nav1.7 mutation. Pain. 2014;155:1702-707.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic hemicorporal prurigo related to a posttraumatic Brown-Sequard syndrome. Dermatology. 2008;217:45-7.
- [CrossRef] [PubMed] [Google Scholar]
- Paroxysmal Itching in syringomyelia with chiari malformation (type I): A case report. Orthop Trauma. 2008;57:647-51.
- [Google Scholar]
- Intractable localized pruritus as the sole manifestation of intramedullary tumor in a child: Case report and review of the literature. JAMA Dermatol. 2013;149:446-9.
- [CrossRef] [PubMed] [Google Scholar]
- Central neuropathic itch from spinal-cord cavernous hemangioma: A human case, a possible animal model, and hypotheses about pathogenesis. Pain. 2005;113:233-7.
- [CrossRef] [PubMed] [Google Scholar]
- Unilateral neurogenic pruritus: Paroxysmal itching associated with central nervous system lesions. Ann Intern Med. 1982;97:222-3.
- [CrossRef] [PubMed] [Google Scholar]
- Unilateral pruritus following stroke. Indian J Dermatol Venereol Leprol. 2015;81:186-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic pruritus following Wallenberg syndrome. Neurology. 2009;72:676.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy and neurophysiology of pruritus. Semin Cutan Med Surg. 2011;30:64-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Skin manifestations associated with tumours of the brain. Br J Dermatol. 1975;92:675-8.
- [CrossRef] [PubMed] [Google Scholar]
- Brainstem glioma presenting as pruritus in children with neurofibromatosis-1. J Pediatr Hematol Oncol. 2009;31:972-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pruritus in familial Creutzfeldt-Jakob disease: A common symptom associated with central nervous system pathology. J Neurol. 2011;258:89-95.
- [CrossRef] [PubMed] [Google Scholar]
- Trigeminal trophic syndrome-a unique clinical presentation of a rare condition. Ear Nose Throat J. 2019;10:606-8.
- [CrossRef] [PubMed] [Google Scholar]
- Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol. 2014;112:1729-38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Brachioradial pruritus: A trigger for generalization of itch. J Am Acad Dermatol. 2013;68:870-73.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropathic itch: Routes to clinical diagnosis. Front Med (Lausanne). 2021;8:641746.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Central mechanisms of itch. Curr Probl Dermatol. 2016;50:11-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison of characteristics of neuropathic and non-neuropathic pruritus to develop a tool for the diagnosis of neuropathic pruritus: The NP5. Front Med. 2019;6:79.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Initial development and validation of a patient-reported symptom survey for small-fiber polyneuropathy. J Pain. 2017;18:556-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain. 2006;123:231-43.
- [CrossRef] [PubMed] [Google Scholar]
- Microneurographic recording from unmyelinated nerve fibers in neurological disorders: An update. Clin Neurophysiol. 2015;126:437-45.
- [CrossRef] [PubMed] [Google Scholar]
- Neurologic itch management. Curr Probl Dermatol. 2016;50:116-23.
- [CrossRef] [PubMed] [Google Scholar]
- Use of nalbuphine for treatment of neuraxial opioid-induced pruritus: a systematic review and meta-analysis. AANA J. 2019;87:222-30.
- [PubMed] [Google Scholar]
- Role of mast cells and basophils in pruritus. Immunol Rev. 2018;282:248-64.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose ondansetron reduces activation of interoceptive and sensorimotor brain regions. Neuropsychopharmacology. 2019;44:390-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ondansetron for treating itch in healing burns. Internet J Pain Symptom Control Palliative Care. 2006;5
- [Google Scholar]
- Post-herpetic neuralgia: A systematic review of current interventional pain management strategies. J Cutan Aesthet Surg. 2020;13:265-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antipruritic effects of botulinum neurotoxins. Toxins (Basel). 2018;10:143.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peripheral nerve block with a high concentration of tetracaine dissolved in bupivacaine for intractable post-herpetic itch: A case report. JA Clin Rep. 2016;2:43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cutaneous field stimulation in the treatment of severe itch. Arch Dermatol. 2001;137:1323-5.
- [CrossRef] [PubMed] [Google Scholar]
- Transcutaneous electrical nerve stimulation offers partial relief in notalgia paresthetica patients with a relevant spinal pathology. J Dermatol. 2007;34:315-9.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy neuropathy: Clinical presentations. Arq Neuropsiquiatr. 2013;71:661-6.
- [CrossRef] [PubMed] [Google Scholar]
- Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain. 2017;158:2108-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Discovery of selective, orally bioavailable, N-linked arylsulfonamide Nav1.7 inhibitors with pain efficacy in mice. Bioorg Med Chem Lett. 2017;27:2087-93.
- [CrossRef] [PubMed] [Google Scholar]
- Cannabinoids for the treatment of refractory neuropathic pruritus in amyotrophic lateral sclerosis: A case report. Palliat Med. 2022;36:208-211.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis and management of neuropathic itch. Dermatol Clin. 2018;36:213-24.
- [CrossRef] [PubMed] [Google Scholar]
- Holistic approach to treatment of intractable central neuropathic itch. J Am Acad Dermatol. 2011;64:955-9.
- [CrossRef] [PubMed] [Google Scholar]
- Notalgia paresthetica: Successful treatment with exercises. Acta Derm Venereol. 2011;91:356-7.
- [CrossRef] [PubMed] [Google Scholar]






