Translate this page into:
Alveolar soft part sarcoma presenting as metastatic nodule on the nose
Correspondence Address:
A S Sanjana
No 48/22, 2nd Cross, MICO Layout, Attiguppe, Vijayanagar, Bangalore - 560040, Karnataka
India
| How to cite this article: Kumar S, Sanjana A S, Gopal M G, Nandini A S. Alveolar soft part sarcoma presenting as metastatic nodule on the nose. Indian J Dermatol Venereol Leprol 2012;78:666 |
Sir,
The term ′Alveolar Soft Part Sarcoma′ (ASPS) for a distinctive and hitherto unrecognized soft tissue tumor was coined by Christopherson et al. [1] The entity ASPS had been described by a variety of descriptive terminologies including ′malignant myoblastoma′, ′granular cell myoblastoma′ and ′malignant granular cell myoblastoma′. ASPS is a rare, poor prognosis neoplasm of unknown histiogenesis with a distinctive histology, specific molecular characteristics and unique clinical behaviours.
A 44 year old male patient was presented with erythematous nodule on the tip of the nose since 3 months [Figure - 1]a. The nodule was situated over an erythematous telangiectatic base. On general physical examination a diffuse mass on the lower third of the right thigh involving the anterolateral compartment was noticed [Figure - 1]b.
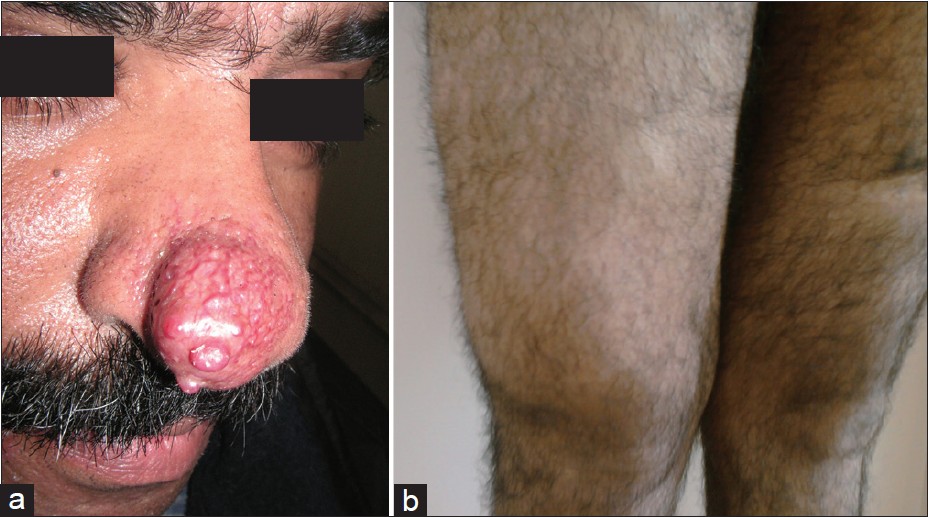 |
| Figure 1: (a) Erythematous nodule on the tip of right side of the nose with telangiectasia (b) Diffuse mass involving the lower 1/3rd of the anterolateral aspect of right thigh |
Incisional biopsy of the erythematous nodule on the nose was done under local anaesthesia. Simultaneously, FNAC of the thigh mass was done. Histopathological examination of the nodule biopsy specimen revealed neoplastic cells predominantly arranged in tubuloalveolar pattern and in nests, separated by fine fibro vascular septae [Figure - 2]a. Individual cells were round to polygonal with clear granular cytoplasm, mild nuclear pleomorphism with prominent nucleoli. Few mitoses were noted. FNAC of the thigh mass revealed similar cytological pattern. Hence, a diagnosis of ASPS of the right thigh with metastatic nodule on the nose was made.
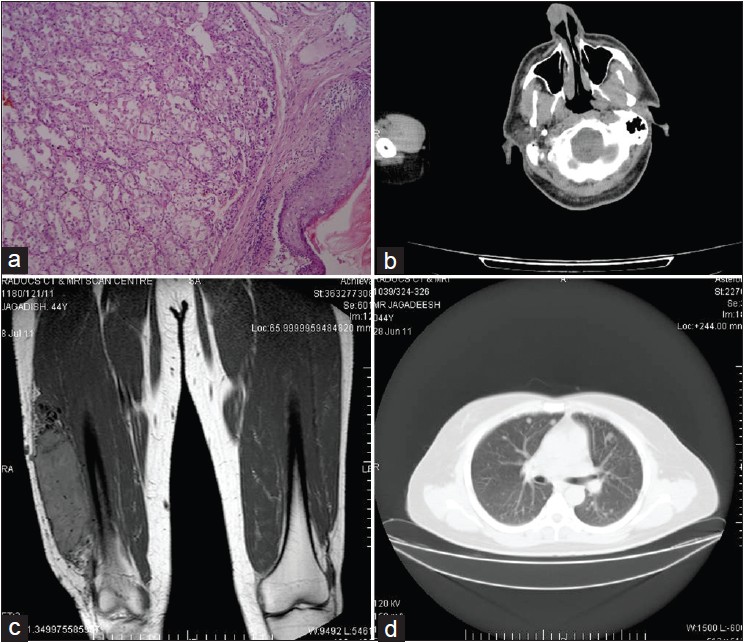 |
| Figure 2: (a) Histopathological picture showing alveolar pattern of arrangement of tumor cells (Haematoxylin and eosin stain, 40× magnification) (b) CT head showing heterogeneously enhancing soft tissue mass on the right side of the nose with septal infiltration (c) MRI thigh showing T2 enhanced well circumscribed lesion involving muscles of the lower 1/3rd of anterolateral aspect of thigh without bone involvement (d) CT Thorax showing multiple varying size metastatic nodules in both the lungs |
On further workup including CT scan head and neck revealed heterogeneously enhancing soft tissue mass on the right side of the nose, moderately vascular, with infiltration of the adjacent nasal septum [Figure - 2]b. Brain imaging was normal. MRI thigh demonstrated well circumscribed malignant lesion involving the muscles of the lower 1/3 rd of the anterolateral aspect of right thigh with no bony infiltration [Figure - 2]c. CT thorax showed multiple well defined rounded nodules measuring 4 mm to 25 mm [Figure - 2]d. Ultrasound abdomen was normal. Complete blood count, peripheral smear, erythrocyte sedimentation rate, blood urea and serum creatinine were within normal limits. Immunohistochemical analysis was positive for desmin and neuron specific enolase, and negative for S-100 and myoverdin.
The patient was counselled regarding the diagnosis and its prognosis and was offered chemotherapy. However, patient refused the treatment and is doing well on follow up at 8 months after the diagnosis.
ASPS is rare, distinctive sarcoma with a relatively indolent clinical course. However, the prognosis is poor and is often characterized by late metastases. Immunohistochemical analysis in general is negative for epithelial markers, neuroendocrine markers and melanocytic markers. Neuron specific enolase and vimentin may be present in 30% to 50%. Anti-bodies to actin and desmin is reported in 50% of cases of ASPS.
Cytogenetic analysis characteristically shows a tumor specific unbalanced translocation: der (17) t (x:7) (p11:25). This translocation causes the fusion of the transcription factor TEF 3 located on xp11.22 with a novel gene at 17q25, named ASPL, also known as ASPSCR1. [2] The translocation creates a novel TFE 3 fusion protein that appears to act as an aberrant transcription factor, inducing unregulated transcription of TFE 3 regulated genes.
The treatment of localised disease is radical resection. Metastatic ASPS is treated by resection of tumor and metastatectomy. The most common metastatic sites include lung, bone, central nervous system and liver. [3]
Survival rates of ASPS are 77% at 2 years, 60% at 5 years, 38% at 10 years and only 15% at 20 years by Lieberman et al. [4] Another study from MD Anderson Cancer Centre, Texas, USA found a 5 year disease free survival of 71% in localised disease group as compared with only 20% survival in metastatic disease group.
ASPS is resistant to conventional chemotherapy and radiotherapy, hence the search for novel therapies and their evaluation is being done in clinical trials. Molecular targeted treatment has been increasingly utilized. The c-Met receptor is activated in ASPS. ARQ 197, a novel c-Met inhibitor has shown a disease control rate of 80%. KRX-0401 (perifosine) an AKT inhibitor is also under evaluation. Anti-angiogenic agents under evaluation include bevacizumab, cediranib (AZD2171) and sunitinib malate. [5]
| 1. |
Christopherson WM, Foote FW Jr, Stewart FW. Alveolar soft-part sarcomas; structurally characteristic tumors of uncertain histogenesis. Cancer 1952;5;100-11.
[Google Scholar]
|
| 2. |
Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, et al. The der (17) t (X;17) (p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 2001;20;48-57.
[Google Scholar]
|
| 3. |
Portera CA Jr, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM, et al. Alveolar soft part sarcoma: Clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer 2001;91;585-91.
[Google Scholar]
|
| 4. |
Lieberman PH, Brennan MF, Kimmel M, Erlandson RA, Garin-Chesa P, Flehinger BY, et al. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer 1989;63;1- 13.
[Google Scholar]
|
| 5. |
Stacchiotti S, Tamborini E, Marrari A, Brich S, Rota SA, Orsenigo M, et al. Response to sunitinib malate in advanced alveolar soft part sarcoma. Clin Cancer Res 2009;15;96-104.
[Google Scholar]
|
Fulltext Views
2,549
PDF downloads
2,493





