Translate this page into:
AIDS vaccine: Present status and future challenges
Correspondence Address:
P K Nigam
Department of Dermatology and Venereology, Pt. JNM Medical College and associated Dr. BRAM Hospital, Raipur, Chattisgarh 492001
India
| How to cite this article: Nigam P K, Kerketta M. AIDS vaccine: Present status and future challenges. Indian J Dermatol Venereol Leprol 2006;72:8-18 |
Abstract
Development of a preventive vaccine for HIV is the best hope of controlling the AIDS pandemic. HIV has, however, proved a difficult pathogen to vaccinate against because of its very high mutation rate and capability to escape immune responses. Neutralizing antibodies that can neutralize diverse field strains have so far proved difficult to induce. Adjuvanting these vaccines with cytokine plasmids and a "prime-boost," approach is being evaluated in an effort to induce both CTL and antibody responses and thereby have immune responses active against both infected cells and free viral particles, thereby necessitating fewer doses of recombinant protein to reach maximum antibodies titers. Although obstacles exist in evaluation of candidate HIV vaccines, evidence from natural history studies, new molecular tools in virology and immunology, new adjuvants, new gene expression systems, new antigen delivery systems, recent discoveries in HIV entry and pathogenesis, and promising studies of candidate vaccines in animal models have provided reasons to hope that developing a safe and effective AIDS vaccine is possible and within reach.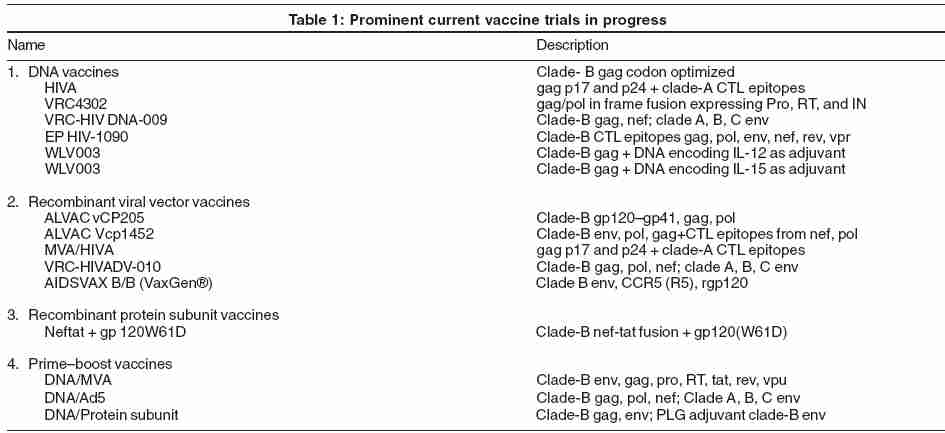

INTRODUCTION
0Human immunodeficiency virus (HIV)-1 was identified in 1983 and subsequently found to be the etiologic agent of the acquired immunodeficiency syndrome (AIDS). UNAIDS and the World Health Organization (WHO) estimate that more than 40 million persons have been infected with HIV-1, with the largest burden in sub-Saharan Africa and in Asia. Although intensive anti-retroviral therapy has been able to slow or halt expansion of the epidemic in some industrialized countries, these treatments are poorly accessible in developing countries. Development of a preventive vaccine for HIV is the best hope of controlling the AIDS pandemic. HIV has, however, proved a difficult pathogen to vaccinate against. This is largely because HIV has a very high mutation rate and can escape immune responses; it has a latent stage where it can rest silently integrated into host DNA, and neutralizing antibodies that can neutralize diverse field strains have so far proved difficult to induce. Evidence from natural history studies, new molecular tools in virology and immunology, new adjuvants, new gene expression systems, new antigen delivery systems, recent discoveries in HIV entry and pathogenesis, and promising studies of candidate vaccines in animal models have provided reasons to hope that developing a safe and effective AIDS vaccine is possible.
EEFFECTORS OF ANTIVIRAL IMMUNITY: CURRENT CONCEPTS
The primary effector mechanisms important for protection against viruses are neutralizing antibodies produced by B-cells and cytolytic activity mediated primarily by CD8+ T-cells. In addition, there are soluble factors produced by activated CD4+ and CD8+ T-cells that have antiviral activity and can influence the differentiation, expansion, and duration of T-cell responses. There are specific neutralizing epitopes, suggesting the site of antibody binding,[1],[2] or neutralization occurs when a threshold level of the virion surface is covered by antibody that binds the native envelope oligomer regardless of specificity.[3]
Antibodies are the only component of the adaptive immune response that can neutralize a virus particle prior to infection of a cell and antibody formation is the only immune response associated with protection for any currently licensed vaccine. Antibody titers can be sustained at high levels in serum and mucosal secretions and can be present at the time of infection. This is unlike T-cells, which only recognize virus in the context of an already infected cell by specific interactions between the T-cell receptor and 8-10 amino acid peptides processed from viral antigens and presented in the context of major histocompatibility complex (MHC) molecules. Therefore, T-cells can only clear virus effectively after infection has occurred. The recognition is restricted by the MHC molecule, which means that the particular epitopes recognized by a given individual will depend on the set of inherited alleles encoding the MHC molecules. Although each person should have the capacity to recognize multiple epitopes among the antigens included in HIV-1, the hierarchy of recognition or epitope dominance may vary even among individuals who share MHC haplotypes. These issues suggest that the epitope repertoire in a vaccine will need to have enough breadth to encompass all the relevant MHC haplotypes of potential vaccinees. In addition, it will be important to induce a broad response in each individual against several viral antigens to diminish the possibility of immune escape through genetic variation and to allow for host selection of dominant epitopes.
CD8+ T-cells are the principal effector mechanism of the adaptive immune response to clear virus-infected cells.[4],[5] The CD8+ lymphocyte recognizes a virus-infected cell through a cognate interaction between the T-cell receptor and a processed peptide epitope presented in the groove of a MHC class-I molecule. Lysis of the infected cells occurs through the production and secretion of perforin and granzymes that penetrate the target cell membrane and induce apoptosis. FasL is also upregulated on the activated CD8+ T-cell, which can bind Fas on the target cell and induce apoptosis through other pathways. CD8+ T-cells also produce cytokines with antiviral properties such as interferon (IFN-g) and tumor necrosis factor-a in addition to other soluble factors that may play a role in virus inhibition.
Although CD4+ T-cells may have some capacity for lysis of HIV-infected cells[6] and production of antiviral cytokines, their major role is in shaping the immune response by establishing a microenvironment with a particular cytokine composition. For HIV and most other viruses, induction of a type-1 cytokine profile [production of interleukin (IL)-12, IL-2, and IFN-a] is more likely to provide protection than induction of type-2 cytokines (IL-4, IL-5, and IL-13).[5]
T-CELLS CAN CONTROL HIV INFECTION
The most compelling evidence for the importance of CD8+ cytotoxic T-lymphocytes (CTLs) for controlling lentivirus infection comes from studies of pathogenesis and vaccine evaluation in nonhuman primate models. The CD8+ CTL response is the best correlate of viremia control after primary simian immunodeficiency virus (SIV) infection in macaques, similar to the findings in HIV-infected humans as discussed above.[7] There are now several studies using nucleic-acid or other recombinant vector approaches that have demonstrated induction of CD8+ CTL responses with a weak or absent antibody response, does not protect from lentivirus infection, but reduces viral load and delays disease progression. With approaches optimizing the CD8+ CTL response, such as by the addition of an IL-2 adjuvant to a recombinant DNA-vaccine regimen, nearly complete control of subsequent simian HIV (SHIV) infection can be achieved.[8]
Control of the initial viremia associated with primary HIV infection temporally correlates with the appearance of CD8+ cytotoxic T-lymphocytes, and mutations in specific CTL epitopes can be detected in the residual virus population.[9] In addition, HIV-specific CD8+ CTL activity has been demonstrated in a small subset of uninfected, seronegative commercial sex workers, suggesting transient infection may have occurred inducing protective immunity mediated by CD8+ CTL. In persons who remain uninfected despite significant occupational exposure to HIV-1-contaminated material, studies have also focused on HIV-specific T cell responses. Although HIV-specific antibodies could not be detected, peripheral blood mononuclear cells show lymphoproliferative activity when stimulated with HIV-specific peptides.[10] Another subset of persons infected with HIV-1 have persistent infection, but do not progress to AIDS. Some of these individuals are infected with virus isolates that replicate poorly.[11] However, others are infected with viruses that have normal replication capacity, but have maintained a strong and broad set of humoral and cellular HIV-specific immune responses that appears to be responsible for their delayed disease progression. This has been associated with HIV-specific CD4+ T-cell proliferation and strong CD8+ CTL activity against multiple epitopes.[12]
VACCINE-INDUCED NEUTRALIZING ANTIBODY RESPONSES IN CLINICAL TRIALS
Neutralizing antibody responses have been induced by immunization with recombinant envelope glycoproteins alone or in combination with poxvirus vectors. The antibody response to immunization with recombinant gp 120 (rgp120) alone is maximal after the third or fourth injection and is dose-dependent. Serum antibody titers have a relatively short half-life. Repeated boosting does not prolong the half-life significantly. Therefore, it is likely that recombinant envelope glycoprotein products may find their greatest utility in boosting antibody responses in subjects primed with recombinant vector vaccines.[13]
The initial recombinant envelope glycoprotein products were derived from sequences of syncytium-inducing, T-cell-line-adapted (TCLA), CXCR4-utilizing X4 viruses from clade B. Newer products, such as the VaxGen B/B product, incorporate sequences from primary isolates which utilize CCR5 (R5) combining the rgp 120 from HIV-1MN and HIV-1GNE8.[14] Although type-specific vaccine antigen neutralization can be induced, neutralization of typical primary R5 HIV isolates is not induced.[15] Recombinant gp120 products induce less binding antibody, but more neutralizing antibody than rgp160 products.[16],[17]
A four-dose immunization regimen using envelope glycoprotein is more effective for antibody induction when there is an interval of several months among doses.[16],[17] The half-life of vaccine antigen-specific antibody titers is 3 months in subjects receiving only rgp120 envelope glycoprotein, regardless of number of doses. The half-life is extended with gp160 antigens, and is also more prolonged when priming with poxvirus vectors precedes rgp120 immunization. Priming with one subtype and boosting with another demonstrates subtype-specificity in the antibody response.[18] When a subject is initially immunized with rgp120 derived from a clade B, TCLA X4 HIV strain, subsequent boosting with another clade B strain does not broaden the response significantly, and does not boost the response to the new envelope antigen as well as to the original rgp120 .[19]
The effects of the antigen dose on the magnitude of antibody production are dependent on the adjuvant formulation. One of the adjuvant QS21 appears to allow a reduction in the antigen dose by more than 10-100 fold without affecting the magnitude of the antibody response.[19] The HIV-specific antibody response after recombinant canarypox immunization alone is weak, but subsequent boosting with purified recombinant envelope subunit protein induces HIV-specific antibody titers of the same magnitude and quality as three or four inoculations of the purified recombinant envelope subunit protein alone.[20],[21]
VACCINE INDUCED CD8+ CTL RESPONSES IN CLINICAL TRIALS
Induction of HIV-specific CD8+ CTL responses requires the delivery of vaccine antigens into the cytoplasmic compartment of an antigen-presenting cell (APC) for display in a MHC class-I molecule on the cell surface. Therefore, vector-based approaches or nucleic-acid vaccines that rely on antigen production within the target cell are most effective. Delivering vaccine antigens as purified proteins or even whole inactivated virus will primarily access the endocytic pathway for antigen presentation and lead to CD4+ T-cell activation. Although this is critical for antibody production and important for supporting CD8+ CTL development, it is not sufficient for inducing CD8+ CTL. In some cases, a novel adjuvant or delivery system is able to provide access for these types of vaccines into the cytoplasmic compartment, but in general, vector-based vaccines, including nucleic acids, are more potent methods for inducing CD8+ CTL.
Recombinant vaccinia expressing envelope glycoprotein alone or multiple antigens has consistently induced long-lived CD8+ CTL responses in vaccinia-naive subjects[22] HIV-specific CD8+ CTL can be detected in a majority of subjects receiving recombinant poxvirus vectors, and, in a subset, CTL activity is detectable for 18 months. However, unlike antibody responses, vaccine-induced CTL responses are broadly reactive.[23] CTL induced by recombinant canarypox vectors has been shown to lyse target cells infected with primary R5 HIV-1 isolates from multiple clades.[23] The classical MHC class-I-restricted cytolytic activity is induced and noncytolytic CD8+-mediated suppression of HIV-1 replication has been demonstrated in recipients of recombinant canarypox vaccines.[24] Envelope subunits can induce CD4+ CTL, but rarely induce CD8+ CTL, even when formulated with novel adjuvants.[16]
COMPLEX STRATEGIES FOR NEUTRALIZATION ESCAPE
The induction of strong cross-reactive neutralizing antibody responses is considered the ultimate goal for an effective HIV vaccine. Observations in chimpanzees[25] and in macaque infection models support the view that humoral immune responses may contribute to the control of HIV or SIV replication.[26] However, it has been difficult to elicit antibodies with the capacity to neutralize a diversity of primary HIV-1 isolates. The antigenic variation of envelope has been one stated reason for this difficulty. However, recent studies have focused attention on other even more complex methods of neutralization resistance such as oligomerization and glycosylation of receptor-binding sites located between the inner and outer domains of gp120[27] and glycosylation of the HIV envelope.[28]
Several groups have observed that during HIV infection, virus in the blood continues to evolve away from the host, neutralizing antibody response.[29],[30] Over time, early viral populations, which are neutralization-sensitive, evolve, and are replaced by neutralization-resistant viruses. Wei et al.[29] determined that the escaped virus (neutralization-resistant viruses) contained mutations in the env gene involving primarily changes at glycosylation sites, suggesting that HIV often escapes from humoral responses by modifying its "heavy glycan shield."
Recent studies have focused interest on the Gordian knot of HIV vaccines. The V3 loop of gp120 has long been considered the principal neutralizing HIV-1 domain, evoking type-specific and minimally cross-neutralizing antibodies. However, such responses are often poor at neutralizing primary HIV-1 isolates and its dramatic variability has been the downfall of many creative and complex HIV immunogens targeting this area. This loop plays a key role in determining cell tropism of HIV through differential binding to the CXCR4 and/or CCR5 coreceptors on the target cell. Delineating the three-dimensional structure of this loop could be a key element for designing vaccine components able to induce more broadly neutralizing responses. By studying V3 peptides complexed with monoclonal antibodies that bind to and neutralize virions, Zolla-Pazner et al. elucidated a V3 loop structure.[31] Their studies revealed an apparent homology between the structure of V3 and those of chemokines, which are ligands for the HIV coreceptors, indicating that one of two alternative conformations of the V3 loop exist for viruses that bind to either CCR5 or CXCR4.[31]
PRIME-BOOST IN HIV VACCINES
In an effort to induce both CTL and antibody responses and thereby have immune responses active against both infected cells and free viral particles, attention has turned to evaluating a combination approach, called "prime-boost," where a few doses of a recombinant viral vector (the "prime") are followed by or combined with several doses of a recombinant protein (the "boost"). Several recombinant-attenuated vaccinia vectors and recombinant canarypox vectors have been evaluated in phase-I trials alone and in combination with a recombinant protein envelope boost.
To date, all recombinant viral vectors have been safe and immunogenic and have been shown to prime the immune response to an envelope boost, thereby necessitating fewer doses of recombinant protein to reach maximum antibody titers. However, the antibodies elicited in prime-boost protocols so far have a limited breadth of reactivity.[29] In general, vaccinia -immune individuals have not responded as well to vaccinia vectors as vaccinia -naive individuals have, although there has been no difference in the response of these groups to recombinant canarypox vectors.[28] For this reason, as well as the fact that canarypox does not replicate completely in human cells and is therefore considered safe, canarypox vectors have been the focus of recent clinical studies. Interestingly, at least some CTLs induced by recombinant canarypox vectors based on clade-B HIV and directed against the gag protein were able to kill cells infected with HIV from other clades, because of the more conserved nature of gag and other internal proteins.[32]
Prime-boost approach of plasmid DNA followed by Ad5 vector (both expressing SIV-gag) elicited strong immunogenicity in rhesus macaques after challenge with SHIV-89.6P and these animals exhibited pronounced attenuation of the virus infection.[33] More recent macaque studies in a collaboration between Merck and Aventis Pasteur tested the utility of Ad5 priming followed by a canarypox vector boost (ALVAC vCP205). Other studies have also reported that several different poxviral vectors could function well as boosting agents.[34]
Another important new recombinant vector that is under investigation is vesicular stomatitis virus (VSV). Recently, it was reported that VSV delivered as a mucosal vaccine vector can prime for strong CD8+ T-cell responses and impact viral load in the macaque model system.[35] It has also been demonstrated that recombinant VSV vectors containing SIV antigens can be effectively primed by recombinant pox viral vectors.[36]
CYTOKINE ADJUVANTS
DNA vaccines for HIV-1 appeared on the horizon over a decade ago full of luster and promise. However, recent developments in adjuvanting these vaccines have generated renewed interest in this approach. Cytokine plasmids, which adjuvant DNA vaccines for HIV-1, have been under investigation for several years.[37] The first primate challenge study of an adjuvanted DNA vaccine was reported by Barouch et al.[38] These studies demonstrated that immune responses elicited in rhesus macaques by DNA vaccines can be augmented about twice by the administration of IL-2/Ig fusion protein or a plasmid encoding IL-2/Ig. Moreover, cytokine-augmented DNA-vaccine-immunized macaques managed to control viral replication to a greater extent than animals immunized with DNA only.
Several recombinant envelope products, rgp120 or rgp160 , produced in insect, yeast, or mammalian cells formulated with a variety of adjuvants have been evaluated in clinical trials. Peptides tested have been derived from envelope V3 loop or gag sequences of clade B or multiple clades. They have been presented conjugated to an oligolysine backbone, as a lipopeptide conjugate, mixed with adjuvant, or as a fusion protein with the self-assembling yeast protein Ty as a particle. They have been administered intramuscularly in the deltoid or anterior thigh, rectally and orally as Ty-gag-virus-like particles, and orally encapsulated in polylactide copolymers.
Live recombinant vectors including vaccinia , canarypox , salmonella , as well as nucleic-acid-based vaccines have been evaluated. These vectors have been delivered by a variety of routes and have been constructed to express either single or multiple HIV-1 antigens. In addition, there have been studies evaluating schedule of administration and combination approaches using more than one product in the immunization regimen.
IDEAL HIV VACCINE
An ideal HIV vaccine should induce a wide variety of immune responses against multiple viral antigens to combat infectious viral particles as well as HIV-infected cells at any time of HIV replication. Recently, there has been an enormous boost of new vaccine approaches to improve the immunogenicity of vaccine antigens and their delivery into appropriate immunological compartments. In addition to conventional recombinant protein immunization strategies, the use of synthetic peptides, recombinant proteins, whole-killed HIV, non-replicating-HIV-like particles ( pseudovirions ), and live-attenuated HIV are under study. Genetic immunization techniques using naked or liposomal entrapped DNA , live bacterial or viral vectors, as well as replication-defective replicons that carry selective viral genes represent second- and third-generation delivery systems with promising concepts of innovative vaccination strategies. Eventually, a complex, multicomponent vaccine containing multiple, precisely selected antigen-encoding genes, as well as proteins or peptides of representative and diverse subtypes, could possibly result in an additive or synergistic effect capable of inducing stronger, broader, or more prolonged immune responses. Moreover, an HIV vaccine should induce immune responses that are broadly reactive in order to confer protection against almost all genetic HIV subtypes. Although such a candidate vaccine does not exist so far, it remains a realistic and achievable goal.
CLINICAL EVALUATION OF CANDIDATE HIV VACCINES
More than 60 phase-I trials of approx 30 candidate vaccines have been conducted in uninfected volunteers worldwide. Most of the initial approaches focused on the HIV envelope protein because the envelope is the primary target for neutralizing antibodies in HIV-infected persons. These candidates were immunogenic in diverse populations and induced neutralizing antibody in nearly 100% of recipients. Mammalian-derived envelope candidates induced higher titers of neutralizing antibody than candidates produced in yeast, insect cells, or bacteria. However, the antibodies induced by these early envelope preparations were largely specific for clade-B isolates. In addition, antibodies induced by these early envelope preparations rarely neutralized primary isolates of HIV derived from patient blood with minimal manipulations. In addition, recombinant proteins alone rarely induced CD8+ CTLs, which recognize and kill cells that have been infected with HIV.[39]
The first phase-I trial of a candidate vaccine in Africa was launched early in 1999. This trial will determine the safety and immunogenicity of vCP205 in Ugandan volunteers and the extent to which immunized Ugandan volunteers have CTLs that are active against the subtypes A and D of HIV, which are prevalent in Uganda.
A phase-II trial of a recombinant canarypox vector, vCP205, and an envelope vaccine, gp120 (SF2), was concluded in 1999 and demonstrated the safety and immunogenicity of that vaccine.[40] Another phase-II trial of a canarypox vector, vCP1452, and AIDSVAX, the bivalent gp120, will soon be under way in the United States, Haiti, Trinidad, and Brazil to expand safety information on that combination and to address schedule questions.
Other strategies that have progressed to phase-I trials in uninfected persons include HIV peptides, HIV lipopeptides, DNA expressing one or more HIV proteins, and an attenuated Salmonella - vector-expressing envelope p24 . To date, none has proved as effective in eliciting human CTLs and/or antibody as the recombinant canarypox - gp120 combination. Recently, Merck advanced a candidate DNA vaccine containing a codon-optimized gag gene to phase-I trials. Other approaches to increasing the immunogenicity of DNA vaccines are being pursued and may enter phase-I trials in the next few years.
VaxGen ® [Table - 1] announced the results of the first AIDS vaccine candidate to complete a phase-III trial.[41] Their vaccine is a bivalent HIV monomeric envelope gp120 from clade B (AIDSVAX B/B). A total of 5009 participants, predominantly gay men at high risk, were part of this randomized, double-blind, placebo-controlled trial. They reported that 5.7% of the vaccinated group and 5.8% of the placebo group became HIV-infected by end of the 3-year period, indicating that the vaccine offered no benefit. There is also the question of protection bias with gender differences in these subsets.[39],[42] A trend toward more favorable results in women was observed. In the meantime, the results from a second VaxGen ® phase-III trial in Thailand (AIDSVAX B/E, which uses a combination of clade-B and -E gp120 ), are awaited with interest.
The impact of the cellular immune response is also under evaluation in mostly early phase-I or -II studies. In a compelling study, Merck just reviewed the results from ongoing preventive trials focused on two vaccine candidates, one based on DNA and the other based on a recombinant Ad5 vector.[43] Preliminary data from safety and immunogenicity in uninfected humans suggest that both vaccine modalities were generally well tolerated. DNA- gag by itself can elicit a moderate gag-specific cellular immune response by enzyme-linked immuno-SPOT in 31-40% of the volunteers at the 30-week time point. A gag -expressing Ad5 vector proved to be more immunogenic, with about 60% of the volunteers responding at both 8 and 30 weeks.
Canarypox vectors have also been evaluated for safety and immunogenicity in humans. ALVAC-HIV vCP205 was given in combination with rgp120 to both high-risk and low-risk persons[44] or with rgp160 to HIV-1-uninfected adults.[45] These vaccines raised specific-CTL responses through increasing the dose of the vaccine, higher titers of neutralizing antibodies resulted after rgp120 boost rather than from simultaneous administration of both modalities.[46] ALVAC-HIV vCP205 prime or rgp160 boost generated a strong and broad env T-helper response compared with subjects receiving vCP205 vaccine alone.[47] Preclinical studies of the DNA-MVA/HIVA, consisting of a consensus clade-A gag p24/p17 and a string of clade-A-derived CTL epitopes, showed the induction of cellular immune responses specific for multiple HIV-derived epitopes.[46] Phase-I trials in low-risk volunteers have commenced in Oxford and Nairobi. Preliminary immunogenicity data from the Oxford site indicated that the vaccine induced T-cell responses in some volunteers.[47]
Owing to an enormous amount of preclinical work over the past several years, many other vaccines are being studied and include important concepts worth watching. These include the Venezuelan equine encephalitis virus vectors (encoding gag from clade C), the heat-killed recombinant Saccharomyces cerevisiae (expressing gag from clade B), the IL-2/Ig cytokine adjuvanted DNA of the VRC in collaboration with Harvard, the IL-12 cytokine adjuvanted gag subtype B vaccine of Wyeth and the DNA prime/MVA boost strategy being developed by Harriet Robinson at Emory.[48]
AIDS VACCINE INTERNATIONAL CONFERENCE, LAUSANNE, SWITZERLAND, 2004
Some of the observations made at the AIDS Vaccine 2004 International Conference in Lausanne, Switzerland, 26th-28th August, 2004, organized by the WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS) are as follows:
1. SIV is the virus equivalent of HIV in many nonhuman primate species. Preliminary data presented show that immune responses specific for multiple cytotoxic T-lymphocyte (CTL) and helper T-lymphocyte (HTL) epitopes were induced by the study vaccine and CTL-specific responses were increased by fivefold to tenfold following SIV viral challenge.
2. Duration of infection, not viral load, is responsible for the decrease in HIV-specific CD4 and CD8 cells.
3. Greater participation of women and adolescents is needed in HIV-vaccine clinical trials.
The group expressed hope that a viable first-generation vaccine would be ready for large-scale trials in the next 4 years.
OBSTACLES AND FUTURE PRIORITIES
The major priorities for AIDS vaccine research and development are to design vaccines to induce strong neutralizing antibody responses and strong CTL responses, to continue a focus on mucosal immunity and correlates of immunity, and performance of large-scale clinical trials. These include the following:
1. Identification of antigenic structures- This can induce neutralizing antibody activity against primary HIV-1 isolates.
2. Mucosal immunity -Mucosal immunization in several monkey systems has resulted in protection from rectal or vaginal mucosal challenge.
3. Adjuvants- Methods to enhance the immunogenicity of HIV antigens and increase induction of immune memory. Various approaches are being explored to enhance cellular responses and induce humoral responses with DNA vaccines encoding HIV antigens, for example, by including DNA plasmids directing the production of cytokines, such as IL-12, or purified IL-12 protein, coadministration of IL-4, granulocyte-macrophage colony-stimulating factor, CpG-containing oligodeoxynucleotides, etc.
4. Dendritic cell targeting - Dendritic cells are the APCs of the immune system and certain viral vectors can specifically target dendritic cells. Research groups are actively exploring alternative approaches to target candidate vaccines to dendritic cells.
5. Animal models - Efforts to develop an appropriate small-animal model to expedite vaccine evaluation continue. With increased understanding of the interaction of HIV proteins with host cells, investigators are hopeful that a transgenic small animal may well be on the horizon.
6. Development of new approaches -To optimize the duration, kinetics, magnitude, and breadth of vaccine-induced memory CTL response .
7. Evaluation of candidate vaccines -Another important area of active research is in the evaluation of candidate HIV/AIDS vaccines in animal models. There are good animal models of HIV/AIDS, namely, SIV and SHIV infection in monkeys. SHIVs are chimeric SIV/HIV viruses that contain the HIV env and associated tat, vpu, and rev genes, along with the full complement of the remaining genes of SIV. However, because these models are not HIV itself, concept testing of a candidate vaccine requires testing the SIV or SHIV analog, which may or may not correlate with how the human vaccine will perform in humans.
8. The challenge of conducting efficacy trials - High-risk populations are among the hardest to recruit and retain in vaccine efficacy trials. Community education and support have proved essential to the conduct of clinical trials.
9. Infrastructure - Few developing countries have trained investigators and infrastructure to conduct trials of HIV vaccines.
10. Social challenges -In addition to the primary goal of ensuring volunteer safety, it is to ensure that volunteers do not suffer social harm because of trial participation. For example, persons who test positive for HIV on serologic assays may suffer discrimination in employment, health insurance, life insurance, and immigration.
11. Others -Other challenges to HIV-vaccine development cross into the financial, political, and social realms, and require new approaches and legislative proposals to address them.
AIDS VACCINE TRIAL IN INDIA
National AIDS Research Institute (NARI), Pune, is conducting an international multicentric phase-I HIV-vaccine trial in healthy volunteers under the joint auspices of the National AIDS Control Organization (NACO), the Indian Council of Medical Research (ICMR), and sponsored by the International AIDS Vaccine Initiative (IAVI). The vaccine being tested is called tgAAC09 . The vaccine does not contain HIV virus. Therefore, the volunteers cannot get infected with HIV from this vaccine. This is a preventive vaccine intended for people who are not infected with HIV. It has been tested in animals prior to this trial. Data from animal and preclinical studies indicate that the vaccine is safe and well tolerated, allowing testing in human beings. The phase-I trial of this vaccine is also ongoing in Belgium and Germany.
CONCLUSIONS
Despite all the challenges inherent in HIV-vaccine development and delivery, a preventive vaccine for AIDS remains the best hope to end the global epidemic. Researchers, public health leaders, governments, private organizations and companies, and affected communities must work together closely to accelerate research and delivery of HIV vaccines. The ultimate vaccine that can prevent persistent HIV-1 infection will probably require a conceptual breakthrough in the understanding of how to elicit a broadly neutralizing antibody response against primary R5 HIV-1 isolates, and an optimal HIV-specific CD8+ CTL response. Large clinical trials will remain a critical component of the search for safe and effective HIV vaccines. However, a vaccine aimed at control of viremia, delayed disease progression, and reduced transmission based on induction of HIV-specific CD8+ CTL could have a significant impact on the AIDS epidemic and may be within our grasp using currently available biotechnology.
MULTIPLE-CHOICE QUESTIONS
1. The HIV-1 virus was identified in the year
(a) 1981 (c) 1983
(b) 1982 (d) 1984
2. The primary effector mechanisms important for protection against viruses are
(a) Neutralizing antibodies produced by B-cells
(b) Cytolytic activity mediated primarily through CD8+ T-cells
(c) Soluble factors produced by activated CD4+ and CD8+ T-cells
(d) All of the above
3. The neutralization of a virus particle prior to infection of the cell can be mediated by
(a) Antibody in the serum (c) T-cells
(b) MHC molecules (d) All of the above
4. CD4+ T-cells cause lysis of HIV-infected cells mainly through
(a) Production and secretion of perforin and granzymes
(b) Production of antiviral cytokines
(c) Shaping the immune response with a particular cytokine composition
(d) FasL
5. The best correlate of viremia control after primary SIV infection is
(a) CD8+ CTL response (b) Neutralizing antibody response
(c) Both (d) None
6. The augmentation of DNA vaccine for HIV-1 can be done through
(a) Cytokine plasmids (b) Peptides derived from envelope
(c) Live recombinant vectors (d) All of the above
7. All of these are vectors used in vaccine preparation, except
(a) Canarypox (b) Salmonella
(c) Venezuelan equine encephalitis virus (d) Cowpox
8. The half-life of vaccine antigen-specific antibody titers in subjects receiving only rgp120 envelope glycoprotein, regardless of number of doses is about
(a) Three months (b) One month
(c) Nine months (d) Six months
9. The vaccine being tested at NARI, Pune, is
(a) WLV003 (b) Vcp1452
(c) DNA/Ad5 (d) tgAAC09
10. In clinical trials, the neutralizing antibody response to immunization with rgp120 alone is observed to be dose dependent and maximum after
(a) First injection (b) Second injection
(c) Third or fourth injection (d) No neutralizing antibody response is induced
ANSWERS
1-(c), 2-(d), 3-(a), 4-(c), 5-(a), 6-(d), 7-(d), 8-(b), 9-(d), 10-(c)
| 1. |
Goudsmit J, Boucher CA, Meloen RH, Epstein LG, Smit L, van der Hoek L, et al. Human antibody response to a strain-specific HIV-1 gp120 epitope associated with cell fusion inhibition. AIDS 1988;2:157-64.
[Google Scholar]
|
| 2. |
Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, et al . Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 1993;67:3978-88.
[Google Scholar]
|
| 3. |
Burton DR, Williamson RA, Parren PW. Antibody and virus: binding and neutralization. Virology 2000;270:1-3.
[Google Scholar]
|
| 4. |
Ada G, Leung KN, Erti H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses . Immunol Rev 1981;58:5-24.
[Google Scholar]
|
| 5. |
Sethi KK, Omata Y, Schneweis KE. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol 1983;64:443-7.
[Google Scholar]
|
| 6. |
Siliciano RF, Lawton T, Knall C, Karr RW, Berman P, Gregory T, et al . Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell 1988;54:561-75.
[Google Scholar]
|
| 7. |
Reimann KA, Tenner RK, Racz P, Montefiori DC, Yasutomi Y, Lin W, et al . Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol 1994;68:2362-70.
[Google Scholar]
|
| 8. |
Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Tong-Ming F, Wagner W, et al . Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000;290:486-92.
[Google Scholar]
|
| 9. |
Price DA, Goulder PJ, Klenerman P, Sewell AK, Easterbrook PJ, Troop M, et al . Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA 1997;94:1890-5.
[Google Scholar]
|
| 10. |
Clerici M, Levin JM, Kessler HA, Harris A, Berzofsky JA, Landay AL, et al . HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA 1994;271:42-6.
[Google Scholar]
|
| 11. |
Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med 1995;332:228-32.
[Google Scholar]
|
| 12. |
Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, et al . Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997;278:1447-50.
[Google Scholar]
|
| 13. |
Graham BS, Matthews TJ, Belshe RB, Clements ML, Dolin R, Wright PF, et al . Augmentation of human immunodeficiency virus type1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. The NIAID AIDS vaccine clinical trials network. J Infect Dis 1993;167:533-7.
[Google Scholar]
|
| 14. |
Berman PW, Huang W, Riddle L, Gray AM, Wrin T, Vennari J, et al . Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology 1999;265:1-9.
[Google Scholar]
|
| 15. |
Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, et al . Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis 1996;173:340-8.
[Google Scholar]
|
| 16. |
Graham BS, Keefer MC, McElrath MJ, Gorse GJ, Schwartz DH, Weinhold K, et al . Safety and immunogenicity of a candidate HIV-1 vaccine in healthy adults: Recombinant glycoprotein (rgp) 120-a randomized, double-blind trial. Ann Intern Med 1996;125:270-9.
[Google Scholar]
|
| 17. |
Belshe RB, Graham BS, Keefer MC, Gorse GJ, Wright P, Dolin R , et al . Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. NIAID AIDS vaccine clinical trials network. JAMA 1994;272:475-80.
[Google Scholar]
|
| 18. |
Corey L, McElrath MJ, Weinhold K, Matthews T, Stablein D, Graham B, et al . Cytotoxic T cell and neutralizing antibody responses to HIV-1 envelope with a combination vaccine regimen. J Infect Dis 1998;177:301-9.
[Google Scholar]
|
| 19. |
Gorse GJ, Patel GB, Newman FK. Antibody to native human immunodeficiency virus type 1 envelope glycoproteins induced by IIIB and MN recombinant gp120 vaccines. Clin Diag Lab Immunol 1996;3:378-86.
[Google Scholar]
|
| 20. |
Clements-Mann ML, Matthews TJ, Weinhold K. HIV-1 immune responses induced by canarypox (ALVAC)-gp160 MN, SF-2 rgp120, or both vaccines in seronegative adults. J Infect Dis 1998;177:1230-46.
[Google Scholar]
|
| 21. |
Evans TG, Keefer MC, Weinhold KJ, Wolff M, Montefiori DM, Gorse GJ, et al . A canarypox vaccine expressing multiple HIV-1 genes given alone or with rgp120 elicits broad and durable CD8+ CTL responses in seronegative volunteers. J Infect Dis 1999;180:290-98.
[Google Scholar]
|
| 22. |
El-Daher N, Keefer MC, Reichman RC, Dolin R, Roberts NJ. Persisting human immunodeficiency virus type 1 gp160-specific human T lymphocyte responses including CD8+ cytotoxic activity after receipt of envelope vaccines. J Infect Dis 1993;168:306-13.
[Google Scholar]
|
| 23. |
Ferrari G, Humphrey W, McElrath MJ. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA 1997;94:1396-401.
[Google Scholar]
|
| 24. |
Castillo RC, Arango-Jaramillo S, John R, Weinhold K, Kanki P, Carruth L, et al . Resistance to human immunodeficiency virus type 1 in vitro as a surrogate of vaccine-induced protective immunity. J Infect Dis 2000;181:897-903.
[Google Scholar]
|
| 25. |
Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van Opstal O, et al . HIV-1 envelope-elicited neutralizing antibody titers correlate with protection and virus load in chimpanzees. Vaccine 1994;12:1141-8.
[Google Scholar]
|
| 26. |
Schmitz JE, Kuroda MJ, Santra S, Simon MA, Lifton MA, Lin W, et al . Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J Virol 2003;77:2165-73.
[Google Scholar]
|
| 27. |
Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, et al . HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 2002;420:678-82.
[Google Scholar]
|
| 28. |
Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat Med 1998;4:679-84.
[Google Scholar]
|
| 29. |
Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al . Antibody neutralization and escape by HIV-1. Nature 2003;422:307-12.
[Google Scholar]
|
| 30. |
Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA 2003;100:4144-9.
[Google Scholar]
|
| 31. |
Zolla-Pazner S, Sharon M, Kessier N, Levy R, G φrlach M, Anglister J. Alternative conformations of HIV-1 V3 loops mimic b hairpins in chemokines, suggesting a mechanism for coreceptor selectivity. Structure 2003;11:225-36.
[Google Scholar]
|
| 32. |
Quinones-Kochs MI, Buonocore L, Rose JK. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J Virol 2002;76:4199-211.
[Google Scholar]
|
| 33. |
Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al . Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002;415:331-5.
[Google Scholar]
|
| 34. |
Baig J, Levy DB, McKay PF, Schmitz JE, Santra S, Subbramanian RA, et al . Elicitation of simian immunodeficiency virus-specific cytotoxic T lymphocytes in mucosal compartments of rhesus monkeys by systemic vaccination. J Virol 2002;76:1484-90.
[Google Scholar]
|
| 35. |
Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, et al . An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 2001;106:539-49.
[Google Scholar]
|
| 36. |
Ramsburg E, Rose NF, Mefford M, Haglund K, Buonocore L, Nixon DF, et al . A single VSV/SHIV immunization followed by one heterologous MVA/SHIV boost results in effective control of peak virus load after SHIV challenge. AIDS 2003;17:S117-24.
[Google Scholar]
|
| 37. |
Boyer JD, Cohen AD, Ugen KE, Edgeworth RL, Bennett M, Shah A, et al . Therapeutic immunization of HIV-infected chimpanzees using HIV-1 plasmid antigens and interleukin-12 expressing plasmids. AIDS 2000;14:1515-22.
[Google Scholar]
|
| 38. |
Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, et al . Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000;290:486-92.
[Google Scholar]
|
| 39. |
Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, et al . Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol 2002;76:5357-68.
[Google Scholar]
|
| 40. |
Belshe RB, Stevens C, Gorse GJ, Buchbinder S, Weinhold K, Sheppard H, et al . Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J Infect Dis 2001;183:1343-52.
[Google Scholar]
|
| 41. |
Cohen J. AIDS vaccine trial produces disappointment and confusion. Science 2003;299:1290-1.
[Google Scholar]
|
| 42. |
Calarota SA, Leandersson AC, Bratt G, Hinkula J, Klinman M, Weinhold KJ, et al . Immune responses in asymptomatic HIV-1-infected patients after HIV-DNA immunization followed by highly active antiretroviral treatment. J Immunol 1999;163:2330-8.
[Google Scholar]
|
| 43. |
Kahn P. Update on Mercks AIDS vaccine program. IAVI Report February April 2003. Quoted by Calarota SA, Weiner DB. Present status of human HIV vaccine development. AIDS 2003;17:S73-S84.
[Google Scholar]
|
| 44. |
Gupta K, Hudgens M, Corey L, McElrath J, Weinhold K, Montefiori DC, et al . Safety and immunogenicity of a high-titred canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS Vaccine Evaluation Group Protocol 022A. J Acquir Immune Defic Syndr 2002;29:254-61.
[Google Scholar]
|
| 45. |
Ratto-Kim S, Loomis-Price LD, Aronson N, Grimes J, Hill C, Williams C, et al . Comparison between env-specific T-cell epitopic responses in HIV-1-uninfected adults immunized with combination of ALVAC-HIV (vCP205) plus or minus rgp160MN/LAI-2 and HIV-1-infected adults. J Acquir Immune Defic Syndr 2003;32:9-17.
[Google Scholar]
|
| 46. |
Wee EG, Patel S, McMichael AJ, Hanke T. A DNA/MVA-based candidate human immunodeficiency virus vaccine induces multi-specific T cell responses in rhesus macaques. J Gen Virol 2002;83:75-80.
[Google Scholar]
|
| 47. |
Hanke T, McMichael AJ, Mwau M, Wee EG, Ceberej I, Patel S, et al . Development of a DNA-MVA/HIVA vaccine for Kenya. Vaccine 2002;20:1995-8.
[Google Scholar]
|
| 48. |
Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, et al . Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 2001;292:69-74. Quoted by Newman MJ. Heterologous prime-boost vaccination strategies for HIV-1: Augmenting cellular immune responses. Curr Opin Invest Drugs 2002;3:374-8.
et al . Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 2001;292:69-74. Quoted by Newman MJ. Heterologous prime-boost vaccination strategies for HIV-1: Augmenting cellular immune responses. Curr Opin Invest Drugs 2002;3:374-8.'>[Google Scholar]
|
Fulltext Views
5,162
PDF downloads
4,075





